Thermal Degradation Kinetics of ZnO/polyester Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ZnO Nanoparticles
2.3. UPR/ZnO Nanocomposites
2.4. Characterization Techniques
2.5. Kinetic Models
3. Results and Discussion
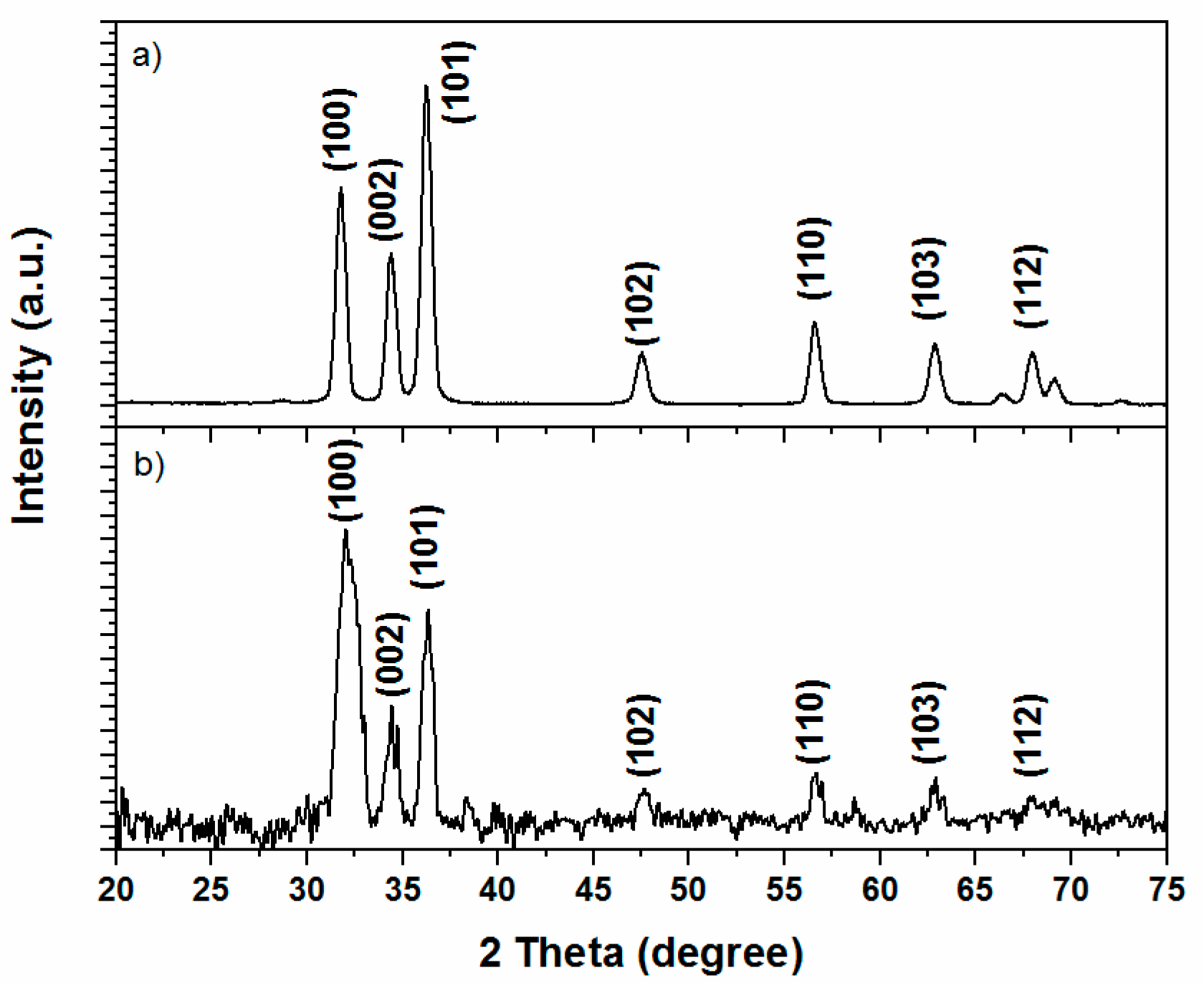
3.1. ZnO Microstructure
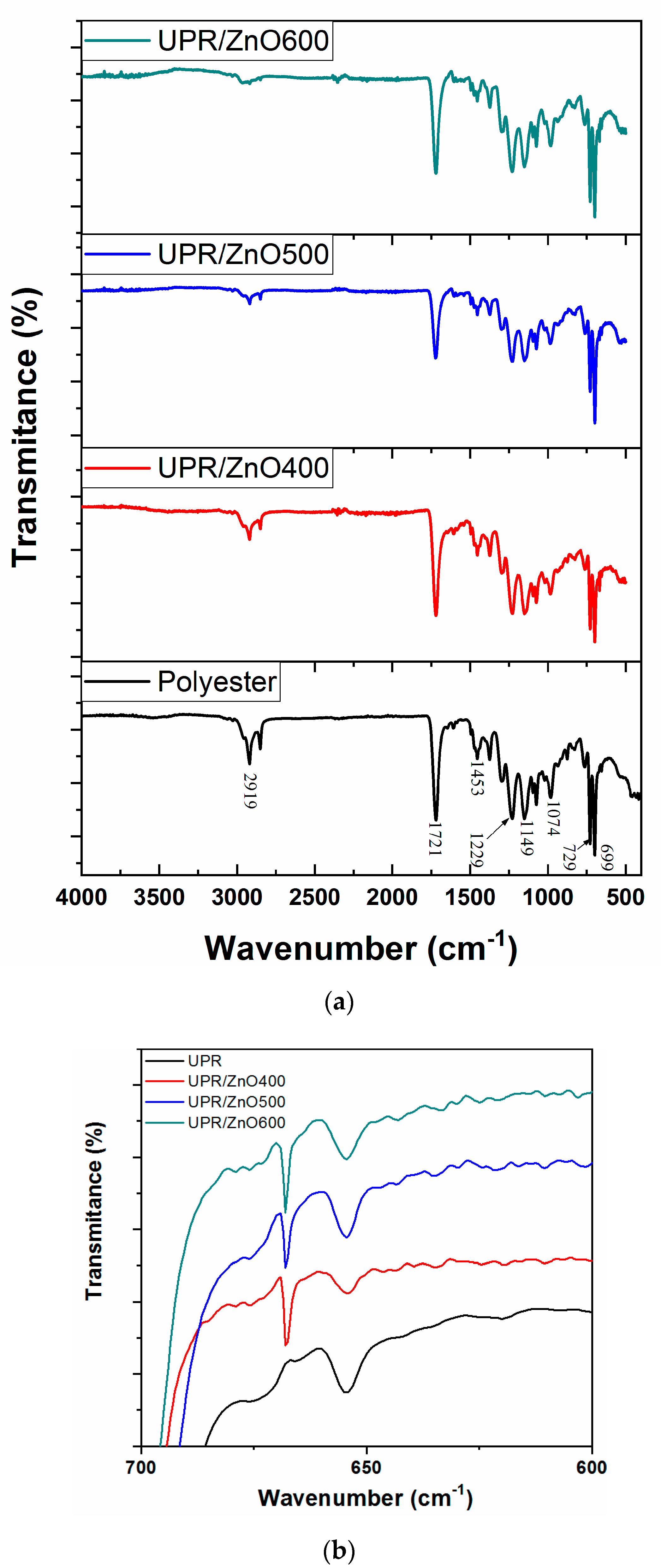
3.2. FT-IR Spectroscopy
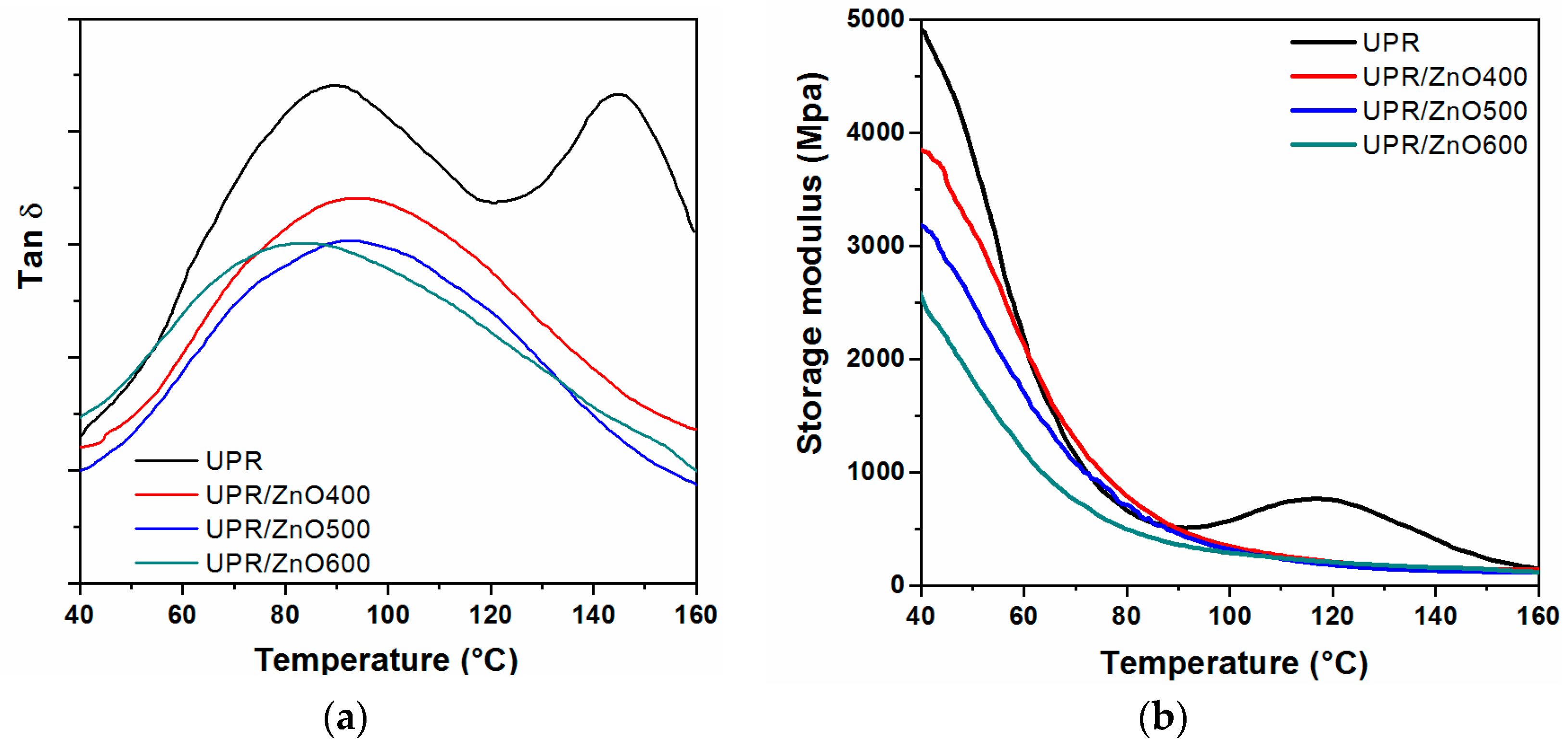
3.3. Dynamic Mechanical Analysis
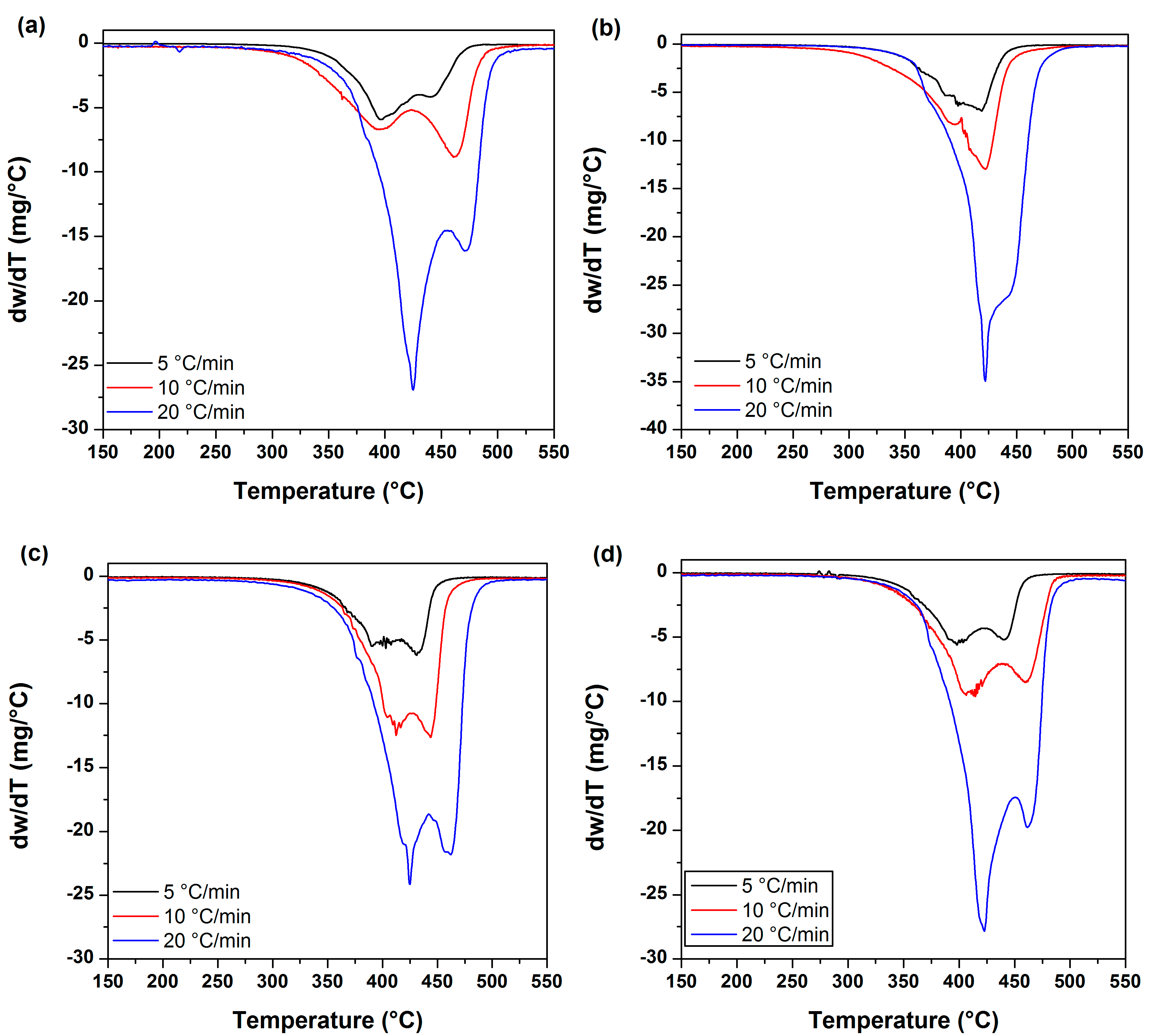
3.4. Thermal Stability
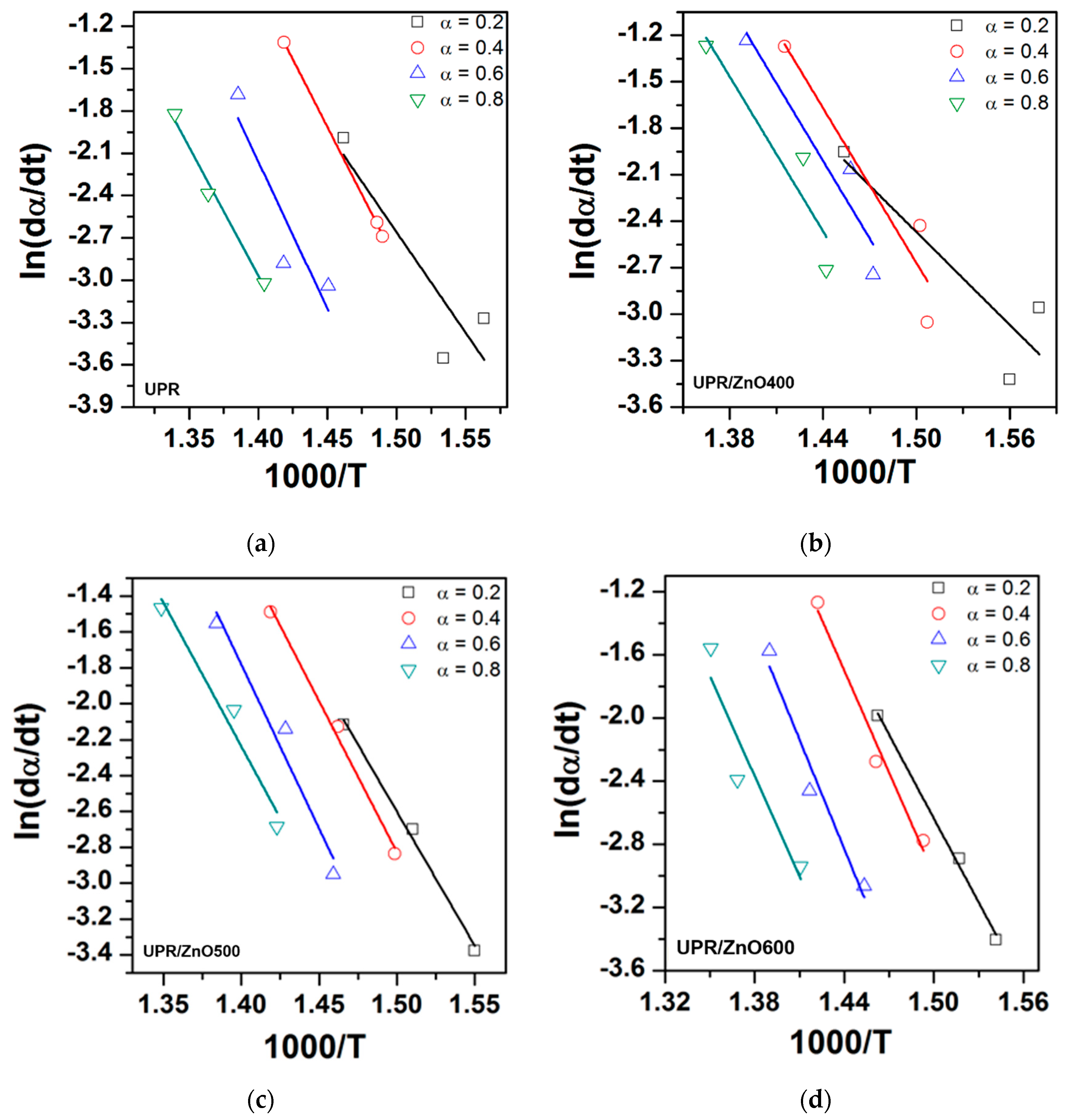
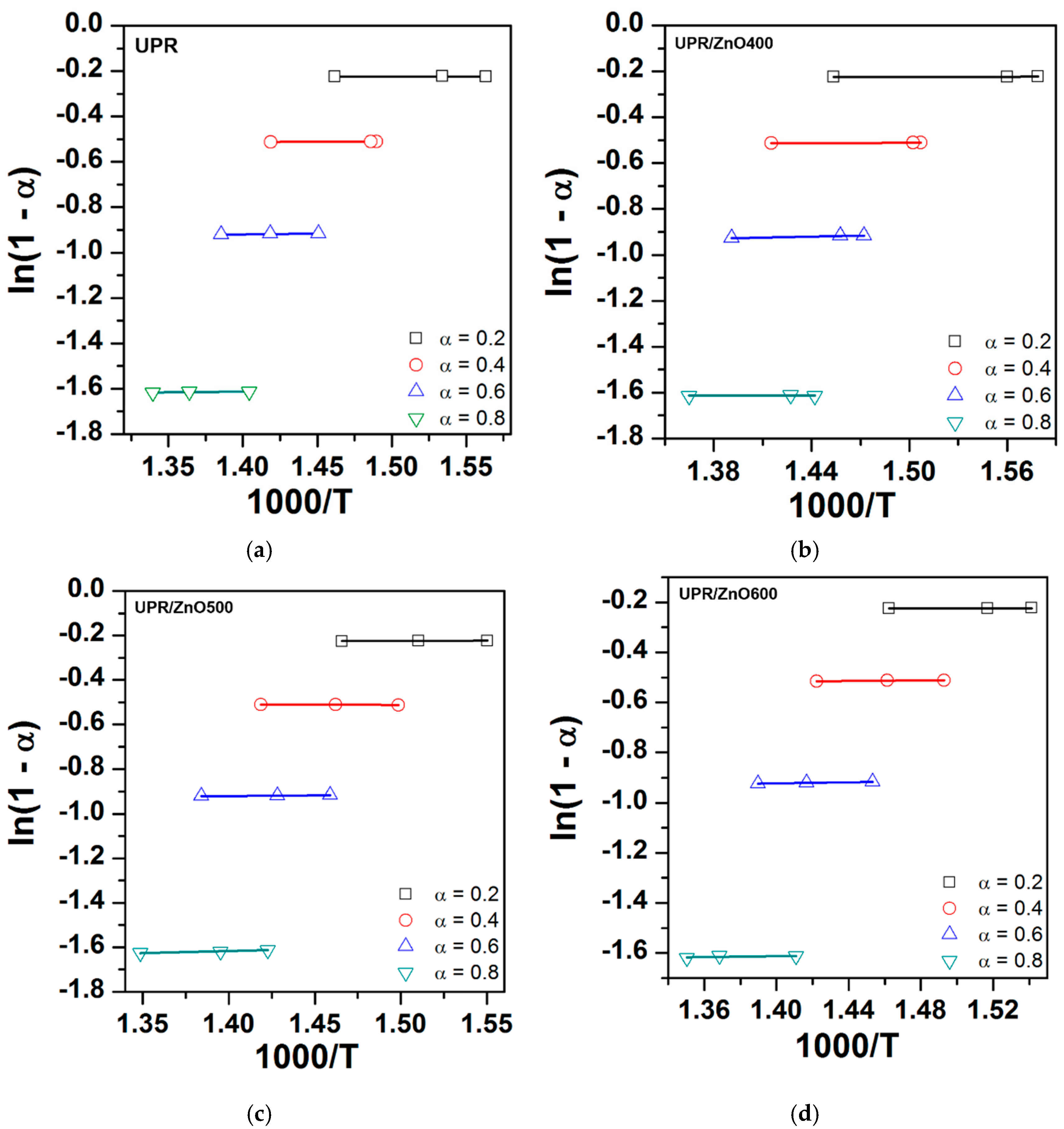
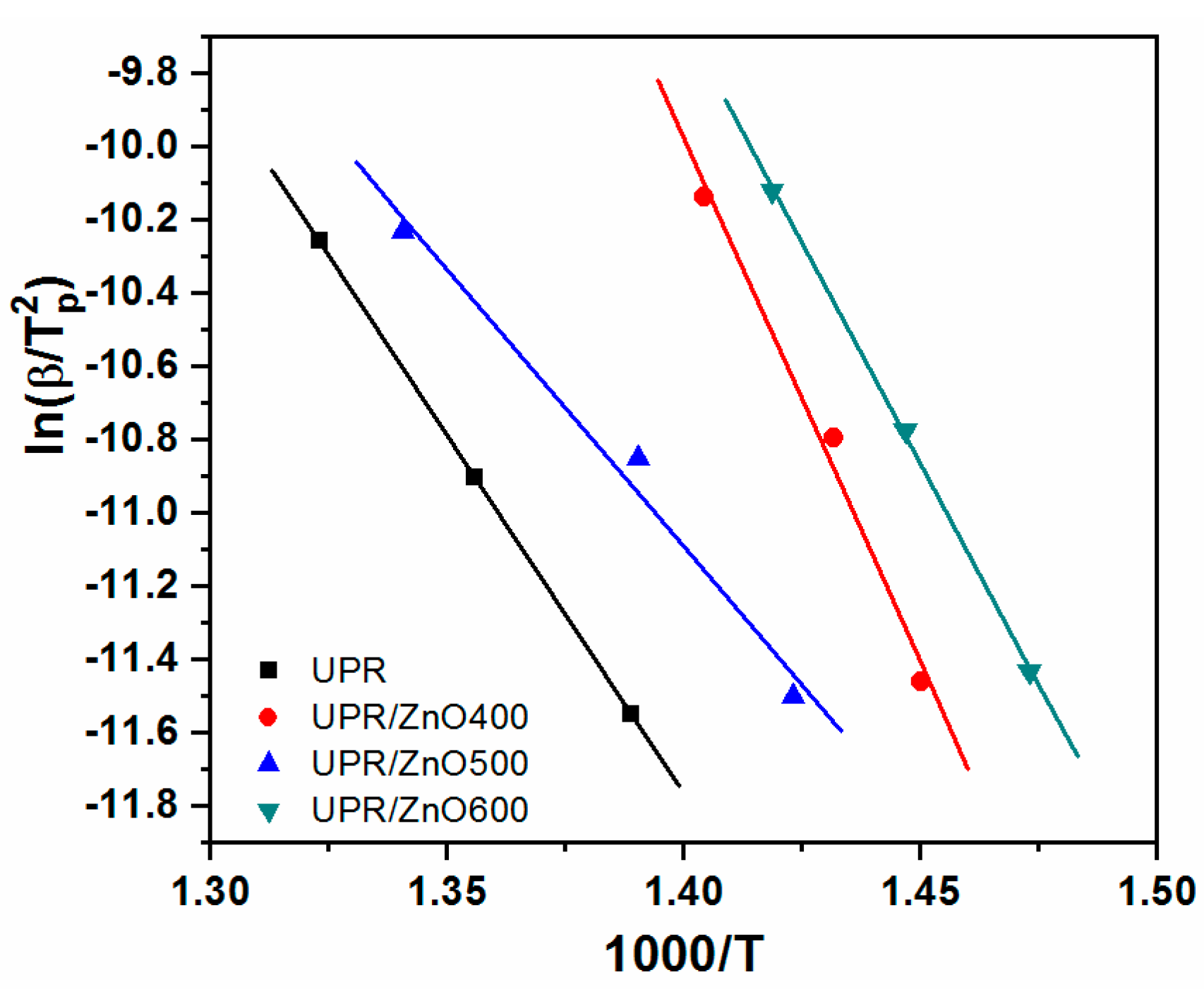
3.5. Kinetics of Thermal Degradation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sirohi, S.; Singh, R.; Jain, N.; Pani, B.; Dutt, K.; Nain, R. Synthesis and characterization of multifunctional ZnO/polyester green composite films. J. Polym. Res. 2017, 24. [Google Scholar] [CrossRef]
- Fu, S.; Sun, Z.; Huang, P.; Li, Y.; Hu, N. Some basic aspects of polymer nanocomposites: A critical review. Nano Mater. Sci. 2019, 1, 2–30. [Google Scholar] [CrossRef]
- Baskaran, R.; Sarojadevi, M.; Vijayakumar, C.T. Mechanical and thermal properties of unsaturated polyester/calcium carbonate nanocomposites. J. Reinf. Plas. Compos. 2011, 30, 1549–1556. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, M.; Chen, Z.; Chen, T.; Yu, Y.; Jiang, J. Preparation and characterization of a microencapsulated flame retardant and its flame-retardant mechanism in unsaturated polyester resins. Powder Technol. 2019, 354, 71–81. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. (Eds.) 29—zinc, cadmium and mercury. In Chemistry of the Elements, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 1997; pp. 1201–1226. ISBN 978-0-7506-3365-9. [Google Scholar]
