Synthesis and Characterization of Copoly(Ether Sulfone)s with Different Percentages of Diphenolic Acid Units
Abstract
:1. Introduction
2. Experimental
2.1. Materials
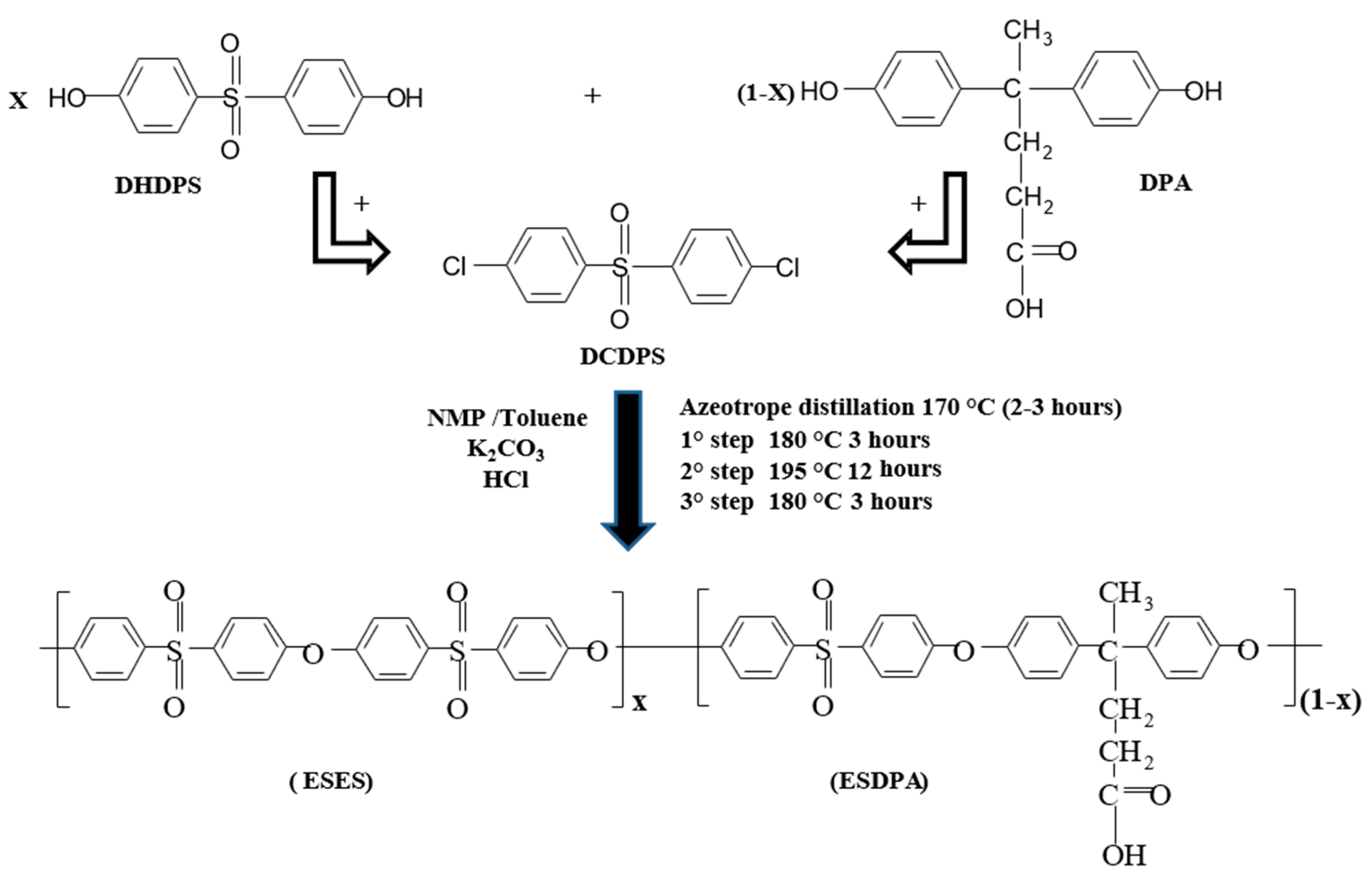
2.2. Synthesis
2.3. Methods of Analysis
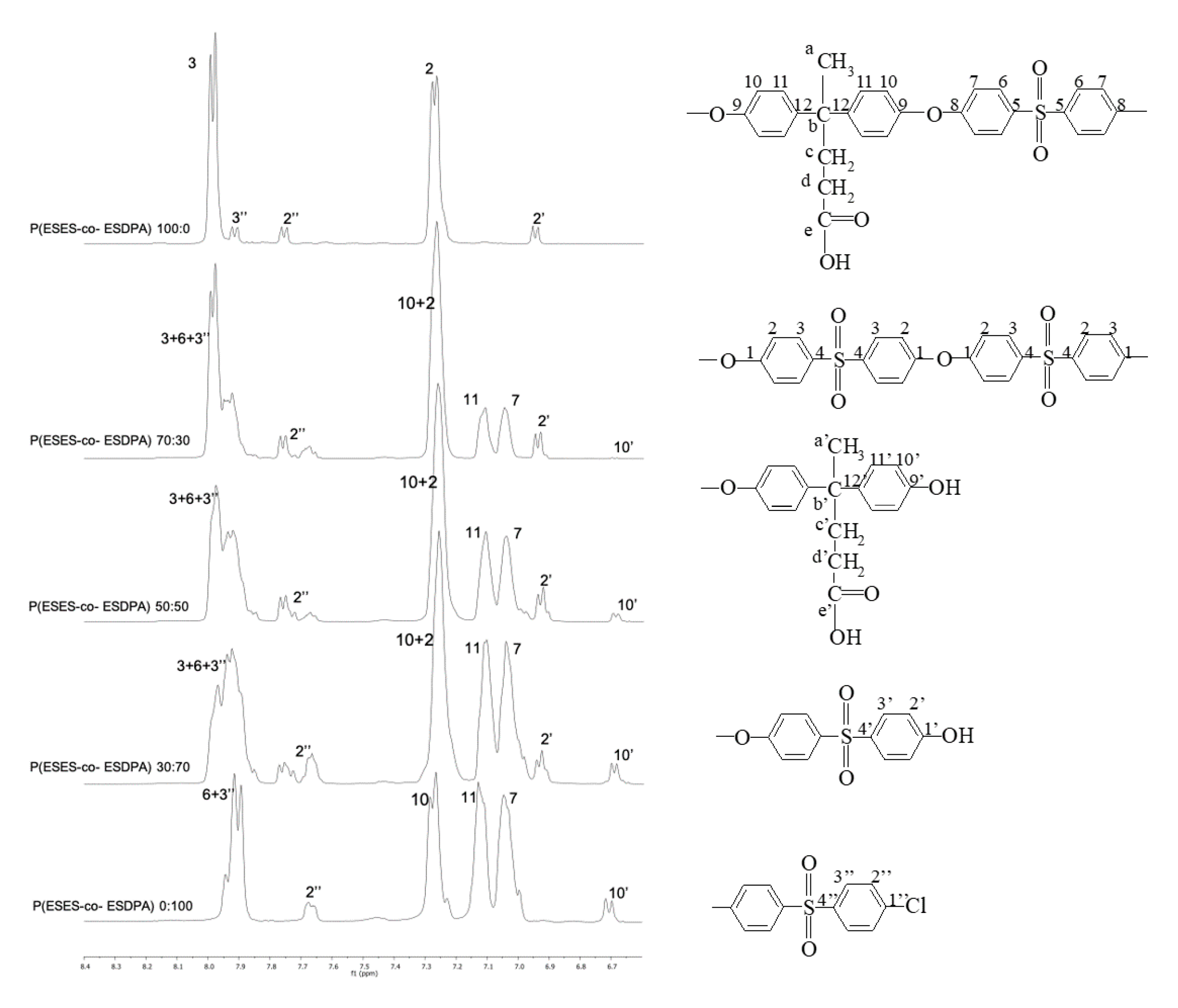
2.3.1. H-NMR and 13C-NMR
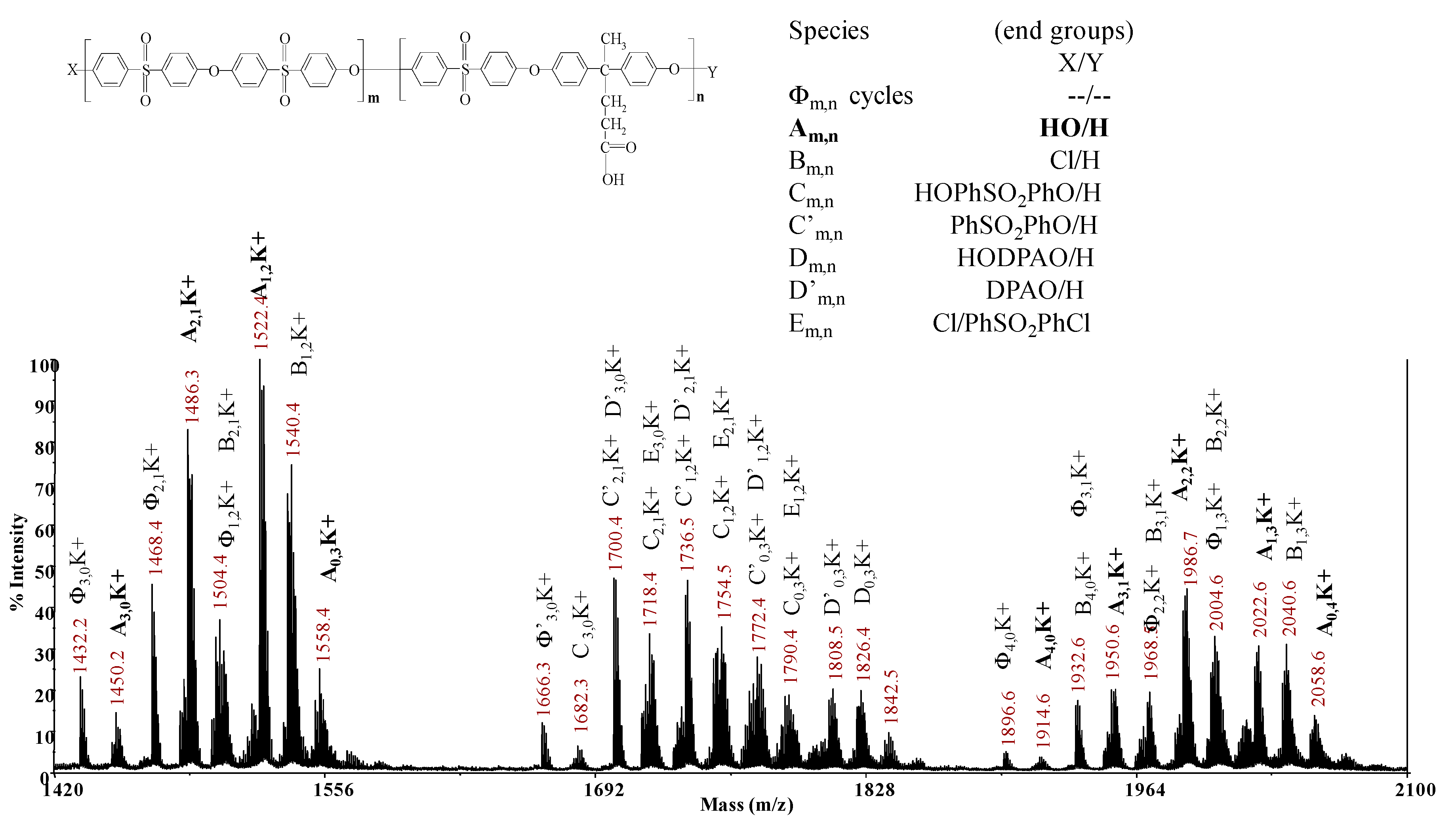
2.3.2. MALDI-TOF MS
2.3.3. FT-IR
2.3.4. Differential Scanning Calorimetry
2.3.5. Dinamo Mechanical Analysis (DMA)
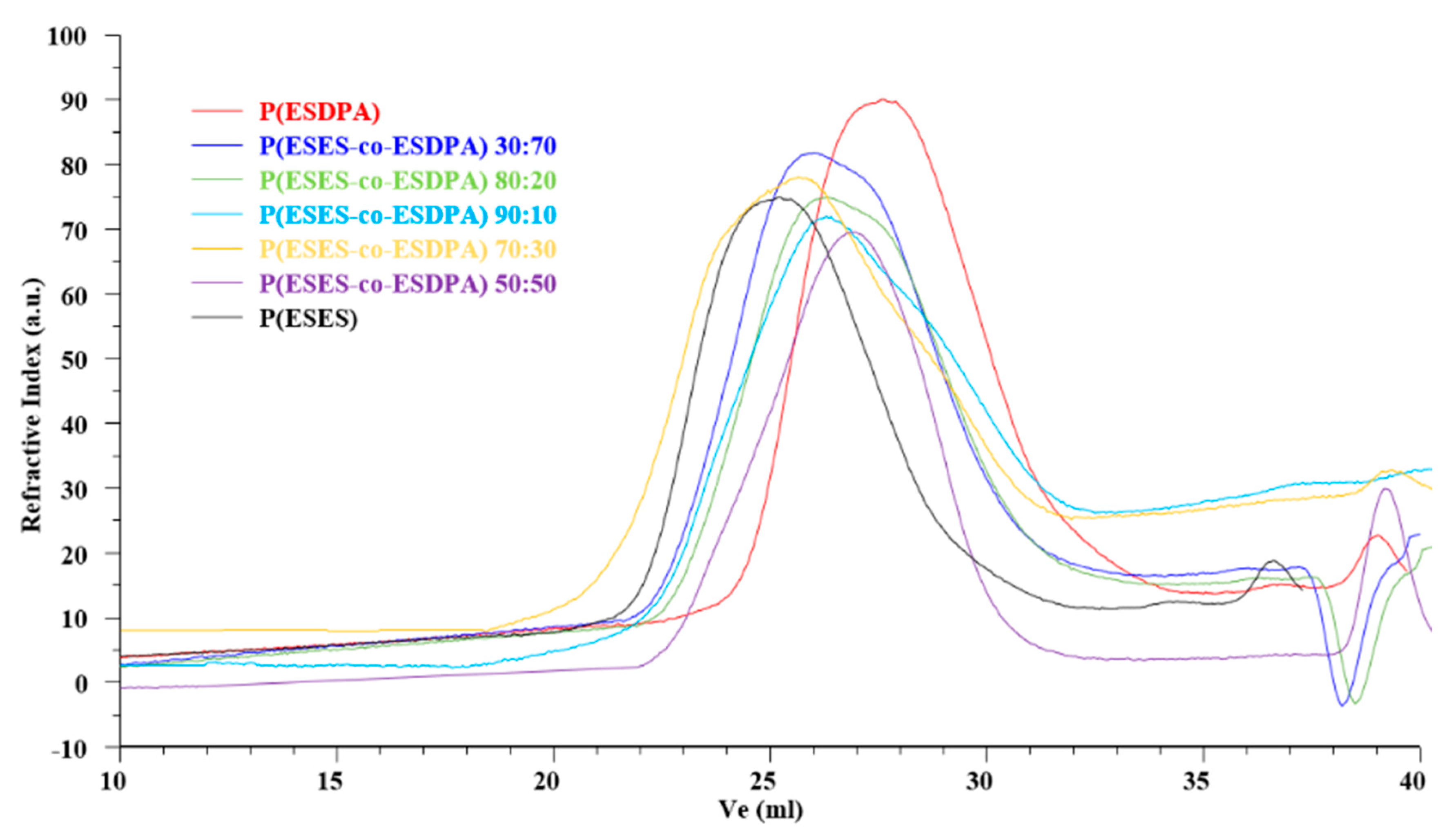
2.3.6. Size Exclusion Chromatography (SEC)
2.3.7. Wettability Analysis
2.3.8. Viscosimetry
2.3.9. Chemical Microstructural Analysis and Sequence Distribution of P(ESES-co-ESDPA) Copolymers
3. Results and Discussion
3.1. Infrared Spectroscopy
3.2. NMR Analysis
3.3. MALDI-TOF MS
3.4. Size Exclusion Chromatography
3.5. DSC
3.6. DMA Analysis
3.7. Wettability Analysis
3.8. Selective Extraction on Heavy Metals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maes, C.; Devaux, J.; Legras, R.; Parsons, W.; McGrail, P.T. Characterization of novel modified amorphous poly(ether sulphone)s. J. Polym. Sci. Part A 1994, 32, 3171–3182. [Google Scholar] [CrossRef]
- Sugama, T.; Carciello, N.R. Polyphenyletherketone and polyphenylethersulphone adhesive for metal-to-metal joints. Int. J. Adhes. Adhes. 1993, 13, 257–266. [Google Scholar] [CrossRef]
- Pinnau, I.; Koros, J.W. Structues and gas separation properties of asymmetric polysulfone membrane made by dry, wet, and dry wet phase inversion. J. Appl. Polym. Sci. 1991, 43, 1491–1502. [Google Scholar] [CrossRef]
- Dizman, C.; Tasdelenc, M.A.; Yagcia, Y. Recent advances in the preparation of functionalized polysulfones. Polym. Int. 2013, 62, 991–1007. [Google Scholar] [CrossRef]
- McGrail, P.T. Polyaromatics. Polym. Int. 1996, 41, 103–121. [Google Scholar] [CrossRef]
- Kim, B.S.; Chiba, T.; Inoue, T. Morphology development via reaction-induced phase-separation in epoxy poly(ether sulfone) blends-morphology control using poly(ether sulfone) with functional end groups. Polymer 1995, 36, 43–47. [Google Scholar] [CrossRef]
