A Review on Plant Cellulose Nanofibre-Based Aerogels for Biomedical Applications
Abstract
:1. Introduction
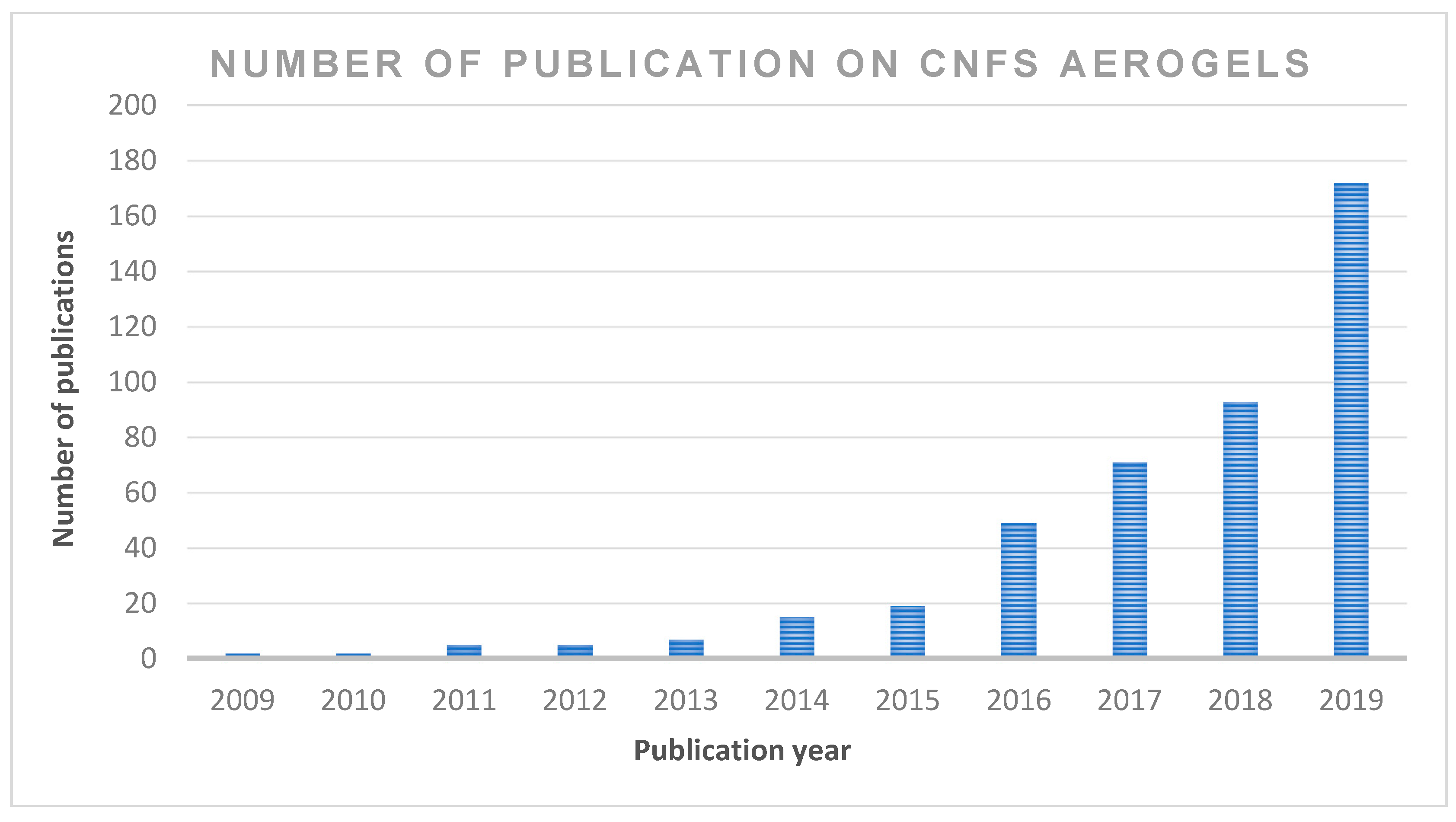
2. Chronological Studies
3. Preparation of CNF Aerogel and Properties
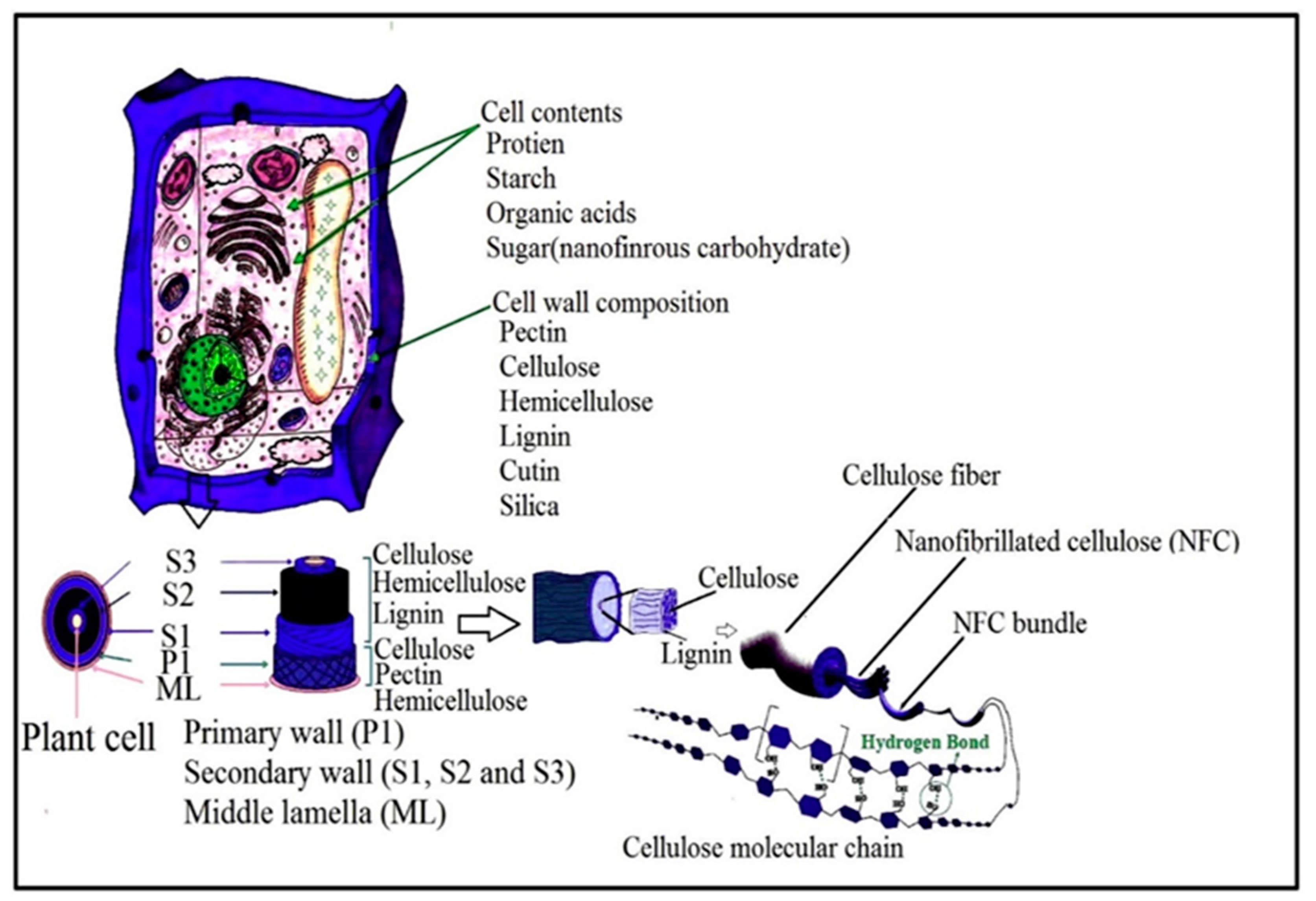
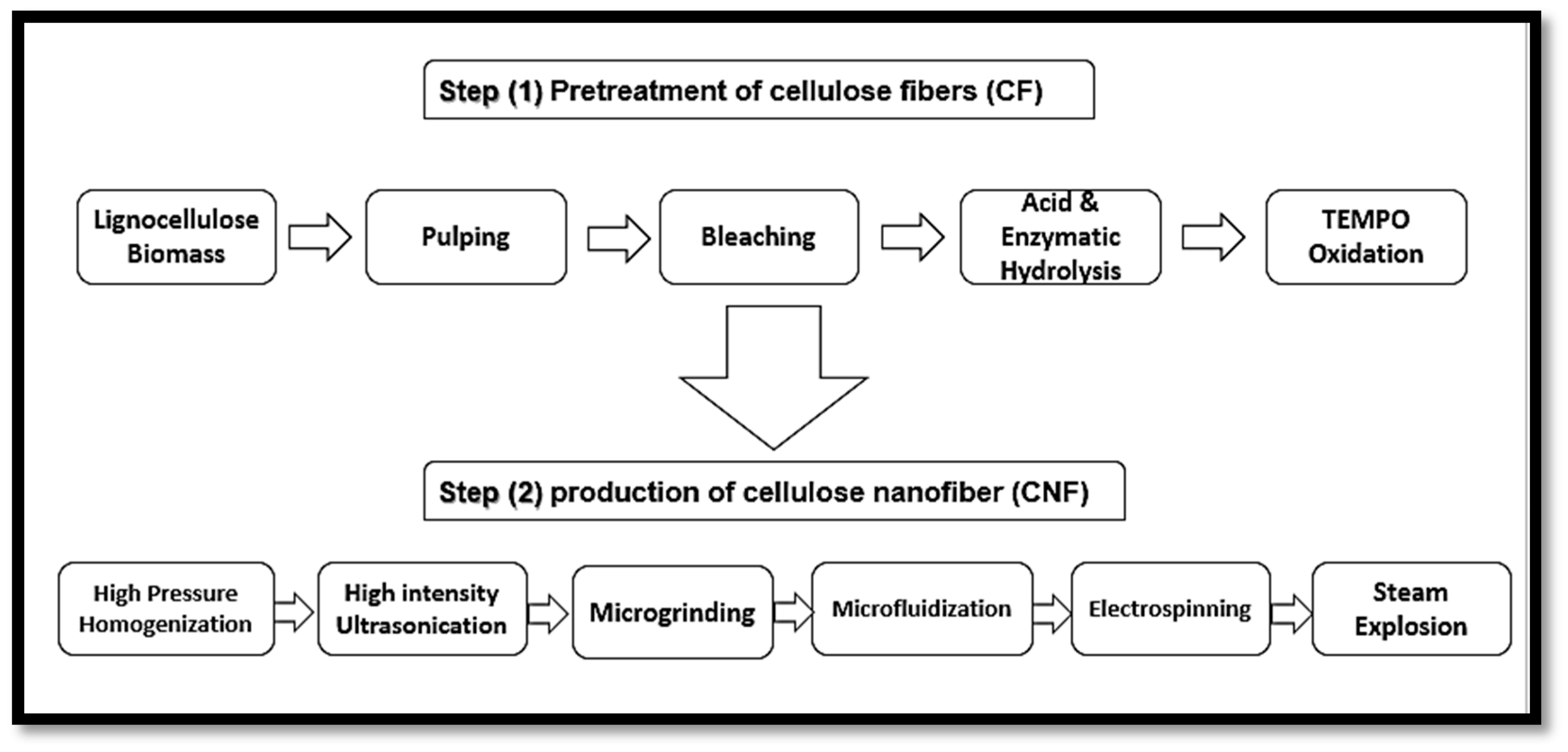
3.1. Isolation of Plant Cellulose Nanofibres (CNFs)
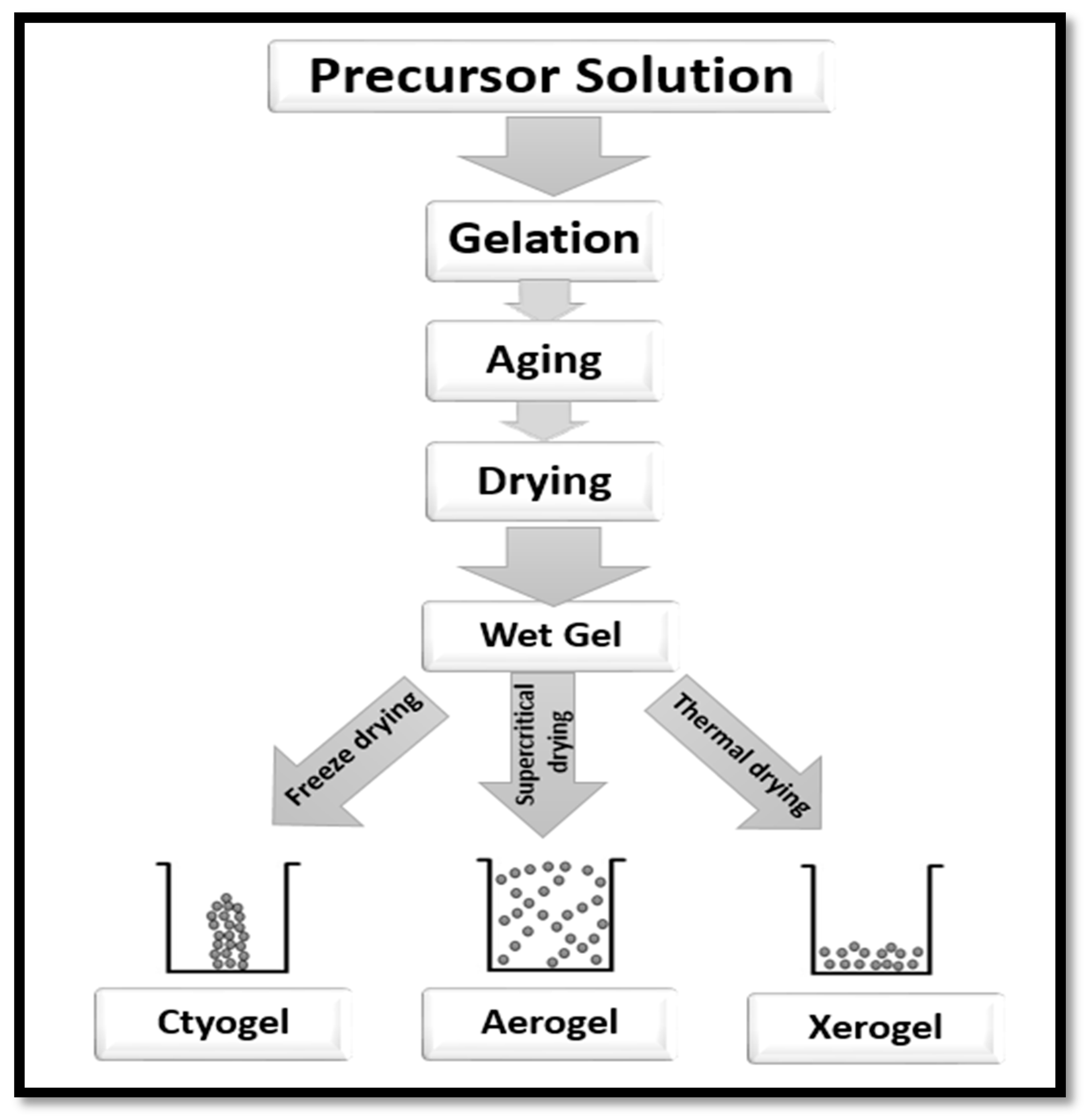
3.2. Isolation of CNF Aerogel
3.3. Properties of Aerogel and CNF-Based Aerogels
3.3.1. Physical Properties and Surface Area
3.3.2. Mechanical and Morphological Properties
3.3.3. Biocompatibility and Toxicity
4. Cellulose Nanofibre (CNF) Aerogels in Medical Application
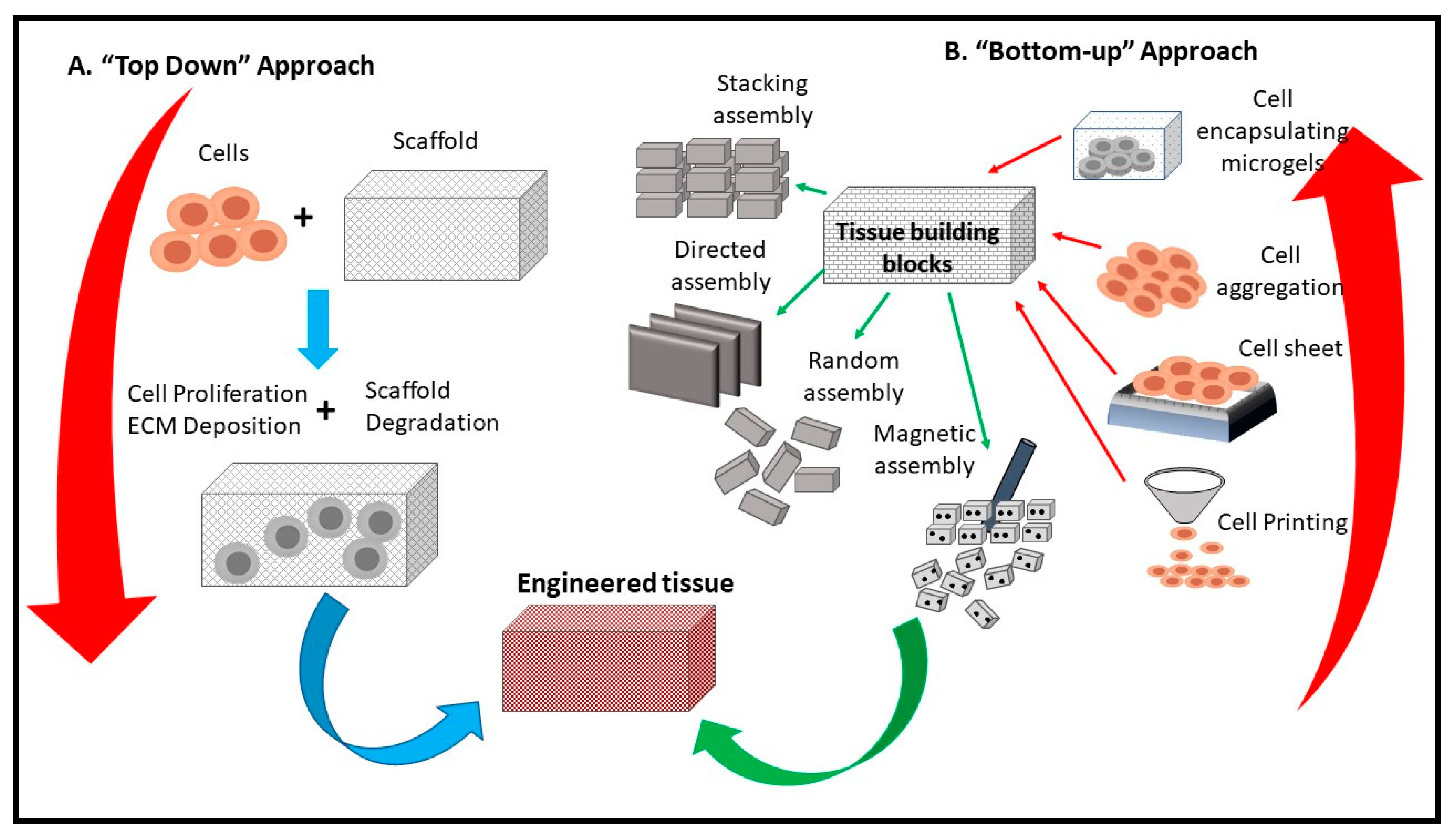
4.1. Tissue Engineering
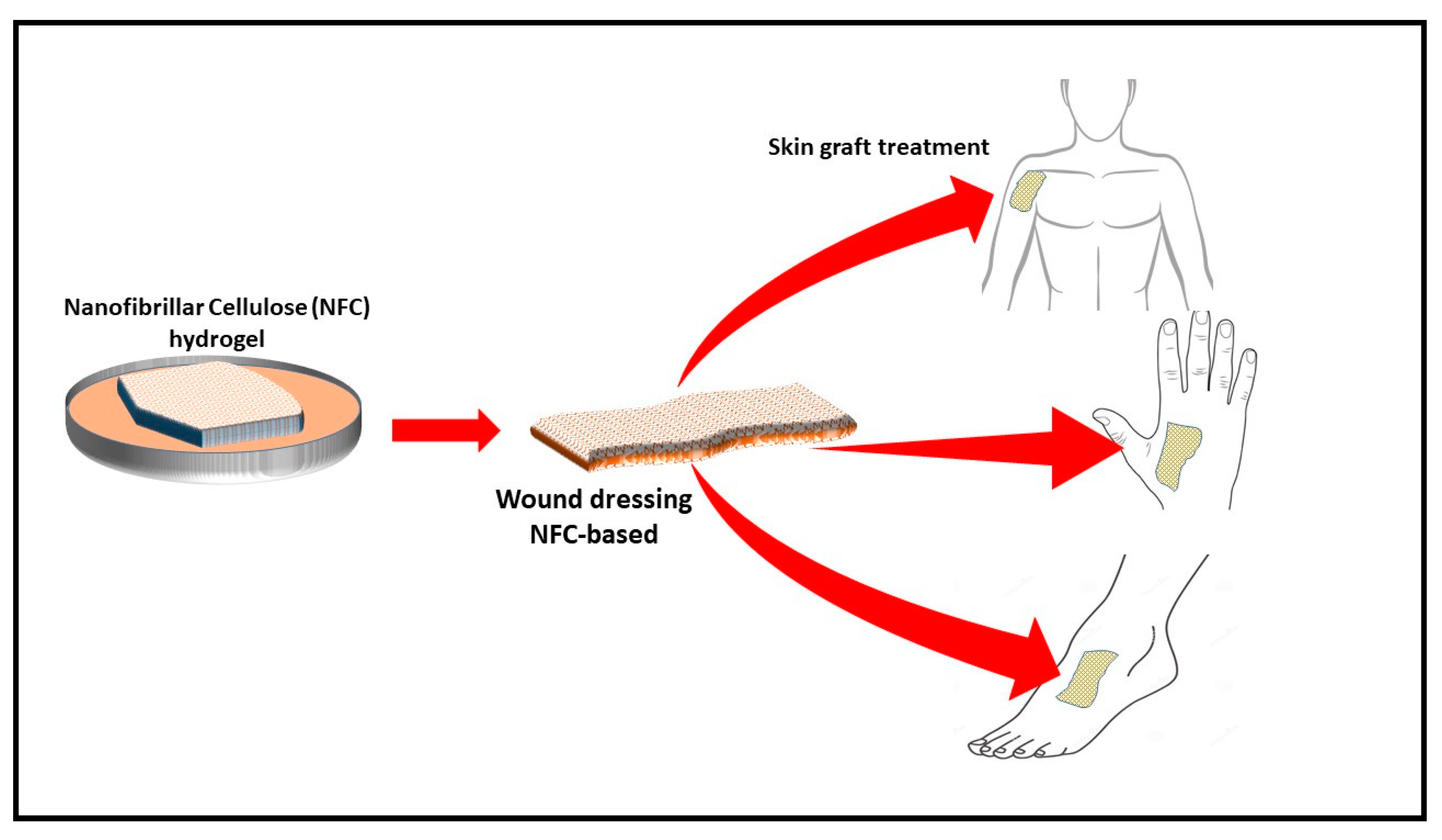
4.1.1. Wound Healing
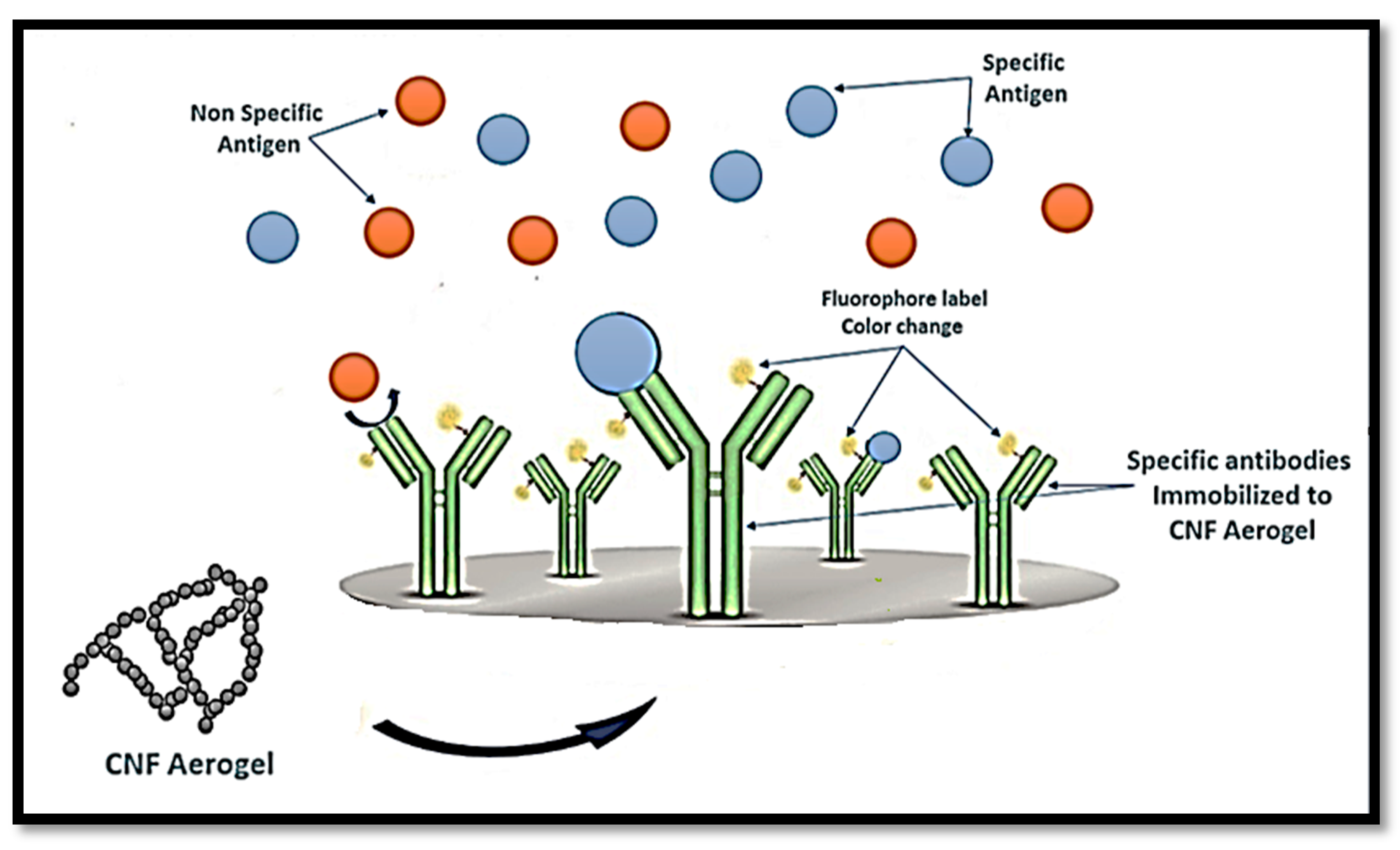
4.1.2. Biosensing and Diagnostics
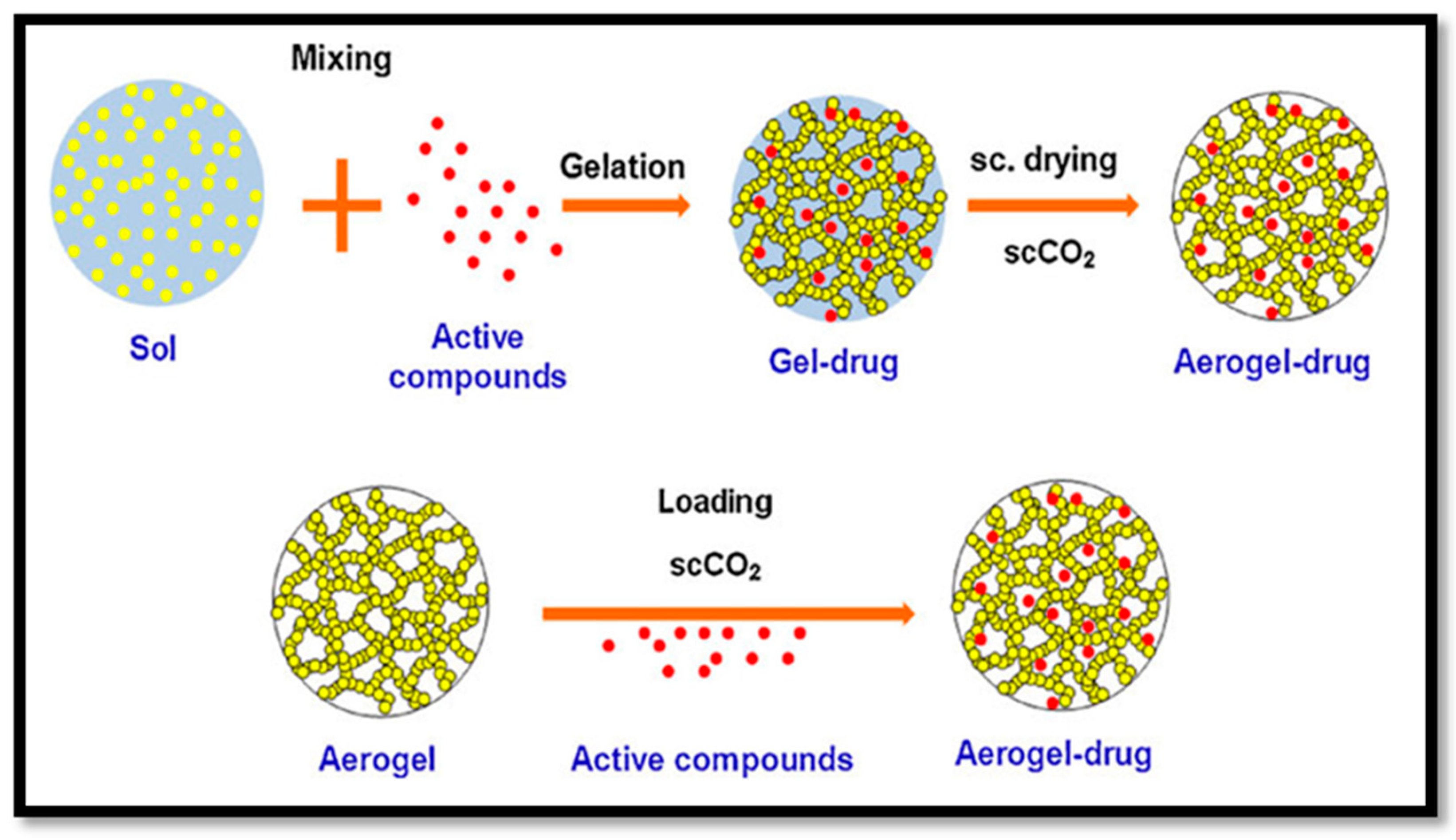
4.1.3. Drug Delivery
4.1.4. Antimicrobial Immobilisation
4.2. Potential and Challenges of with CNF Aerogel in Biomedical Application
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maleki, H.; Durães, L.; García-González, C.A.; del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and biomedical applications of aerogels: Possibilities and challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef]
- García-González, C.A.; López-Iglesias, C.; Concheiro, A.; Alvarez-Lorenzo, C. Biomedical applications of polysaccharide and protein based aerogels. Biobased Aerogels 2018, 295–323. [Google Scholar]
- Kistler, S.S. Coherent expanded aerogels and jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Manuspiya, H. A critical review on cellulose: From fundamental to an approach on sensor technology. Renew. Sustain. Energy Rev. 2015, 41, 402–412. [Google Scholar] [CrossRef]
- Rehman, N.; Alam, S.; Amin, N.U.; Mian, I.; Ullah, H. Ecofriendly isolation of cellulose from eucalyptus lenceolata: A novel approach. Int. J. Polym. Sci. 2018. [Google Scholar] [CrossRef] [Green Version]
- Draman, S.F.S.; Daik, R.; Mohd, N. Eco-friendly extraction and characterization of cellulose from lignocellulosoic fiber. ARPN J. Eng. Appl. Sci. 2016, 11, 9591–9595. [Google Scholar]
- Chávez-Guerrero, L.; Sepúlveda-Guzmán, S.; Silva-Mendoza, J.; Aguilar-Flores, C.; Pérez-Camacho, O. Eco-friendly isolation of cellulose nanoplatelets through oxidation under mild conditions. Carbohydr. Polym. 2018, 181, 642–649. [Google Scholar] [CrossRef]
- Rizal, S.; Gopakumar, D.A.; Huzni, S.; Thalib, S.; Syakir, M.; Owolabi, F.T.; Aprilla, N.S.; Paridah, M.; Abdul Khalil, H.P.S. Tailoring the effective properties of typha fiber reinforced polymer composite via alkali treatment. BioResources 2019, 14, 5630–5645. [Google Scholar]
- Jawaid, M.; Khan, M.M. Polymer-Based Nanocomposites for Energy and Environmental Applications; Woodhead Publishing: Cambridge, UK, 2018. [Google Scholar]
- Wang, Q.; Wei, W.; Chang, F.; Sun, J.; Xie, S.; Zhu, Q. Controlling the size and film strength of individualized cellulose nanofibrils prepared by combined enzymatic pretreatment and high pressure microfluidization. BioResources 2016, 11, 2536–2547. [Google Scholar] [CrossRef] [Green Version]
- Desmaisons, J.; Boutonnet, E.; Rueff, M.; Dufresne, A.; Bras, J. A new quality index for benchmarking of different cellulose nanofibrils. Carbohydr. Polym. 2017, 174, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Long, L.-Y.; Weng, Y.-X.; Wang, Y.-Z. Cellulose aerogels: Synthesis, applications, and prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopakumar, D.A.; Arumughan, V.; Pottathara, Y.B.; KS, S.; Pasquini, D.; Bračič, M.; Seantier, B.; Nzihou, A.; Thomas, S.; Rizal, S.; et al. Robust superhydrophobic cellulose nanofiber aerogel for multifunctional environmental applications. Polymers 2019, 11, 495. [Google Scholar]
- Kistler, S. The relation between heat conductivity and structure in silica aerogel. J. Phys. Chem. 2002, 39, 79–86. [Google Scholar] [CrossRef]
- Gurav, J.L.; Jung, I.-K.; Park, H.-H.; Kang, E.S.; Nadargi, D.Y. Silica aerogel: Synthesis and applications. J. Nanomater. 2010. [Google Scholar] [CrossRef] [Green Version]
- Bheekhun, N.; Talib, A.; Rahim, A.; Hassan, M.R. Aerogels in aerospace: An overview. Adv. Mater. Sci. Eng. 2013. [Google Scholar] [CrossRef] [Green Version]
- Tillotson, T.; Hrubesh, L. Transparent ultralow-density silica aerogels prepared by a two-step sol-gel process. J. Non-Cryst. Solids 1992, 145, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Poelz, G.; Riethmüller, R. Preparation of silica aerogel for Cherenkov counters. Nucl. Instrum. Methods Phys. Res. 1982, 195, 491–503. [Google Scholar] [CrossRef]
- Pekala, R. Organic aerogels from the polycondensation of resorcinol with formaldehyde. J. Mater. Sci. 1989, 24, 3221–3227. [Google Scholar] [CrossRef]
- Roth, T.B.; Anderson, A.M.; Carroll, M.K. Analysis of a rapid supercritical extraction aerogel fabrication process: Prediction of thermodynamic conditions during processing. J. Non-Cryst. Solids 2008, 354, 3685–3693. [Google Scholar] [CrossRef]
- Leventis, N.; Sotiriou-Leventis, C.; Zhang, G.; Rawashdeh, A.-M.M. Nanoengineering strong silica aerogels. Nano Lett. 2002, 2, 957–960. [Google Scholar] [CrossRef]
- Fischer, F.; Rigacci, A.; Pirard, R.; Berthon-Fabry, S.; Achard, P. Cellulose-based aerogels. Polymer 2006, 47, 7636–7645. [Google Scholar] [CrossRef]
- Pääkkö, M.; Vapaavuori, J.; Silvennoinen, R.; Kosonen, H.; Ankerfors, M.; Lindström, T.; Berglund, L.A.; Ikkala, O. Long and entangled native cellulose I nanofibers allow flexible aerogels and hierarchically porous templates for functionalities. Soft Matter 2008, 4, 2492–2499. [Google Scholar] [CrossRef]