- Lee, S. Developing UV-protective textiles based on electrospun zinc oxide nanocomposite fibers. Fibers Polym. 2009, 10, 295–301. [Google Scholar] [CrossRef]
- Thomas, V.; Yallapu, M.M.; Sreedhar, B.; Bajpai, S.K. A versatile strategy to fabricate hydrogel–silver nanocomposites and investigation of their antimicrobial activity. J. Colloid Interface Sci. 2007, 315, 389–395. [Google Scholar] [CrossRef]
- Ji, P.; Wang, C.; Jiang, Z.; Wang, H. Influence of surface modification of zinc oxide nanoparticles on thermal behavior and hydrophilic property of PET–PEG composites. Polym. Compos. 2016, 37, 1830–1838. [Google Scholar] [CrossRef]
- Xu, B.; Cai, Z. Fabrication of a superhydrophobic ZnO nanorod array film on cotton fabrics via a wet chemical route and hydrophobic modification. Appl. Surf. Sci. 2008, 254, 5899–5904. [Google Scholar] [CrossRef]
- Wan, H.; Ruda, H.E. A study of the growth mechanism of CVD-grown ZnO nanowires. J. Mater. Sci. Mater. Electron. 2010, 21, 1014–1019. [Google Scholar] [CrossRef]
- Mauricio, M.; Manso, F.; Kunita, M.; Velasco, D.; Bento, A.; Muniz, E.; Carvalho, G.; Rubira, A. Synthesis and characterization of ZnO/PET composite using supercritical carbon dioxide impregnation technology. Compos. Part A Appl. Sci. Manuf. 2011, 42, 757–761. [Google Scholar] [CrossRef]