- Blanco, I.; Cicala, G.; Motta, O.; Recca, A. Influence of a selected hardener on the phase separation in epoxy/thermoplastic polymer blends. J. Appl. Polym. Sci. 2004, 94, 361–371. [Google Scholar] [CrossRef]
- Puglisi, C.; Samperi, F.; Cicala, G.; Recca, A.; Restuccia, C.L. Combined MALDI-TOF MS and NMR characterization of copoly(arylen ether sulphone)s. Polymer 2006, 47, 1861–1874. [Google Scholar] [CrossRef]
- Abate, L.; Blanco, I.; Cicala, G.; La Spina, R.; Restuccia, C.L. Thermal and rheological behaviour of some random aromatic polyethersulfone/polyethersulfone copolymers. Polym. Deg. Stab. 2006, 91, 924–930. [Google Scholar] [CrossRef]
- Abate, L.; Blanco, I.; Cicala, G.; Recca, A.; Restuccia, C.L. Thermal and rheological behaviours of some random aromatic amino-ended polyethersulfone/polyethersulfone copolymers. Polym. Deg. Stab. 2006, 91, 3230–3236. [Google Scholar] [CrossRef]
- Kim, J.P.; Lee, W.Y.; Kang, J.W.; Kwon, S.K.; Kim, J.J.; Lee, J.S. Fluorinated poly(arylene ether sulfide) for polymeric optical waveguide devices. Macromolecules 2001, 34, 7817–7821. [Google Scholar] [CrossRef]
- Theil, F. Synthesis of diaryl ethers: A long-standing problem has been solved. Angew. Chem. 1999, 38, 2345–2347. [Google Scholar] [CrossRef]
- Samperi, F.; Puglisi, C.; Ferreri, T.; Messina, R.; Cicala, G.; Recca, A.; Restuccia, C.L.; Scamporrino, A. Thermal decomposition products of copoly(arylene ether sulfone)s characterized by direct pyrolysis mass spectrometry. Polym. Deg. Stab. 2007, 92, 1304–1315. [Google Scholar] [CrossRef]
- Weber, M.; Rajak, A.; Maletzko, C. Polyethersulfone block copolymers for membrane applications. Macromol. Chem. Phys. 2019, 220, 1900305. [Google Scholar] [CrossRef]
- Naderi, A.; Yong, W.F.; Xiao, Y.; Chungv, T.-S.; Weber, M.; Maletzko, C. Effects of chemical structure on gas transport properties of polyethersulfone polymers. Polymer 2018, 135, 76–84. [Google Scholar] [CrossRef]