- Leventis, N.; Chandrasekaran, N.; Sotiriou-Leventis, C.; Mumtaz, A. Smelting in the age of nano: Iron aerogels. J. Mater. Chem. 2009, 19, 63–65. [Google Scholar] [CrossRef]
- Dash, R.; Li, Y.; Ragauskas, A.J. Cellulose nanowhisker foams by freeze casting. Carbohydr. Polym. 2012, 88, 789–792. [Google Scholar] [CrossRef]
- Buratti, C.; Moretti, E.; Belloni, E.; Agosti, F. Development of innovative aerogel based plasters: Preliminary thermal and acoustic performance evaluation. Sustainability 2014, 6, 5839–5852. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.H.; Youn, H.J.; Lee, H.L. Preparation of cross-linked cellulose nanofibril aerogel with water absorbency and shape recovery. Cellulose 2015, 22, 3715–3724. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Li, Y.; Xu, Y.; Lu, Z.; Chen, L.; Huang, L.; Fan, M. Versatile fabrication of a superhydrophobic and ultralight cellulose-based aerogel for oil spillage clean-up. Phys. Chem. Chem. Phys. 2016, 18, 28297–28306. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Saelices, C.; Seantier, B.; Cathala, B.; Grohens, Y. Spray freeze-dried nanofibrillated cellulose aerogels with thermal superinsulating properties. Carbohydr. Polym. 2017, 157, 105–113. [Google Scholar] [CrossRef]
- Wu, X.; Fan, M.; Mclaughlin, J.F.; Shen, X.; Tan, G. A novel low-cost method of silica aerogel fabrication using fly ash and trona ore with ambient pressure drying technique. Powder Technol. 2018, 323, 310–322. [Google Scholar] [CrossRef]
- Kaya, M.; Tabak, A. Recycling of an agricultural bio-waste as a novel cellulose aerogel: A green chemistry study. J. Polym. Environ. 2020, 28, 323–330. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, Y.-n.; Wan, J.; Cui, R.; Yu, X.; Zhao, G.; Lin, K. A novel multifunctional carbon aerogel-coated platform for osteosarcoma therapy and enhanced bone regeneration. J. Mater. Chem. B 2020, 8, 368–379. [Google Scholar] [CrossRef]
- Leventis, N.; Sotiriou-Leventis, L.; Mulik, S. Crosslinking 3D assemblies of silica nanoparticles (aerogels) by surface-initiated free radical polymerization of styrene and methylmethacrylate. Polym. Prepr. 2007, 48, 950–951. [Google Scholar]
- Tappan, B.C.; Huynh, M.H.; Hiskey, M.A.; Chavez, D.E.; Luther, E.P.; Mang, J.T.; Son, S.F. Energetic decomposition of high-nitrogen metal complexes and the formation of low-density nano-structured metal monoliths. MRS Online Proc. Libr. Arch. 2005, 896. [Google Scholar] [CrossRef]
- Guo, H.; Meador, M.A.B.; McCorkle, L.; Quade, D.J.; Guo, J.; Hamilton, B.; Cakmak, M. Tailoring properties of cross-linked polyimide aerogels for better moisture resistance, flexibility, and strength. ACS Appl. Mater. Interfaces 2012, 4, 5422–5429. [Google Scholar] [CrossRef] [PubMed]
- Meador, M.A.B.; Malow, E.J.; Silva, R.; Wright, S.; Quade, D.; Vivod, S.L.; Guo, H.; Guo, J.; Cakmak, M. Mechanically strong, flexible polyimide aerogels cross-linked with aromatic triamine. ACS Appl. Mater. Interfaces 2012, 4, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Khedkar, M.V.; Somvanshi, S.B.; Humbe, A.V.; Jadhav, K. Surface modified sodium silicate based superhydrophobic silica aerogels prepared via ambient pressure drying process. J. Non-Cryst. Solids 2019, 511, 140–146. [Google Scholar] [CrossRef]
- Tang, A.; Li, J.; Li, J.; Zhao, S.; Liu, W.; Liu, T.; Wang, J.; Liu, Y. Nanocellulose/PEGDA aerogel scaffolds with tunable modulus prepared by stereolithography for three-dimensional cell culture. J. Biomater. Sci. Polym. Ed. 2019, 30, 797–814. [Google Scholar] [CrossRef]
- Ge, Q.; Li, Z.; Wang, Z.; Zhang, W.; He, X.; Zhou, J.; Fang, N. Projection micro stereolithography based 3D printing and its applications. Int. J. Extrem. Manuf. 2020. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, J.; Wu, L.; Weng, Z.; Zheng, L.; Peng, S.; Zhang, X. High performance POSS filled nanocomposites prepared via UV-curing based on 3D stereolithography printing. Compos. Part A Appl. Sci. Manuf. 2019, 117, 276–286. [Google Scholar] [CrossRef]
- Saoud, K.M.; Saeed, S.; Bertino, M.F.; White, L.S. Fabrication of strong and ultra-lightweight silica-based aerogel materials with tailored properties. J. Porous Mater. 2018, 25, 511–520. [Google Scholar] [CrossRef]
- Chua, C.K.; Leong, K.F.; An, J. Introduction to rapid prototyping of biomaterials. In Rapid Prototyping of Biomaterials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–15. [Google Scholar]
- Jiang, Y.; Xu, Z.; Huang, T.; Liu, Y.; Guo, F.; Xi, J.; Gao, W.; Gao, C. Direct 3D printing of ultralight graphene oxide aerogel microlattices. Adv. Funct. Mater. 2018, 28, 1707024. [Google Scholar] [CrossRef]
- Maleki, H.; Montes, S.; Hayati-Roodbari, N.; Putz, F.; Huesing, N. Compressible, thermally insulating, and fire retardant aerogels through self-assembling silk fibroin biopolymers inside a silica structure—An approach towards 3D printing of aerogels. ACS Appl. Mater. Interfaces 2018, 10, 22718–22730. [Google Scholar] [CrossRef] [PubMed]
- Kam, D.; Chasnitsky, M.; Nowogrodski, C.; Braslavsky, I.; Abitbol, T.; Magdassi, S.; Shoseyov, O. Direct CRYO writing of aerogels via 3D Printing of aligned cellulose nanocrystals inspired by the plant cell wall. Colloids Interfaces 2019, 3, 46. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F. Starch based aerogels: Production, properties and applications. Trends Food Sci. Technol. 2019, 89, 1–10. [Google Scholar] [CrossRef]
- Fernandes, S.C.; Freire, C.S.; Silvestre, A.J.; Pascoal Neto, C.; Gandini, A. Novel materials based on chitosan and cellulose. Polym. Int. 2011, 60, 875–882. [Google Scholar] [CrossRef]