- Carrión, F.J.; Sanes, J.; Bermúdez, M.-D. Influence of ZnO nanoparticle filler on the properties and wear resistance of polycarbonate. Wear 2007, 262, 1504–1510. [Google Scholar] [CrossRef]
- Somwangthanaroj, A.; Suwanchatchai, K.; Ando, S.; Tanthapanichakoon, W. Effect of zinc precursor on thermal and light emission properties of ZnO nanoparticles embedded in polyimide films. Mater. Chem. Phys. 2009, 114, 751–755. [Google Scholar] [CrossRef]
- Tibiletti, L.; Longuet, C.; Ferry, L.; Coutelen, P.; Mas, A.; Robin, J.-J.; Lopez-Cuesta, J.-M. Thermal degradation and fire behaviour of unsaturated polyesters filled with metallic oxides. Polym. Degrad. Stab. 2011, 96, 67–75. [Google Scholar] [CrossRef]
- Pistor, V.; dos Santos Soares, S.S.S.; Ornaghi, H.L.; Fiorio, R.; Zattera, A.J. Influence of glass and sisal fibers on the cure kinetics of unsaturated polyester resin. Mater. Res. 2012, 15, 650–656. [Google Scholar] [CrossRef] [Green Version]
- Scaccia, S. TG–FTIR and kinetics of devolatilization of Sulcis coal. J. Anal. Appl. Pyrolysis 2013, 104, 95–102. [Google Scholar] [CrossRef]
- Jiang, G.; Nowakowski, D.J.; Bridgwater, A. V A systematic study of the kinetics of lignin pyrolysis. Thermochim. Acta 2010, 498, 61–66. [Google Scholar] [CrossRef]
- Heydari, M.; Rahman, M.; Gupta, R. Kinetic study and thermal decomposition behavior of lignite coal. Int. J. Chem. Eng. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Paik, P.; Kar, K.K. Kinetics of thermal degradation and estimation of lifetime for polypropylene particles: Effects of particle size. Polym. Degrad. Stab. 2008, 93, 24–35. [Google Scholar] [CrossRef]