- Ioan, S. (Ed.) Functionalized Polysulfones Synthesis, Characterization, and Applications; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9780429170713. [Google Scholar] [CrossRef]
- Koch, T.; Ritter, H. Functionalized polysulfones from 4,4-bis(4-hydroxyphenyl)pentanoic acid, 2,2-isopropylidenediphenol and bis(4-chlorophenyl) sulfone: Synthesis, analogous amidation of the carboxylic groups. Macromol. Chem. Phys. 1994, 195, 1709–1717. [Google Scholar] [CrossRef]
- Esser, I.C.H.M.; Parsons, I.W. Modified poly(ether sulfone)/poly(ether ether sulfone) polymers: Approaches to pendent carboxyl groups. Polymer 1993, 34, 2836–2844. [Google Scholar] [CrossRef]
- Weisse, H.; Keul, H.; Höcher, H. A new route to carboxylated poly(ether sulfone)s: Synthesis and characterization. Polymer 2001, 42, 5973–5978. [Google Scholar] [CrossRef]
- Ganesh, S.D.; Harish, M.N.K.; Madhu, B.J.; Maqbool, H.; Pai, K.V.; Kariduraganavar, M.Y. Poly(arylene ether sulfone)s with HEPES pendants: Synthesis, thermal, and dielectric studies. ISRN Polym. Sci. 2013. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Vakhtangishvili, L.; Fritsch, D. Synthesis and Functionalization of Poly(ether sulfone)s Based on 1,1,1-Tris(4-hydroxyphenyl)ethane. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 2967–2977. [Google Scholar] [CrossRef]
- Alenazi, N.A.; Hussein, M.A.; Alamry, K.A.; Asiri, A.M. Modified polyether-sulfone membrane: A mini review. Des. Monomers Polym. 2017, 20, 532–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scamporrino, A.; Samperi, F.; Zampino, D.; Dattilo, S.; Puglisi, C.; Spina, A. Synthesis and characterization of PES copolymers with carboxyl units as metal recovering materials. In The Fiftieth Anniversary of the Institute for Polymers Composites and Biomaterials; IPCB CNR: Catania, Italy, 2019; p. 87. ISBN 9788880803652. [Google Scholar]