- Peterson, J.J.; Willgert, M.; Hansson, S.; Malmström, E.; Carter, K.R. Surface-grafted conjugated polymers for hybrid cellulose materials. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3004–3013. [Google Scholar] [CrossRef]
- Tayeb, A.H.; Amini, E.; Ghasemi, S.; Tajvidi, M. Cellulose nanomaterials—Binding properties and applications: A review. Molecules 2018, 23, 2684. [Google Scholar] [CrossRef] [Green Version]
- Abduk Khalil, H.P.S.; Davoudpour, Y.; Saurabh, C.K.; Hossain, M.S.; Adnan, A.; Dungani, R.; Paridah, M.; Sarker, M.Z.I.; Fazita, M.N.; Syakir, M. A review on nanocellulosic fibres as new material for sustainable packaging: Process and applications. Renew. Sustain. Energy Rev. 2016, 64, 823–836. [Google Scholar] [CrossRef]
- Madsen, B.; Gamstedt, E.K. Wood versus plant fibers: Similarities and differences in composite applications. Adv. Mater. Sci. Eng. 2013. [Google Scholar] [CrossRef] [Green Version]
- Fall, A.B.; Lindstrom, S.B.; Sundman, O.; Ödberg, L.; Wagberg, L. Colloidal stability of aqueous nanofibrillated cellulose dispersions. Langmuir 2011, 27, 11332–11338. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Bhat, A.; Yusra, A.I. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Mishra, R.K.; Sabu, A.; Tiwari, S.K. Materials chemistry and the futurist eco-friendly applications of nanocellulose: Status and prospect. J. Saudi Chem. Soc. 2018, 22, 949–978. [Google Scholar] [CrossRef]
- Gupta, P.; Singh, B.; Agrawal, A.K.; Maji, P.K. Low density and high strength nanofibrillated cellulose aerogel for thermal insulation application. Mater. Des. 2018, 158, 224–236. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, Q.; Liu, J.; Sun, J.; Zhu, Q.; Chen, H. Processing nanocellulose to bulk materials: A review. Cellulose 2019, 26, 1–33. [Google Scholar] [CrossRef]
- Toivonen, M.S.; Kaskela, A.; Rojas, O.J.; Kauppinen, E.I.; Ikkala, O. Ambient-dried cellulose nanofibril aerogel membranes with high tensile strength and their use for aerosol collection and templates for transparent, flexible devices. Adv. Funct. Mater. 2015, 25, 6618–6626. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Gardner, D.J.; Han, Y. Drying cellulose nanofibrils: In search of a suitable method. Cellulose 2012, 19, 91–102. [Google Scholar] [CrossRef]
- Peng, Y.; Han, Y.; Gardner, D.J. Spray-drying cellulose nanofibrils: Effect of drying process parameters on particle morphology and size distribution. Wood Fiber Sci. 2012, 44, 448–461. [Google Scholar]
- Beck, S.; Bouchard, J.; Berry, R. Dispersibility in water of dried nanocrystalline cellulose. Biomacromolecules 2012, 13, 1486–1494. [Google Scholar] [CrossRef]
- Nissilä, T.; Karhula, S.S.; Saarakkala, S.; Oksman, K. Cellulose nanofiber aerogels impregnated with bio-based epoxy using vacuum infusion: Structure, orientation and mechanical properties. Compos. Sci. Technol. 2018, 155, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Capadona, L.A.; Meador, M.A.B.; Alunni, A.; Fabrizio, E.F.; Vassilaras, P.; Leventis, N. Flexible, low-density polymer crosslinked silica aerogels. Polymer 2006, 47, 5754–5761. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The sol-gel process chem. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.; Wang, K. Polyimide aerogels cross-linked with aminated Ag nanowires: Mechanically strong and tough. Polymers 2017, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Cervin, N.T.; Johansson, E.; Larsson, P.A.; Wågberg, L. Strong, water-durable, and wet-resilient cellulose nanofibril-stabilized foams from oven drying. ACS Appl. Mater. Interfaces 2016, 8, 11682–11689. [Google Scholar] [CrossRef]
- Chen, W.; Li, Q.; Wang, Y.; Yi, X.; Zeng, J.; Yu, H.; Liu, Y.; Li, J. Comparative study of aerogels obtained from differently prepared nanocellulose fibers. ChemSusChem 2014, 7, 154–161. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Saito, T.; Isogai, A. Aerogels with 3D ordered nanofiber skeletons of liquid-crystalline nanocellulose derivatives as tough and transparent insulators. Angew. Chem. Int. Ed. 2014, 53, 10394–10397. [Google Scholar] [CrossRef]
- Yang, X.; Cranston, E.D. Chemically cross-linked cellulose nanocrystal aerogels with shape recovery and superabsorbent properties. Chem. Mater. 2014, 26, 6016–6025. [Google Scholar] [CrossRef]
- Sakai, K.; Kobayashi, Y.; Saito, T.; Isogai, A. Partitioned airs at microscale and nanoscale: Thermal diffusivity in ultrahigh porosity solids of nanocellulose. Sci. Rep. 2016, 6, 20434. [Google Scholar] [CrossRef]
- Lavoine, N.; Bergström, L. Nanocellulose-based foams and aerogels: Processing, properties, and applications. J. Mater. Chem. A 2017, 5, 16105–16117. [Google Scholar] [CrossRef] [Green Version]
- Martoïa, F.; Cochereau, T.; Dumont, P.J.; Orgéas, L.; Terrien, M.; Belgacem, M. Cellulose nanofibril foams: Links between ice-templating conditions, microstructures and mechanical properties. Mater. Des. 2016, 104, 376–391. [Google Scholar] [CrossRef]
- Lee, J.; Deng, Y. The morphology and mechanical properties of layer structured cellulose microfibril foams from ice-templating methods. Soft Matter 2011, 7, 6034–6040. [Google Scholar] [CrossRef]
- Pan, Z.-Z.; Nishihara, H.; Iwamura, S.; Sekiguchi, T.; Sato, A.; Isogai, A.; Kang, F.; Kyotani, T.; Yang, Q.-H. Cellulose nanofiber as a distinct structure-directing agent for xylem-like microhoneycomb monoliths by unidirectional freeze-drying. ACS Nano 2016, 10, 10689–10697. [Google Scholar] [CrossRef] [PubMed]
- Bendahou, D.; Bendahou, A.; Seantier, B.; Grohens, Y.; Kaddami, H. Nano-fibrillated cellulose-zeolites based new hybrid composites aerogels with super thermal insulating properties. Ind. Crop. Prod. 2015, 65, 374–382. [Google Scholar] [CrossRef]