- Kalaee, M.; Akhlaghi, S.; Mazinani, S.; Sharif, A.; Jarestani, Y.C.; Mortezaei, M. Effect of ZnO nanoparticles on kinetics of thermal degradation and final properties of ethylene-propylene-diene rubber systems. J. Therm. Anal. Calorim. 2012, 110, 1407–1414. [Google Scholar] [CrossRef]
- Gu, H.; He, J.M.; Hu, J.; Huang, Y.D. Thermal degradation kinetics of semi-aromatic polyamide containing benzoxazole unit. J. Therm. Anal. Calorim. 2012, 107, 1251–1257. [Google Scholar] [CrossRef]
- Esthappan, S.K.; Nair, A.B.; Joseph, R. Effect of crystallite size of zinc oxide on the mechanical, thermal and flow properties of polypropylene/zinc oxide nanocomposites. Compos. Part B: Eng. 2015, 69, 145–153. [Google Scholar] [CrossRef]
- Bindu, P.; Thomas, S. Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J. Theor. Appl. Phys. 2014, 8, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Kicko-Walczak, E. Studies on the mechanism of thermal decomposition of unsaturated polyester resins with reduced flammability. Polym. Polym. Compos. 2004, 12, 127–134. [Google Scholar] [CrossRef]
- Sapkota, K.P.; Lee, I.; Hanif, M.A.; Islam, M.A.; Hahn, J.R. Solar-light-driven efficient zno–single-walled carbon nanotube photocatalyst for the degradation of a persistent water pollutant organic dye. Catalysts 2019, 9, 498. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.J.; Saion, E.; Al-Hada, N.M.; Soltani, N. A simple up-scalable thermal treatment method for synthesis of ZnO nanoparticles. Metals 2015, 5, 2383–2392. [Google Scholar] [CrossRef] [Green Version]