- Dattilo, S.; Puglisi, C.; Mirabella, E.; Spina, A.; Scamporrino, A.A.; Zampino, D.C.; Blanco, I.; Cicala, G.; Ognibene, G.; Di Mauro, C.; et al. Thermal Degradation Processes of aromatic poly(ether sulfone) random copolymers bearing pendant carboxyl groups. Polymers 2020. submitted. [Google Scholar]
- Ognibene, G.; Gangemi, C.M.A.; D’Urso, A.; Purrello, R.; Cicala, G.; Fragalà, M.E. Combined Approach to Remove and Fast Detect Heavy Metals in Water Based on PES–TiO2 Electrospun Mats and Porphyrin Chemosensors. ACS Omega 2018, 3, 7182–7190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ognibene, G.; Gangemi, C.M.A.; Spitaleri, L.; Gulino, A.; Purrello, R.; Cicala, G.; Fragalà, M.E. Role of the surface composition of the polyethersulfone-TiiP-H2T4 fibers on lead removal: From electrostatic to coordinative binding. J. Mater. Sci. 2019, 54, 8023–8033. [Google Scholar] [CrossRef]
- Lu, Z.; Li, J.; Hua, J.; Li, X.; Qin, J.; Qin, A.; Ye, C. Two new poly(arylene ether sulfone)s containing second-order nonlinear optical chromophores. Synth. Met. 2005, 152, 217–220. [Google Scholar] [CrossRef]
- Wu, X.; Xie, Z.; Wang, H.; Zhao, C.; Ng, D.; Zhang, K. Improved filtration performance and antifouling properties of polyethersulfone ultrafiltration membranes by blending with carboxylic acid functionalised polysulfone. RSC Adv. 2018, 8, 7774–7784. [Google Scholar] [CrossRef] [Green Version]
- Jadranka, B.-G.; Zdenek, B.; Jan, S. IR laser ablative degradation of poly(phenylene ether-sulfone): Deposition of films containing ether, sulfone, sulfoxide and sulfide groups. Polym. Deg. Stab. 2009, 94, 196–200. [Google Scholar] [CrossRef]
- Guiver, M.D.; Zhang, H.; Robertson, G.P.; Dai, Y. Modified polysulfones. III. Synthesis and Characterization of polysulfone aldehydes for reactive membrane materials. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 675–682. [Google Scholar] [CrossRef] [Green Version]
- Hussein, M.A.; Alam, M.M.; Alenazi, N.A.; Alamry, K.A.; Asiri, A.A.; Rahman, M.M. Nanocomposite base functionalization polyethersulfone abd conjugated ternary ZnYCdO nanomaterials foe the fabrication of selective Cd2+ sensor probe. J. Polym. Res. 2018, 25, 262. [Google Scholar] [CrossRef]