- Svagan, A.J.; Jensen, P.; Dvinskikh, S.V.; Furó, I.; Berglund, L.A. Towards tailored hierarchical structures in cellulose nanocomposite biofoams prepared by freezing/freeze-drying. J. Mater. Chem. 2010, 20, 6646–6654. [Google Scholar] [CrossRef]
- Parmenter, K.E.; Milstein, F. Mechanical properties of silica aerogels. J. Non-Cryst. Solids 1998, 223, 179–189. [Google Scholar] [CrossRef]
- Way, A.E.; Hsu, L.; Shanmuganathan, K.; Weder, C.; Rowan, S.J. pH-responsive cellulose nanocrystal gels and nanocomposites. ACS Macro Lett. 2012, 1, 1001–1006. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Huang, J.; Lin, N.; Ahmad, I.; Mariano, M.; Dufresne, A.; Thomas, S.; Gałęski, A. Recent developments in nanocellulose-based biodegradable polymers, thermoplastic polymers, and porous nanocomposites. Prog. Polym. Sci. 2018, 87, 197–227. [Google Scholar] [CrossRef]
- Ramesh, M.; Palanikumar, K.; Reddy, K.H. Plant fibre based bio-composites: Sustainable and renewable green materials. Renew. Sustain. Energy Rev. 2017, 79, 558–584. [Google Scholar] [CrossRef]
- Gourlay, S.J.; Rice, R.M.; Hegyeli, A.F.; Wade, C.W.; Dillon, J.G.; Jaffe, H.; Kulkarni, R. Biocompatibility testing of polymers: In vivo implantation studies. J. Biomed. Mater. Res. 1978, 12, 219–232. [Google Scholar] [CrossRef]
- Fernández-Cossío, S.; León-Mateos, A.; Sampedro, F.G.; Oreja, M.T.C. Biocompatibility of agarose gel as a dermal filler: Histologic evaluation of subcutaneous implants. Plast. Reconstr. Surg. 2007, 120, 1161–1169. [Google Scholar] [CrossRef]
- Rodrigues, S.; Dionísio, M.; López, C.R.; Grenha, A. Biocompatibility of chitosan carriers with application in drug delivery. J. Funct. Biomater. 2012, 3, 615–641. [Google Scholar] [CrossRef] [Green Version]
- Kumari, P.; Pathak, G.; Gupta, R.; Sharma, D.; Meena, A. Cellulose nanofibers from lignocellulosic biomass of lemongrass using enzymatic hydrolysis: Characterization and cytotoxicity assessment. DARU J. Pharm. Sci. 2019, 27, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.M.; Raposo, N.; Brayner, R.; Teixeira, E.; Oliveira, V.; Quintão, C.C.R.; Camargo, L.; Mattoso, L.; Brandão, H. Cytotoxicity and expression of genes involved in the cellular stress response and apoptosis in mammalian fibroblast exposed to cotton cellulose nanofibers. Nanotechnology 2013, 24, 075103. [Google Scholar] [CrossRef] [PubMed]
- Vartiainen, J.; Pöhler, T.; Sirola, K.; Pylkkänen, L.; Alenius, H.; Hokkinen, J.; Tapper, U.; Lahtinen, P.; Kapanen, A.; Putkisto, K. Health and environmental safety aspects of friction grinding and spray drying of microfibrillated cellulose. Cellulose 2011, 18, 775–786. [Google Scholar] [CrossRef]
- Alexandrescu, L.; Syverud, K.; Gatti, A.; Chinga-Carrasco, G. Cytotoxicity tests of cellulose nanofibril-based structures. Cellulose 2013, 20, 1765–1775. [Google Scholar] [CrossRef]
- Souza, S.F.; Mariano, M.; Reis, D.; Lombello, C.B.; Ferreira, M.; Sain, M. Cell interactions and cytotoxic studies of cellulose nanofibers from Curauá natural fibers. Carbohydr. Polym. 2018, 201, 87–95. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, F.; Liu, J.; Smått, J.-H.; Gepperth, D.; Lastusaari, M.; Xu, C.; Hupa, L. Biocomposites of copper-containing mesoporous bioactive glass and nanofibrillated cellulose: Biocompatibility and angiogenic promotion in chronic wound healing application. Acta Biomater. 2016, 46, 286–298. [Google Scholar] [CrossRef]
- Pértile, R.A.; Moreira, S.; Gil da Costa, R.M.; Correia, A.; Guãrdao, L.; Gartner, F.; Vilanova, M.; Gama, M. Bacterial cellulose: Long-term biocompatibility studies. J. Biomater. Sci. Polym. Ed. 2012, 23, 1339–1354. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zhai, T.; Turng, L.-S. Aerogel microspheres based on cellulose nanofibrils as potential cell culture scaffolds. Cellulose 2017, 24, 2791–2799. [Google Scholar] [CrossRef]
- Qin, L.; Zhao, X.; He, Y.; Wang, H.; Wei, H.; Zhu, Q.; Zhang, T.; Qin, Y.; Du, A. Preparation, characterization, and in vitro evaluation of resveratrol-loaded cellulose aerogel. Materials 2020, 13, 1624. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, T.; Takahashi, S.I.; Ito, H.; Inagaki, H.; Noishiki, Y. Tissue biocompatibility of cellulose and its derivatives. J. Biomed. Mater. Res. 1989, 23, 125–133. [Google Scholar] [CrossRef]
- Czaja, W.; Krystynowicz, A.; Bielecki, S.; Brown, R.M., Jr. Microbial cellulose—The natural power to heal wounds. Biomaterials 2006, 27, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Boda, S.K.; Wang, H.; Teusink, M.J.; Shuler, F.D.; Xie, J. Novel 3D hybrid nanofiber aerogels coupled with BMP-2 peptides for cranial bone regeneration. Adv. Healthc. Mater. 2018, 7, 1701415. [Google Scholar] [CrossRef] [PubMed]
- Salgado, M.; Santos, F.; Rodríguez-Rojo, S.; Reis, R.L.; Duarte, A.R.C.; Cocero, M.J. Development of barley and yeast β-glucan aerogels for drug delivery by supercritical fluids. J. CO2 Util. 2017, 22, 262–269. [Google Scholar] [CrossRef]
- Power, M.; Hosticka, B.; Black, E.; Daitch, C.; Norris, P. Aerogels as biosensors: Viral particle detection by bacteria immobilized on large pore aerogel. J. Non-Cryst. Solids 2001, 285, 303–308. [Google Scholar] [CrossRef]
- Yoshii, F.; Zhao, L.; Wach, R.A.; Nagasawa, N.; Mitomo, H.; Kume, T. Hydrogels of polysaccharide derivatives crosslinked with irradiation at paste-like condition. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2003, 208, 320–324. [Google Scholar] [CrossRef]
- Son, W.K.; Youk, J.H.; Lee, T.S.; Park, W.H. Preparation of antimicrobial ultrafine cellulose acetate fibers with silver nanoparticles. Macromol. Rapid Commun. 2004, 25, 1632–1637. [Google Scholar] [CrossRef]