- Bai, Z.; Jiang, S.; Tang, G.; Hu, Y.; Song, L.; Yuen, R.K.K. Enhanced thermal properties and flame retardancy of unsaturated polyester-based hybrid materials containing phosphorus and silicon. Polym. Adv. Technol. 2014, 25, 223–232. [Google Scholar] [CrossRef]
- Hayami, R.; Endo, N.; Abe, T.; Miyase, Y.; Sagawa, T.; Yamamoto, K.; Tsukada, S.; Gunji, T. Zinc–diethanolamine complex: Synthesis, characterization, and formation mechanism of zinc oxide via thermal decomposition. J. Sol. Gel. Sci. Technol. 2018, 87, 743–748. [Google Scholar] [CrossRef]
- Rosu, D.; Cascaval, C.N.; Rosu, L. Synthesis and characterization of some composites with bioactive properties on the basis of vinyl ester resins. E-Polymers 2008, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.; Jha, R. Analysis of structural, optical and magnetic properties of Fe/Co co-doped ZnO nanocrystals. Ceram. Int. 2017, 43, 8488–8496. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, M.; Kumar, A.; Sharma, S.; Shahi, P.; Chatterjee, S.; Ghosh, A.K. Investigations on structural and optical properties of Al-modified ZnO nanoparticles. J. Mater. Sci. Mater. Electron. 2020, 31, 7715–7723. [Google Scholar] [CrossRef]
- Maiti, M.; Basak, G.C.; Srivastava, V.K.; Jasra, R.V. Influence of synthesized nano-ZnO on cure and physico-mechanical properties of SBR/BR blends. Int. J. Ind. Chem. 2017, 8, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Costa, C.S.M.F.; Fonseca, A.C.; Moniz, J.; Godinho, M.; Coelho, J.F.J.; Serra, A.C. Going