- Wang, D.; Zou, W.; Li, L.; Wei, Q.; Sun, S.; Zhao, C. Preparation and characterization of functional carboxylic polyethersulfone membrane. J. Membr. Sci. 2011, 374, 93–101. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kang, I.K.; Huh, M.W.; Yoon, S.C. Surface characterization and in vitro blood compatibility of poly(ethylene terephthalate) immobilized with insulin and/or heparin using plasma glow discharge. Biomaterials 2000, 21, 121–130. [Google Scholar] [CrossRef]
- Mahlin, D.; Wood, J.; Hawkins, N.; Mahey, J.; Royall, P.G. A novel powder sample holder for the determination of glass transition temperatures by DMA. Int. J. Pharm. 2009, 371, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Cicala, G.; Mamo, A.; Recca, G.; Restuccia, C.L. Synthesis and thermal characterization of some novel ABA block copolymers. Macromol. Mater. Eng. 2007, 292, 588–597. [Google Scholar] [CrossRef]
- Carlier, V.; Sclavons, M.; Legras, R. Supported dynamic mechanical thermal analysis: An easy, powerful and very sensitive technique to assess thermal properties of polymer, coating and even nanocoating. Polymer 2001, 42, 5327–5335. [Google Scholar] [CrossRef]
- Randall, J.C. Polymer Sequence Determination. J. Polym. Sci. Polym. Lett. Ed. 1977, 16, 71. [Google Scholar] [CrossRef]
- Abraham, R.J.; Haworth, I.S.; Bunn, A.; Hearmon, R.A. Substituent effects in the 13C NMR spectra of aryl ether copolymers: 5. Aryl ether ketone copolymers in acid solution. Polymer 1990, 31, 728. [Google Scholar] [CrossRef]
- Abraham, R.J.; Haworth, I.S.; Bunn, A.; Hearmon, R.A. Substituent effects in the 13C NMR spectra of aryl ether copolymers. 6. Sulphonated aryl ether ketones. Polymer 1990, 32, 126. [Google Scholar] [CrossRef]
- Yamadera, Y.; Murano, M. The determination of randomness in copolyesters by high resolution nuclear magnetic resonance. J. Polym. Sci. Part A 1967, 5, 2259. [Google Scholar] [CrossRef]