- Cardea, S.; De Marco, I. Cellulose acetate and supercritical carbon dioxide: Membranes, nanoparticles, microparticles and nanostructured filaments. Polymers 2020, 12, 162. [Google Scholar] [CrossRef] [Green Version]
- Fang, B.; Wan, Y.-Z.; Tang, T.-T.; Gao, C.; Dai, K.-R. Proliferation and osteoblastic differentiation of human bone marrow stromal cells on hydroxyapatite/bacterial cellulose nanocomposite scaffolds. Tissue Eng. Part A 2009, 15, 1091–1098. [Google Scholar] [CrossRef]
- Liebner, F.; Haimer, E.; Wendland, M.; Neouze, M.A.; Schlufter, K.; Miethe, P.; Heinze, T.; Potthast, A.; Rosenau, T. Aerogels from unaltered bacterial cellulose: Application of scCO2 drying for the preparation of shaped, ultra-lightweight cellulosic aerogels. Macromol. Biosci. 2010, 10, 349–352. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, T.-J.; Zhang, D.-W.; Li, H.-Y.; Ma, Y.-R.; Qi, L.-M.; Zhou, Y.-L.; Zhang, X.-X. Amperometric hydrogen peroxide biosensor based on the immobilization of heme proteins on gold nanoparticles–bacteria cellulose nanofibers nanocomposite. Talanta 2011, 84, 71–77. [Google Scholar] [CrossRef]
- Korhonen, J.T.; Kettunen, M.; Ras, R.H.; Ikkala, O. Hydrophobic nanocellulose aerogels as floating, sustainable, reusable, and recyclable oil absorbents. ACS Appl. Mater. Interfaces 2011, 3, 1813–1816. [Google Scholar] [CrossRef] [PubMed]
- Valo, H.; Arola, S.; Laaksonen, P.; Torkkeli, M.; Peltonen, L.; Linder, M.B.; Serimaa, R.; Kuga, S.; Hirvonen, J.; Laaksonen, T. Drug release from nanoparticles embedded in four different nanofibrillar cellulose aerogels. Eur. J. Pharm. Sci. 2013, 50, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Li, Q.; Chen, W.; Yu, H. Composite aerogels based on dialdehyde nanocellulose and collagen for potential applications as wound dressing and tissue engineering scaffold. Compos. Sci. Technol. 2014, 94, 132–138. [Google Scholar] [CrossRef]
- Cai, H.; Sharma, S.; Liu, W.; Mu, W.; Liu, W.; Zhang, X.; Deng, Y. Aerogel microspheres from natural cellulose nanofibrils and their application as cell culture scaffold. Biomacromolecules 2014, 15, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.V.; Fontenot, K.R.; Prevost, N.T.; Pircher, N.; Liebner, F.; Condon, B.D. Preparation, characterization and activity of a peptide-cellulosic aerogel protease sensor from cotton. Sensors 2016, 16, 1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henschen, J.; Illergård, J.; Larsson, P.A.; Ek, M.; Wågberg, L. Contact-active antibacterial aerogels from cellulose nanofibrils. Colloids Surf. B Biointerfaces 2016, 146, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, J.; Mishra, H.; Mishra, P.K.; Wimmer, R.; Ahmad, F.J.; Talegaonkar, S. Cellulose nanofiber aerogel as a promising biomaterial for customized oral drug delivery. Int. J. Nanomed. 2017, 12, 2021. [Google Scholar] [CrossRef] [Green Version]
- Eluszkiewicz, J.; Uymin, G.; Flittner, D.; Cady-Pereira, K.; Mlawer, E.; Henderson, J.; Moncet, J.-L.; Nehrkorn, T.; Wolff, M. A fast code for channel limb radiances with gas absorption and scattering in a spherical atmosphere. J. Quant. Spectrosc. Radiat. Transf. 2017, 193, 31–39. [Google Scholar] [CrossRef]
- Zhou, J.; Hsieh, Y.-L. Conductive polymer protonated nanocellulose aerogels for tunable and linearly responsive strain sensors. ACS Appl. Mater. Interfaces 2018, 10, 27902–27910. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wang, Y.; Zhang, L.; Xu, Z.; Dai, H.; Wu, W. Nanocellulose/gelatin composite cryogels for controlled drug release. ACS Sustain. Chem. Eng. 2019, 7, 6381–6389. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, F.; Grénman, H.; Spoljaric, S.; Seppälä, J.; Eriksson, J.E.; Willför, S.; Xu, C. Development of nanocellulose scaffolds with tunable structures to support 3D cell culture. Carbohydr. Polym. 2016, 148, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, N.; Joseph, J.P.; Hussain, J.; Adeeb, M.A.M.; Wakim, M.J.Y.; Yahya, E.B.; Arif, A.; Saleem, A.; Sharif, N. Prevalence of human papilloma virus sub genotypes following head and neck squamous cell carcinomas in asian continent, a systematic review Article. Asian Pac. J. Cancer Prev. APJCP 2019, 20, 3269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, T.; Li, Y.; Chen, T. Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering. Int. J. Nanomed. 2013, 8, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Iglesias, C.; Barros, J.; Ardao, I.; Monteiro, F.J.; Alvarez-Lorenzo, C.; Gómez-Amoza, J.L.; García-González, C.A. Vancomycin-loaded chitosan aerogel particles for chronic wound applications. Carbohydr. Polym. 2019, 204, 223–231. [Google Scholar] [CrossRef]
- Gopi, S.; Amalraj, A.; Sukumaran, N.P.; Haponiuk, J.T.; Thomas, S. Biopolymers and their composites for drug delivery: A brief review. In Macromolecular Symposia; Wiley Online Library: Hoboken, NJ, USA, 2018. [Google Scholar]
- Yahya, E.B.; Alhawari, S.M.; Amhimmid, K.; AbuAeshah, R.H.A.; Saada, A.O. Evaluation of in-vitroantibacterial activity of aqueous and alcoholic extracts of the peels punica granatum and olea europaea leaves. J. Sci. Technol. (Med. Sci.) 2018, 2, 36–44. [Google Scholar]
- Hakkarainen, T.; Koivuniemi, R.; Kosonen, M.; Escobedo-Lucea, C.; Sanz-Garcia, A.; Vuola, J.; Valtonen, J.; Tammela, P.; Mäkitie, A.; Luukko, K. Nanofibrillar cellulose wound dressing in skin graft donor site treatment. J. Control. Release 2016, 244, 292–301. [Google Scholar] [CrossRef]
- Weishaupt, R.; Siqueira, G.; Schubert, M.; Kämpf, M.M.; Zimmermann, T.; Maniura-Weber, K.; Faccio, G. A protein-nanocellulose paper for sensing copper ions at the nano-to micromolar level. Adv. Funct. Mater. 2017, 27, 1604291. [Google Scholar] [CrossRef]