greener: Synthesis of fully biobased unsaturated polyesters for styrene crosslinked resins with enhanced thermomechanical properties. Express Polym. Lett. 2017, 11, 885–898. [Google Scholar] [CrossRef]
- Gouanve, F.; Meyer, M.; Grenet, J.; Marais, S.; Poncin-Epaillard, F.; Saiter, J.M. Unsaturated polyester resin (UPR) reinforced with flax fibers, untreated and cold He plasma-treated: Thermal, mechanical and DMA studies. Compos. Interfaces 2006, 13, 355–364. [Google Scholar] [CrossRef]
- Chabros, A.; Gawdzik, B.; Podkościelna, B.; Goliszek, M.; Paczkowski, P. Composites of unsaturated polyester resins with microcrystalline cellulose and its derivatives. Materials 2020, 13, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashemi, M.; Shojaei, A. Morphology development and mechanical properties of unsaturated polyester resin containing nanodiamonds. Polym. Int. 2017, 66, 950–959. [Google Scholar] [CrossRef]
- Guo, J.; Wang, J.; He, Y.; Sun, H.; Chen, X.; Zheng, Q.; Xie, H. Triply biobased thermoplastic composites of polylactide/succinylated lignin/epoxidized soybean oil. Polymers 2020, 12, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liufu, S.C.; Xiao, H.N.; Li, Y.P. Thermal analysis and degradation mechanism of polyacrylate/ZnO nanocomposites. Polym. Degrad. Stab. 2005, 87, 103–110. [Google Scholar] [CrossRef]
- Wellen, R.M.R.; Canedo, E.L. On the Kissinger equation and the estimate of activation energies for non-isothermal cold crystallization of PET. Polym. Test. 2014, 40, 33–38. [Google Scholar] [CrossRef] [Green Version]