- Zhou, X.-M.; Jiang, Z.-H. Sequence Analysis of Poly(ether sulfone) Copolymers by 13C NMR. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 1624–1630. [Google Scholar] [CrossRef]
- Samperi, F.; Battiato, S.; Recca, G.; Puglisi, C.; Mendichi, R. Reactive melt mixing of PC/PEN blend. Structural characterization of reaction products. Polymer 2015, 74, 108–123. [Google Scholar] [CrossRef]
- Montaudo, G.; Montaudo, M.S.; Samperi, F. Mass Spectrometry of Polymers; Montaudo, G., Lattimer, R.P., Eds.; CRC Press: Boca Raton, FL, USA, 2002; Chapters 2 and 10; ISBN 0-8493-3127-7. [Google Scholar]
- Pasch, H.; Schrepp, W. MALDI–TOF Mass Spectrometry of Synthetic Polymers; Springer: Berlin/Heidelberg, Germany, 2003; p. 298. [Google Scholar]
- Montaudo, G.; Samperi, F.; Montaudo, M.S. Characterization of synthetic polymers by MALDI-MS. Prog. Polym. Sci. 2006, 31, 277–357. [Google Scholar] [CrossRef]
- Rizzarelli, P.; Carroccio, S. Biodegradable Polymers Vol 1: Advancement in Biodegradation Study and Applications; Chu, C.-C., Ed.; Nova Science Publishers: New York, NY, USA, 2015; p. 77. ISBN 978-1-63483-632-6. [Google Scholar]
- Samperi, F.; Battiato, S.; Puglisi, C.; Giovanella, U.; Mendichi, R.; Destri, S. Combined Techniques for the Characterization of Polyfluorene Copolymers and Correlation with their Optical Properties. Macromolecules 2012, 45, 1811–1824. [Google Scholar] [CrossRef]
- Montaudo, M.S.; Puglisi, C.; Battiato, S.; Zappia, S.; Destri, S.; Samperi, F. An innovative approach for the chemical structural characterization of poly(styrene 4-vinylpyridine) copolymers by matrix-assisted laser desorption/ionization time of flight mass spectrometry. J. Appl. Polym. Sci. 2019, 136, 46976. [Google Scholar] [CrossRef]




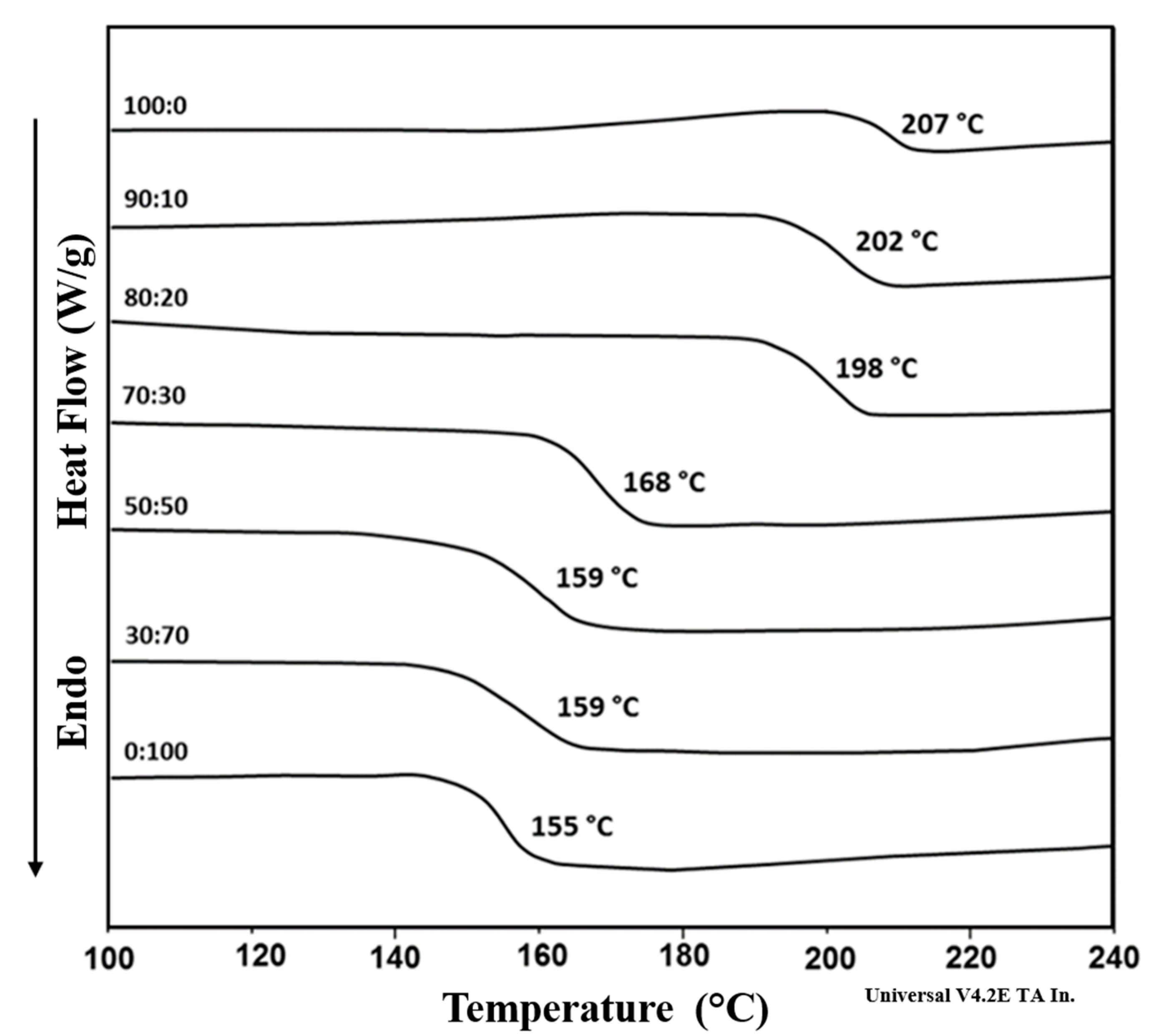


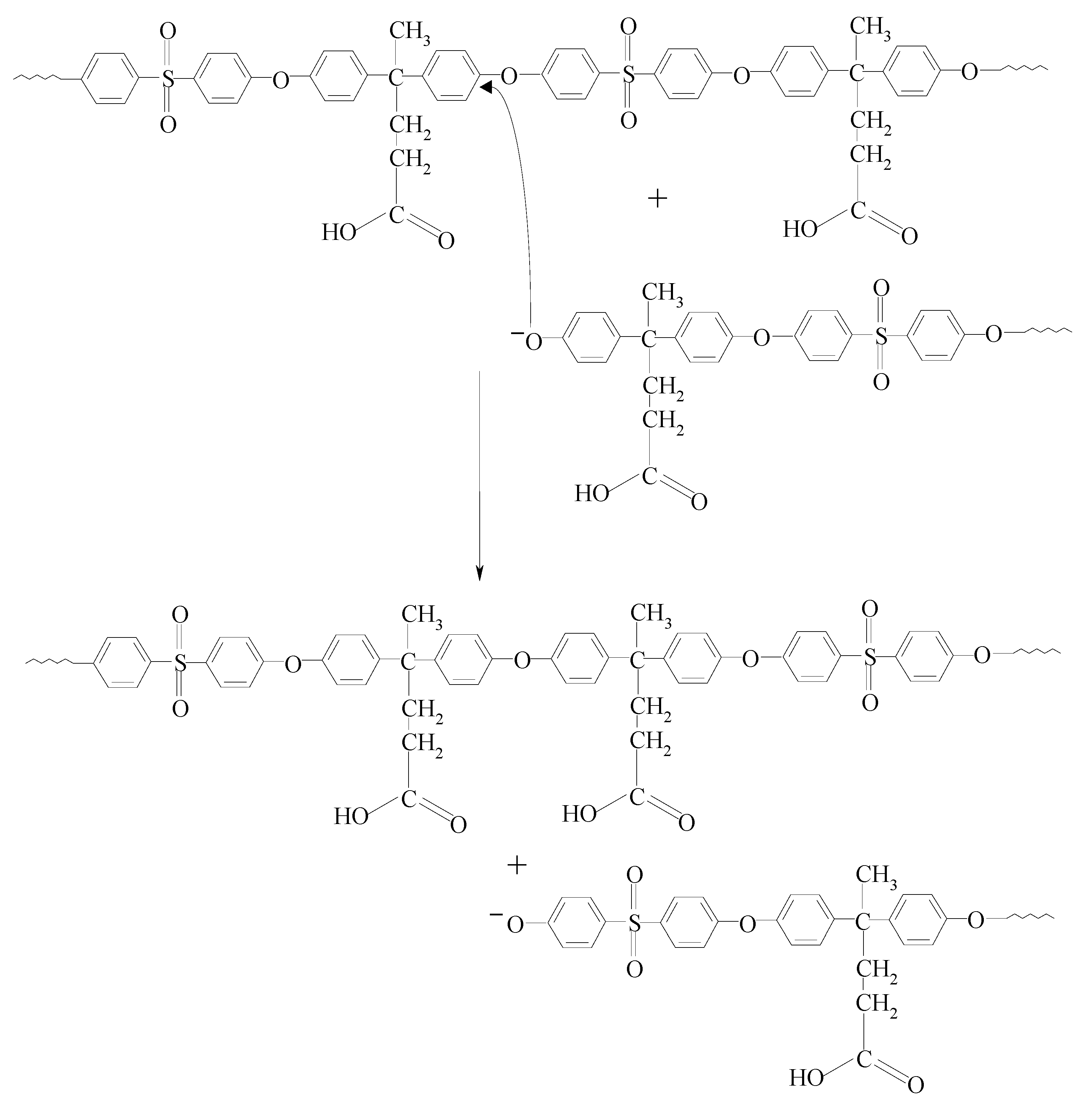
| Sample | Feed Ratio (Mol Fraction) | Molar Composition (%mol) 1 ESES/ESDPA | Mn (g/mol) 2 (1H-NMR) | ηinh 3 | Tg (°C) | PDI (Mw/Mn) 4 | Yield 5 (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| DCDPS | DHDPS | DPA | 1H-NMR | 13C-NMR | ||||||