- Svagan, A.J.; Benjamins, J.-W.; Al-Ansari, Z.; Shalom, D.B.; Müllertz, A.; Wågberg, L.; Löbmann, K. Solid cellulose nanofiber based foams–towards facile design of sustained drug delivery systems. J. Control. Release 2016, 244, 74–82. [Google Scholar] [CrossRef]
- García-González, C.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Xiao, Y.; Rong, L.; Wang, B.; Mao, Z.; Xu, H.; Zhong, Y.; Zhang, L.; Sui, X. A light-weight and high-efficacy antibacterial nanocellulose-based sponge via covalent immobilization of gentamicin. Carbohydr. Polym. 2018, 200, 595–601. [Google Scholar] [CrossRef]
- Son, W.K.; Youk, J.H.; Park, W.H. Antimicrobial cellulose acetate nanofibers containing silver nanoparticles. Carbohydr. Polym. 2006, 65, 430–434. [Google Scholar] [CrossRef]
- Chen, L.; Bromberg, L.; Hatton, T.A.; Rutledge, G.C. Electrospun cellulose acetate fibers containing chlorhexidine as a bactericide. Polymer 2008, 49, 1266–1275. [Google Scholar] [CrossRef]
- Díez, I.; Eronen, P.; Österberg, M.; Linder, M.B.; Ikkala, O.; Ras, R.H. Functionalization of nanofibrillated cellulose with silver nanoclusters: Fluorescence and antibacterial activity. Macromol. Biosci. 2011, 11, 1185–1191. [Google Scholar] [CrossRef]
- Anitha, S.; Brabu, B.; Thiruvadigal, D.J.; Gopalakrishnan, C.; Natarajan, T. Optical, bactericidal and water repellent properties of electrospun nano-composite membranes of cellulose acetate and ZnO. Carbohydr. Polym. 2012, 87, 1065–1072. [Google Scholar] [CrossRef]
- Korehei, R.; Kadla, J.F. Encapsulation of T4 bacteriophage in electrospun poly (ethylene oxide)/cellulose diacetate fibers. Carbohydr. Polym. 2014, 100, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Wu, Q.; Zhang, Z.; Ren, S.; Lei, T.; Negulescu, I.I.; Zhang, Q. Porous carbon nanofibers from electrospun biomass tar/polyacrylonitrile/silver hybrids as antimicrobial materials. ACS Appl. Mater. Interfaces 2015, 7, 15108–15116. [Google Scholar] [CrossRef]
- Deng, Z.; Jung, J.; Zhao, Y. Development, characterization, and validation of chitosan adsorbed cellulose nanofiber (CNF) films as water resistant and antibacterial food contact packaging. LWT-Food Sci. Technol. 2017, 83, 132–140. [Google Scholar] [CrossRef]
- Matsuyama, K.; Morotomi, K.; Inoue, S.; Nakashima, M.; Nakashima, H.; Okuyama, T.; Kato, T.; Muto, H.; Sugiyama, H. Antibacterial and antifungal properties of Ag nanoparticle-loaded cellulose nanofiber aerogels prepared by supercritical CO2 drying. J. Supercrit. Fluids 2019, 143, 1–7. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Gao, M.; Zhao, Y.; Chen, Y. Sustained release of an essential oil by a hybrid cellulose nanofiber foam system. Cellulose 2020, 27, 2709–2721. [Google Scholar] [CrossRef]
- Tan, S.; Li, J.; Zhou, L.; Chen, P.; Xu, D.; Xu, Z. Fabrication of a flexible film electrode based on cellulose nanofibers aerogel dispersed with functionalized graphene decorated with SnO2 for supercapacitors. J. Mater. Sci. 2018, 53, 11648–11658. [Google Scholar] [CrossRef]
- Muthuraj, R.; Sachan, A.; Castro, M.; Feller, J.-F.; Seantier, B.; Grohens, Y. Vapor and pressure sensors based on cellulose nanofibers and carbon nanotubes aerogel with thermoelectric properties. J. Renew. Mater. 2018, 6, 277–287. [Google Scholar] [CrossRef]
- Zheng, Q.; Cai, Z.; Gong, S. Green synthesis of polyvinyl alcohol (PVA)–cellulose nanofibril (CNF) hybrid aerogels and their use as superabsorbents. J. Mater. Chem. A 2014, 2, 3110–3118. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef] [Green Version]


| Year | Types of Aerogel | Precursor Material | Preparation Method | References |
|---|---|---|---|---|
| 1931 | Silica aerogel | Sodium silicate | Supercritical drying | [3] |
| 1932 | Organic and metal oxide aerogels | -Metal oxides -Organic compounds | Supercritical drying | [14] |
| 1950 | Hydrophobic silica aerogels | Single and combinations of metal oxides with silica | Silylation with trichloro-methyl silane to produce water repellents | [16] |
| 1968 | Sol–gel route silica aerogel | Single and combinations of metal oxides with silica | Sol–gel route replaced water glass with TMOS then removed at supercritical conditions | [16] |
| 1974 | Sol–gel route silica aerogel | Single and combinations of metal oxides with silica | Sol–gel route silica aerogel | [18] |
| 1989 | Organic and carbon aerogels | Organic polymer | Sol–gel route silica aerogel | [19] |
| 1992 | Low-density and high-porosity silica aerogel | Single and combinations of metal oxides with silica | Acid–base process and substitution of the alcohol with an aprotic solvent | [17] |
| 1996 | Silica aerogel | -Metal oxide -Organic polymers | Developing rapid supercritical extraction (RSCE) | [20] |
| 1997 | Ultralight aerogels or X-aerogels | -Metals -Organic polymers | Crosslinking di-isocyanates into the silica structures inside the aerogels. | [21] |
| 2006 | Cellulose-based aerogels | Cellulose derivatives | Nontoxic iso-cyanate, via the sole gel route, with a tin-based catalyst. | [22] |
| 2008 | Cellulose nanofibres (CNF) aerogel | Cellulose nanofibres (CNF) | Facile vacuum drying of aqueous CNF gel. | [23] |
| 2009 | Metal aerogel | -Metals -Organic polymers | Smelting interpenetrating of resorcinol-formaldehyde and iron oxide xerogels | [24] |
| 2012 | Cellulose nanowhisker foams | Fully bleached commercial softwood Kraft pulp | Prepared via a freeze-casting method | [25] |
| 2014 | Aerogel-based Plasters | Silica + natural plaster | Granular silica aerogel mixed with natural plaster in different percentages | [26] |