| Material | Heating Rate (°C/min) | Temperature (°C) | Degradation (%) | Heat Resistance Index | ||
|---|---|---|---|---|---|---|
| Onset | Offset | 5 | 30 | |||
| UPR | 5 | 363.6 | 456.6 | 333.1 | 391.3 | 180.3 |
| 10 | 338.5 | 463.7 | 255.1 | 388.3 | 164.2 | |
| 20 | 387.1 | 488.1 | 359.3 | 424.6 | 195.3 | |
| UPR/ZnO400 | 5 | 350.4 | 434.6 | 309.5 | 383.4 | 173.4 |
| 10 | 343.1 | 439.1 | 280.1 | 384.2 | 167.9 | |
| 20 | 381.1 | 459.6 | 380.1 | 428.1 | 200.4 | |
| UPR/ZnO500 | 5 | 351.9 | 445.3 | 315.8 | 387.1 | 175.7 |
| 10 | 371.7 | 454.8 | 333.5 | 404.2 | 184.2 | |
| 20 | 389.9 | 474.7 | 350.6 | 424.3 | 193.5 | |
| UPR/ZnO600 | 5 | 357.2 | 453.3 | 332.1 | 389.7 | 179.6 |
| 10 | 369.5 | 476.9 | 329 | 403.2 | 183.1 | |
| 20 | 388.8 | 476.9 | 368.3 | 423.3 | 196.6 | |
| Material | Heat Hate (°C/min) | Temperatures (°C) | |
|---|---|---|---|
| Transition 1 | Transition 2 | ||
| UPR | 5 | 395.1 | 440.1 |
| 10 | 399.2 | 465.1 | |
| 20 | 424.6 | 470.6 | |
| UPR/ZnO400 | 5 | 416.6 | - |
| 10 | 425.4 | - | |
| 20 | 432.5 | - | |
| UPR/ZnO500 | 5 | 390.9 | 429.6 |
| 10 | 416.1 | 445.9 | |
| 20 | 434.2 | 467.6 | |
| UPR/ZnO600 | 5 | 399.7 | 439.6 |
| 10 | 411.4 | 461.2 | |
| 20 | 430.7 | 469.9 | |
| Material | α | Ea | R2 | n | R2 |
|---|---|---|---|---|---|
| UPR | 0.2 | 118.66 | 0.81 | 0.34 | 0.06 |
| 0.4 | 159.62 | 0.99 | 0.66 | 0.96 | |
| 0.6 | 173.38 | 0.84 | 3.43 | 0.72 | |
| 0.8 | 151.33 | 0.98 | 3.08 | 0.71 | |
| Average | 150.75 | 1.88 | |||