| 1 | 1.0 | 1.0 | 0.0 | 100/0 | 100/0 | 7900 | 0.35 | 207 | 2.19 (18200/8300) | 93.2 |
| 2 | 1.0 | 0.9 | 0.1 | 88/12 | 89/11 | 7000 | 0.34 | 202 | 2.10 (15000/7150) | 94.1 |
| 3 | 1.0 | 0.8 | 0.2 | 77/23 | 82/18 | 7100 | 0.33 | 198 | 2.15 (15700/7300) | 92.7 |
| 4 | 1.0 | 0.7 | 0.3 | 68/32 | 71/29 | 8800 | 0.35 | 168 | 2.20 (19800/9000) | 92.5 |
| 5 | 1.0 | 0.5 | 0.5 | 51/49 | 51/49 | 6600 | 0.32 | 159 | 2.19 (15000/6850) | 94.6 |
| 6 | 1.0 | 0.3 | 0.7 | 32/68 | 33/67 | 7200 | 0.36 | 159 | 2.13 (15800/7400) | 93.4 |
| 7 | 1.0 | 0.0 | 1.0 | 0/100 | 0/100 | 6000 | 0.30 | 155 | 2.26 (13900/6150) | 91.2 |
| Sequence Structures | H/C | Chemical Shifts (ppm) | |
|---|---|---|---|
| Proton | Carbon | ||
ESDPA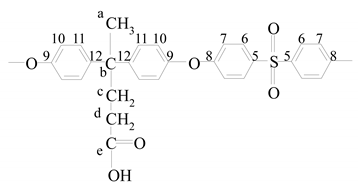 | a | 1.59 (s) | 26.875 |
| b | - | 44.5 | |
| c | 2.11 (t) | 29.5 | |
| d | 2.38 (t) | 35.9 | |
| e | 12.09 (bs) | 173.88 | |
| 5 | - | 135.15 | |
| 6 | 7.91 | 128.5 | |
| 7 | 7.11 | 119.4 | |
| 8 | - | 161.91 | |
| 9 | - | 144.8 | |
| 10 | 7.26 | 117.7 | |
| 11 | 7.04 | 129.4 | |
| 12 | - | 152.3 | |
ESES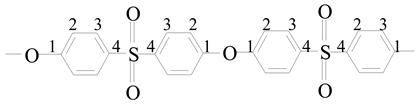 | 1 | - | 159.2 |
| 2 | 7.26–7.29 | 119.63 | |
| 3 | 7.98–8.10 | 129.86 | |
| 4 | - | 136.6 | |
End groups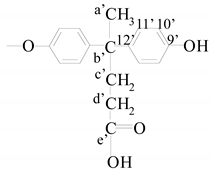 | a′ | 1.53 (s) | 25.48 |
| b′ | - | 44.1 | |
| c′ | 1.88 | 30.2 | |
| d′ | 2.18 (t) | 36.6 | |
| e′ | 12.07 (s) | 172.93 | |
| 9′ | - | 145.4 | |
| 10′ | 6.69 (d) | 114.7 | |
| 11′ | 6.95 (d) | 130.44 | |
| 12′ | - | 153.05 | |
| OH | 9.12/9.19 | - | |
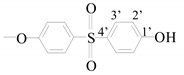 | 1′ | - | 162.12 |
| 2′ | 6.82 (d) | 105.8 | |
| 3′ | 7.70 (d) | 129.45 | |
| 4′ | - | 134.4 | |
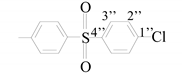 | 1″ | - | 139.33 |
| 2″ | 7.69 (d) | 129.04 | |
| 3″ | 7.92 (d) | 129.62 | |
| 4″ | - | 138.78 | |
| Samples | Normalized Intensity of Carbons | Molar Fractions of Triads | PE-DPA | PDPA-E | LESES | LESDPA | β | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C8 | C1 | |||||||||||||