| 2015 | CNF aerogel with water absorbency and shape recovery | Cellulose nanofibrils (CNF) | Crosslinking of CNF by the reaction between the C–C double bond of maleic acid-functionalised CNF and hypophosphite. | [27] |
| 2016 | Superhydrophobic and ultralight cellulose-based aerogel for oil spill | Cellulose-based aerogel | Novel physical-chemical foaming method, plasma treatment, and subsequent silane modification process. | [28] |
| 2017 | NFC Aerogel with thermal super-insulating | Nanofibrillated cellulose (NFC) | Spray freeze-drying (SFD) of Cellulose nanofibrils | [29] |
| 2018 | Low-cost method of silica aerogel | Fly ash and trona ore | Ambient pressure drying technique | [30] |
| 2020 | Agricultural Bio-waste as a Novel Cellulose Aerogel | Tea stem wastes (TSW) | Pure raw cellulose was isolated, hydrogel formation and then freeze-drying to form cellulose aerogel. | [31] |
| 2020 | A novel multifunctional carbon aerogel | β-TCP powders and sodium carbonate, formaldehyde | Beta-tricalcium phosphate bioceramic was platform-coated with carbon aerogel. | [32] |
| Name | Method | Particle Size | Advantages | Disadvantages |
|---|---|---|---|---|
| Freeze-drying | Freezing of CNF suspension at −65 ℃ then lyophilisation | μm to mm and nanosize thickness | One well-established nanodimension | Expensive agglomeration |
| Supercritical drying | Dehydrating the NFC suspension and replacing the solvent with L(CO2) | Nanosize | Dimensions stay in nanosize | Expensive and complicated method. |
| Spray drying | Concentrating and pumping the liquid then, dehydrating by hot gas | 7.48 μm | Controllable size and not expensive | Agglomeration |
| Oven drying | Put the suspension of NFC inside the oven at 105 ℃ for 24 h. | >100 μm or even mm | Well established for the industry | Loose of nano-D, Bulk material generation |
| Material | Preparation Method | Toxicological Experiment | Conclusion | Reference |
|---|---|---|---|---|
| Micro-fibrillated cellulose (MFC) | Fibrillating the fibres under high compression and shear forces. | Cytotoxicity evaluation with mouse macrophage and human monocyte | No evidence of cytotoxicity from the material nor the method. | [85] |
| Cotton cellulose nanofibres | Acid hydrolysis method | Cytotoxicity evaluation bovine fibroblast cells In-vitro effect on gene expression | Low cytotoxicity at low CNF concentration Reduction in cell viability and affection of expression of stress and apoptosis AMM at high concentration | [84] |
| Poly(vinyl alcohol)/cellulose nanofibril hybrid aerogel | Emulsification and freeze-drying processes | Cytotoxicity investigated with NIH 3T3 cells to explore their potential application as cell culture scaffolds. | Aerogel facilitates cell attachment, differentiation, and proliferation. Moreover, it was nontoxic and biocompatible | [90] |
| Cellulose nanofibril-based structures | Homogeniser without pre-treatment and with 2,2,6,6 tetramethylpiperidine-1-oxy radical | Cytotoxicity evaluation with 3T3 fibroblast cells | No toxic phenomena for pure CNF and slight toxicity for modified CNF | [86] |
| Cellulose nanofibres | Mechanical grinder preceded by mild chemical treatment | Cytotoxicity assays using a Vero cell lineage. | No cytotoxic behaviour of CNF or the method for direct and indirect assays | [87] |
| Cu/mesoporous bioactive glass/CNF membranes and aerogels | EISA method for MBGs, freeze-drying for membrane and solvent-exchange-freeze-drying for aerogel. | Cytotoxicity and biocompatibility evaluation in a 3T3 mouse fibroblast | Low cytotoxicity at low modified CNF concentration and no cell growth in high concentration | [88] |
| Cellulose nanofibres | Enzymatic hydrolysis method | The cytotoxicity of CNF assessed by MTT assay against three different cancer cell lines NCIH460, PA1, and L132 cells. | CNF did not show the cytotoxic effect at the tested concentrations in any of the cell lines. | [83] |
| Resveratrol-loaded cellulose aerogel | Freeze-drying method | Cytotoxicity to cartilage cells by the standard MTT assay | Low toxicity and good biocompatibility. | [91] |
| Material | Advantage | Method | Application | Reference |
|---|---|---|---|---|
| Super critically dried silica sol-gel discs | Facilitate the detection of chemicals and organisms | Use of viruses to trigger a response in immobilised bacteria and chemicals | Biosensors and diagnostics | [96] |
| Cellulose-based hydrogel | Superabsorbent capacity and satisfying biodegradability | Tested for biodegradability and antibacterial activity against E.coli | Antibacterial activity | [97] |
| Ultrafine cellulose acetate fibres with silver nanoparticles | Very strong antimicrobial activity | Direct electrospinning of a CA solution with small amounts of AgNO3 and then photoreduction | Antimicrobial film | [98] |
| Cellulose acetate nanofibre | Inhibit the growth of G+ and G- bacteria | cellulose acetate nanofibre membrane using supercritical carbon dioxide | Strong antibacterial film | [99] |
| Hydroxyapatite/bacterial cellulose (HAp/BC) nanocomposite | Better adhesion and activity and faster proliferated | HAp/BC nanocomposite scaffolds were prepared to utilise the biomimetic technique | Bone tissue engineering. | [100] |
| Bacterial cellulose (BC) aerogel | Easily equipped No aide interactions | BC aerogel matrix loaded with drug and the release behaviour from the matrix were studied | Drug delivery | [101] |
| Bacterial CNF incorporated with gold nanoparticles | Biocatalytic activity and fast response in low conc. of H2O2 | Immobilisation of heme proteins and enzymes | Fabrication of H2O2 biosensors. | [102] |
| Hydrophobic nanocellulose aerogels | Increase oral availability of drugs | Physical adsorption of a drug to aerogel for oral administration | Drug delivery system | [103] |