| UPR/ZnO400 | 0.2 | 83.05 | 0.81 | 0.49 | 0.99 |
| 0.4 | 138.51 | 0.91 | 0.76 | 0.99 | |
| 0.6 | 138.91 | 0.91 | 4.28 | 0.96 | |
| 0.8 | 138.32 | 0.88 | 3.37 | 0.05 | |
| Average | 124.69 | 2.23 | |||
| UPR/ZnO500 | 0.2 | 124.19 | 0.99 | 0.32 | 0.77 |
| 0.4 | 139.74 | 0.99 | 0.76 | 0.80 | |
| 0.6 | 151.88 | 0.96 | 3.51 | 0.99 | |
| 0.8 | 132.81 | 0.96 | 3.71 | 0.97 | |
| Average | 137.15 | 2.13 | |||
| UPR/ZnO600 | 0.2 | 147.14 | 0.99 | 0.76 | 0.79 |
| 0.4 | 178.66 | 0.99 | 2.84 | 0.97 | |
| 0.6 | 191.86 | 0.96 | 4.36 | 0.92 | |
| 0.8 | 175.19 | 0.97 | 4.25 | 0.31 | |
| Average | 173.19 | 3.05 |
| Material | Ea | n | R2 |
|---|---|---|---|
| UPR | 128 | 0.66 | 1.0 |
| UPR/ZnO400 | 139 | 0.63 | 0.987 |
| UPR/ZnO500 | 151 | 3.52 | 0.987 |
| UPR/ZnO600 | 191 | 2.85 | 0.99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco-Urquiza, E.A.; May-Crespo, J.F.; Escalante Velázquez, C.A.; Pérez Mora, R.; González García, P. Thermal Degradation Kinetics of ZnO/polyester Nanocomposites. Polymers 2020, 12, 1753. https://doi.org/10.3390/polym12081753
Franco-Urquiza EA, May-Crespo JF, Escalante Velázquez CA, Pérez Mora R, González García P. Thermal Degradation Kinetics of ZnO/polyester Nanocomposites. Polymers. 2020; 12(8):1753. https://doi.org/10.3390/polym12081753
Chicago/Turabian StyleFranco-Urquiza, E. A., J. F. May-Crespo, C. A. Escalante Velázquez, R. Pérez Mora, and P. González García. 2020. "Thermal Degradation Kinetics of ZnO/polyester Nanocomposites" Polymers 12, no. 8: 1753. https://doi.org/10.3390/polym12081753