| I161.25 | I161.09 | I159.25 | I159.2 | I159.03 | fE-S-E | fE-S-DPA | fDPA-S-E | fDPA-S-DPA | ||||||
| 80/20 | 6.3 | 1.9 | 9.15 | 73.3 | 9.4 | 0.595 | 0.178 | 0.180 | 0.045 | 0.13 | 0.67 | 7.69 | 1.49 | 0.8 |
| 70/30 | 12.6 | 3.2 | 13.0 | 58.0 | 13.2 | 0.425 | 0.255 | 0.257 | 0.060 | 0.23 | 0.68 | 4.35 | 1.47 | 0.81 |
| 50/50 | 21.2 | 12.8 | 16.7 | 35.4 | 17.3 | 0.225 | 0.310 | 0.315 | 0.165 | 0.41 | 0.48 | 2.44 | 2.08 | 0.89 |
| 30/70 | 23.0 | 28.9 | 14.2 | 19.5 | 14.5 | 0.12 | 0.285 | 0.285 | 0.325 | 0.54 | 0.31 | 1.85 | 3.22 | 0.85 |
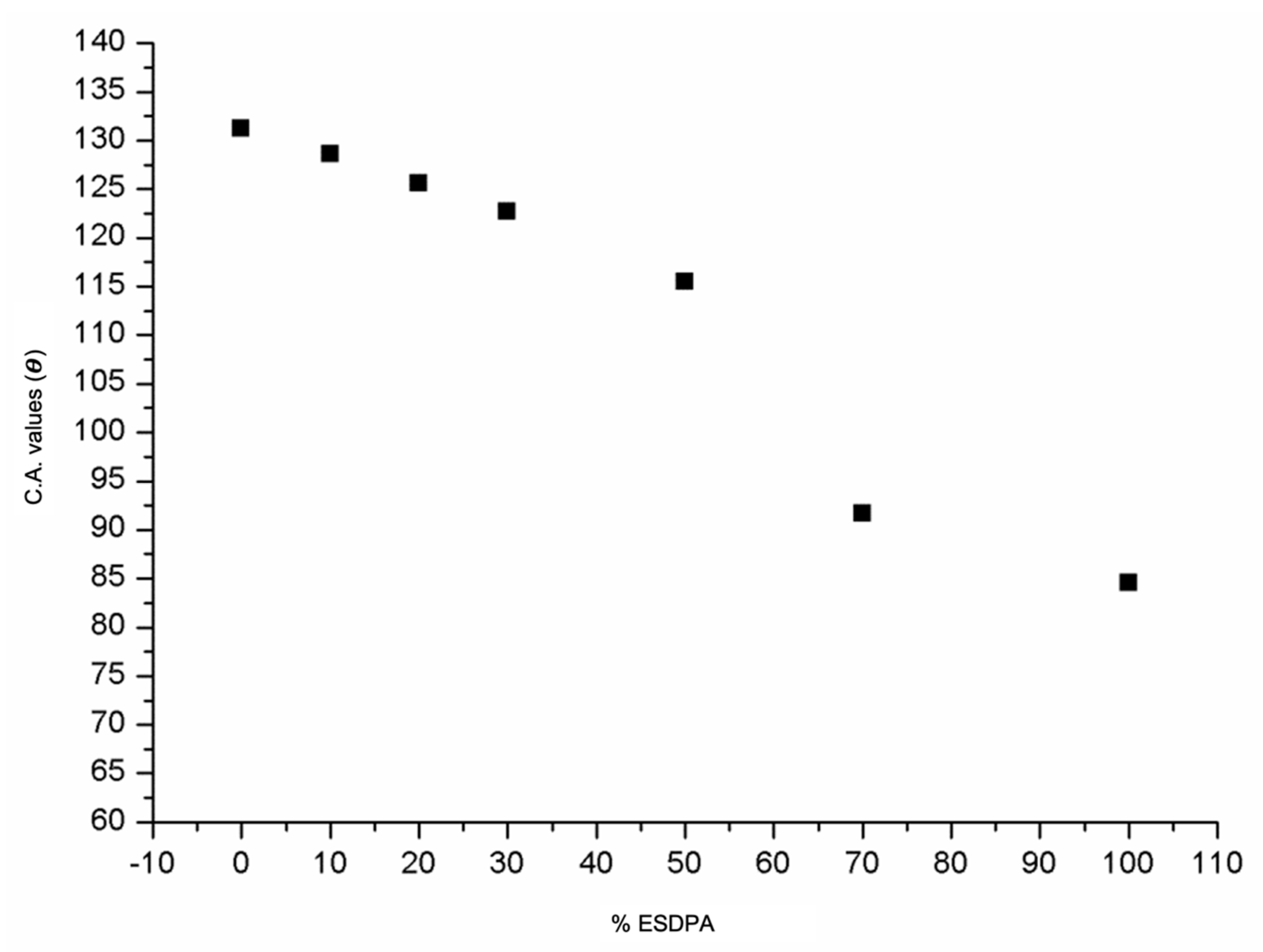
| SAMPLE | AC (θ) |
|---|---|
| P(ESES-co-ESDPA) 0:100 | 84.5° |
| P(ESES-co-ESDPA) 30:70 | 91.7° |
| P(ESES-co-ESDPA) 50:50 | 115.5° |
| P(ESES-co-ESDPA) 70:30 | 122.7° |
| P(ESES-co-ESDPA) 80:20 | 125.6° |
| P(ESES-co-ESDPA) 90:10 | 128.6° |
| P(ESES-co-ESDPA) 100:0 | 131.2° |
| Commercial PES | 137.7° |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scamporrino, A.A.; Puglisi, C.; Spina, A.; Montaudo, M.; Zampino, D.C.; Cicala, G.; Ognibene, G.; Mauro, C.D.; Dattilo, S.; Mirabella, E.F.; et al. Synthesis and Characterization of Copoly(Ether Sulfone)s with Different Percentages of Diphenolic Acid Units. Polymers 2020, 12, 1817. https://doi.org/10.3390/polym12081817
Scamporrino AA, Puglisi C, Spina A, Montaudo M, Zampino DC, Cicala G, Ognibene G, Mauro CD, Dattilo S, Mirabella EF, et al. Synthesis and Characterization of Copoly(Ether Sulfone)s with Different Percentages of Diphenolic Acid Units. Polymers. 2020; 12(8):1817. https://doi.org/10.3390/polym12081817
Chicago/Turabian StyleScamporrino, Andrea A., Concetto Puglisi, Angela Spina, Maurizio Montaudo, Daniela C. Zampino, Gianluca Cicala, Giulia Ognibene, Chiara Di Mauro, Sandro Dattilo, Emanuele F. Mirabella, and et al. 2020. "Synthesis and Characterization of Copoly(Ether Sulfone)s with Different Percentages of Diphenolic Acid Units" Polymers 12, no. 8: 1817. https://doi.org/10.3390/polym12081817