| Nanofibrillated cellulose (NFC) aerogels | Controlled drug delivery | NFC hydrogels are incorporated with the drug then convert it to aerogel | Drug delivery system | [104] |
| NCF/collagen composite aerogels | Strong absorption Biocompatible High proliferation. | Di-aldehyde NCFs and collagen were cross-linked together and formed the composite aerogels. | Tissue engineering and wound dressing | [105] |
| Nanocellulose aerogel (NCA) | Significant increase in cell count. | Cultured NIH 3T3 cells for two weeks on NCA. | Scaffolds for 3D cell culture | [106] |
| Nanocellulose aerogel (NA) | Monitor the level of protease in chronic wounds | The complex of polypeptide-NA (PepNA) to detect the sensitivity of PepNA for human neutrophil. | Biosensors | [107] |
| Antibacterial cellulose-based aerogel | Bacterial inhibition rate of >99.99%. | Fixing antibacterial substances on the surface of cellulose aerogels. | Bacterial growth inhibition | [108] |
| CNF composite aerogel | Significant increase in cell count. | Cultured 3T3 NIH cells on poly (vinyl alcohol). | Scaffolds for 3D cell culture | [90] |
| NFC aerogel | Noticeable increase in drug release | Loaded of NFC aerogel with alkylating antineoplastic agent. | Cancer treatments | [109] |
| Nanocellulose derivate aerogel | Complete inhibition of tested bacteria. | Loading lysozymes and silver nanoparticles on CNF aerogel. | Bacterial growth inhibition | [110] |
| Strain-sensing protonated CNF aerogel | Stretchable and sensitive | Cross-linking CNF surface with PSS in PEDOT/PSS generated PEDOT/PSS/CNF aerogels | Biosensors | [111] |
| Nanocellulose/gelatine composite cryogels | Controllable porosity, and good biocompatibility | Used cross-linked di-aldehyde starch as carriers for controlled 5-fluorouracil (5-FU) release. | Controlled drug release | [112] |
| Antimicrobial Agent | Function | Reference |
|---|---|---|
| Silver nanoparticles (average size of 21 nm) incorporated into the cellulose acetate nanofibre | Excellent antibacterial action against Gram-positive S. aureus and Gram-negative E. coli, K. pneumonia, and P. aeruginosa | [124] |
| Silver nitrate (size ranging from 10 to 20 nm) incorporated into the cellulose acetate nanofibre | Very strong antimicrobial activity against S. aureus, K. pneumonia, E. coli, and P. aeruginosa | [125] |
| Composition of nanofibrillated Cellulose with silver nanoclusters (NFC/AgNC) | Antibacterial activity against E. coli | [126] |
| ZnO incorporated into the cellulose acetate nanofibre | Exhibited strong antibacterial activity against S. aureus, E. coli, and Citrobacter | [127] |
| Silver nanoparticles incorporated into bacterial cellulose nanofibres | Strong antimicrobial potential against E. coli and S. aureus bacteria | [68] |
| T4 bacteriophage incorporated into core/shell electrospun fibres of polyethene oxide, cellulose diacetate (CDA) and their blends | Prevent bacterial growth on contaminated food surfaces | [128] |
| Porous CNFs with biomass tar, polyacrylonitrile (PAN), and silver nanoparticles | Excellent antimicrobial performance against Gram-positive S. aureus and Gram-negative E. coli, | [129] |
| Chitosan adsorbed cellulose nanofibre (CNF) films | Prepared CNF film even with low Mw of chitosan exhibited antibacterial activity against L. innocua and E. coli. | [130] |
| Covalent grafting of gentamicin to nanocellulose-based sponge | Excellent antibacterial performance against E. coli and S. aureus, with bactericidal rates of over 99.9% | [123] |
| Ag nanoparticle/cellulose nanofibre (Ag NP/CNF) composite aerogels | The aerogel exhibited good antibacterial (for E. coli) and antifungal (for A. niger) activity. | [131] |
| Cellulose nanofibres (CNFs) and thyme essential oil (EO) | Sustained antibacterial release for fresh food preservation. | [132] |
| SEM Images of CNF-Based Aerogels | Type | Reference | |
|---|---|---|---|
 |  | (a) and (b) pure CNF aerogels. | [133] |
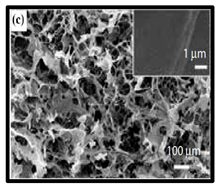 | 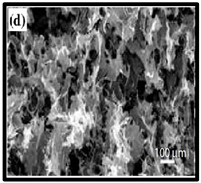 | (c) neat CNF, (d) CNF/ carbon nanotubes (CNTs) (75/25 wt%). | [134] |
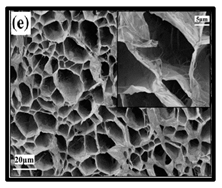 | 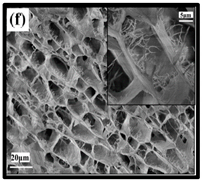 | (e) uncoated Polyvinyl alcohol (PVA)/CNF aerogel, (f) coated PVA/CNF aerogel. | [135] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Khalil, H.P.S.; Adnan, A.S.; Yahya, E.B.; Olaiya, N.G.; Safrida, S.; Hossain, M.S.; Balakrishnan, V.; Gopakumar, D.A.; Abdullah, C.K.; Oyekanmi, A.A.; et al. A Review on Plant Cellulose Nanofibre-Based Aerogels for Biomedical Applications. Polymers 2020, 12, 1759. https://doi.org/10.3390/polym12081759
Abdul Khalil HPS, Adnan AS, Yahya EB, Olaiya NG, Safrida S, Hossain MS, Balakrishnan V, Gopakumar DA, Abdullah CK, Oyekanmi AA, et al. A Review on Plant Cellulose Nanofibre-Based Aerogels for Biomedical Applications. Polymers. 2020; 12(8):1759. https://doi.org/10.3390/polym12081759
Chicago/Turabian StyleAbdul Khalil, H.P.S., A.S. Adnan, Esam Bashir Yahya, N.G. Olaiya, Safrida Safrida, Md. Sohrab Hossain, Venugopal Balakrishnan, Deepu A. Gopakumar, C.K. Abdullah, A.A. Oyekanmi, and et al. 2020. "A Review on Plant Cellulose Nanofibre-Based Aerogels for Biomedical Applications" Polymers 12, no. 8: 1759. https://doi.org/10.3390/polym12081759
APA StyleAbdul Khalil, H. P. S., Adnan, A. S., Yahya, E. B., Olaiya, N. G., Safrida, S., Hossain, M. S., Balakrishnan, V., Gopakumar, D. A., Abdullah, C. K., Oyekanmi, A. A., & Pasquini, D. (2020). A Review on Plant Cellulose Nanofibre-Based Aerogels for Biomedical Applications. Polymers, 12(8), 1759. https://doi.org/10.3390/polym12081759









