Extending Cellulose-Based Polymers Application in Additive Manufacturing Technology: A Review of Recent Approaches
Abstract
:1. Introduction
2. Polymer-Based Additive Manufacturing
2.1. Types of Additive Manufacturing Technologies
2.2. Biodegradable Polymers for Additive Manufacturing
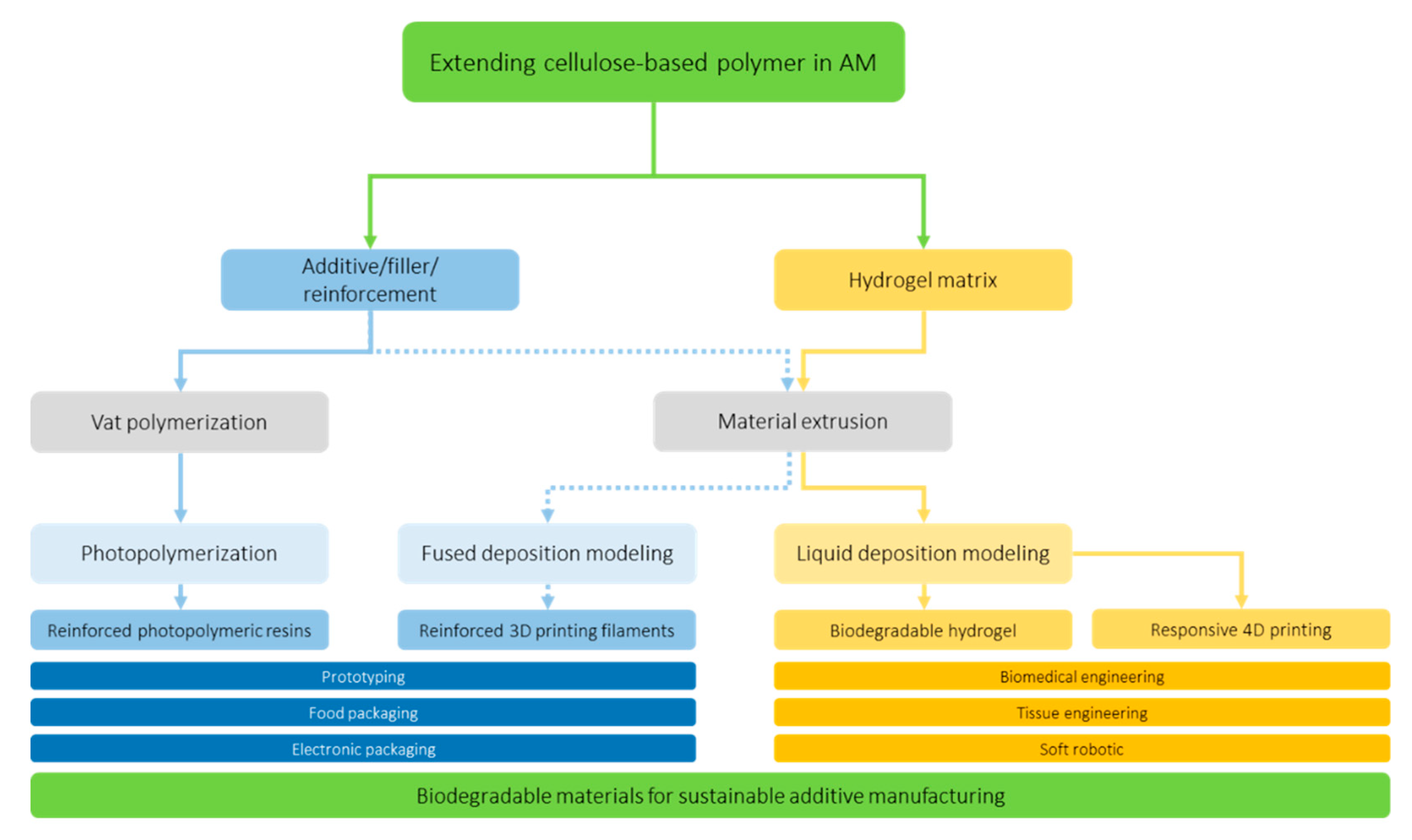
3. Cellulose-Based Polymers in 3D Printing Technology
3.1. Cellulosic Biopolymer
3.2. Fused Deposition Modeling Filament
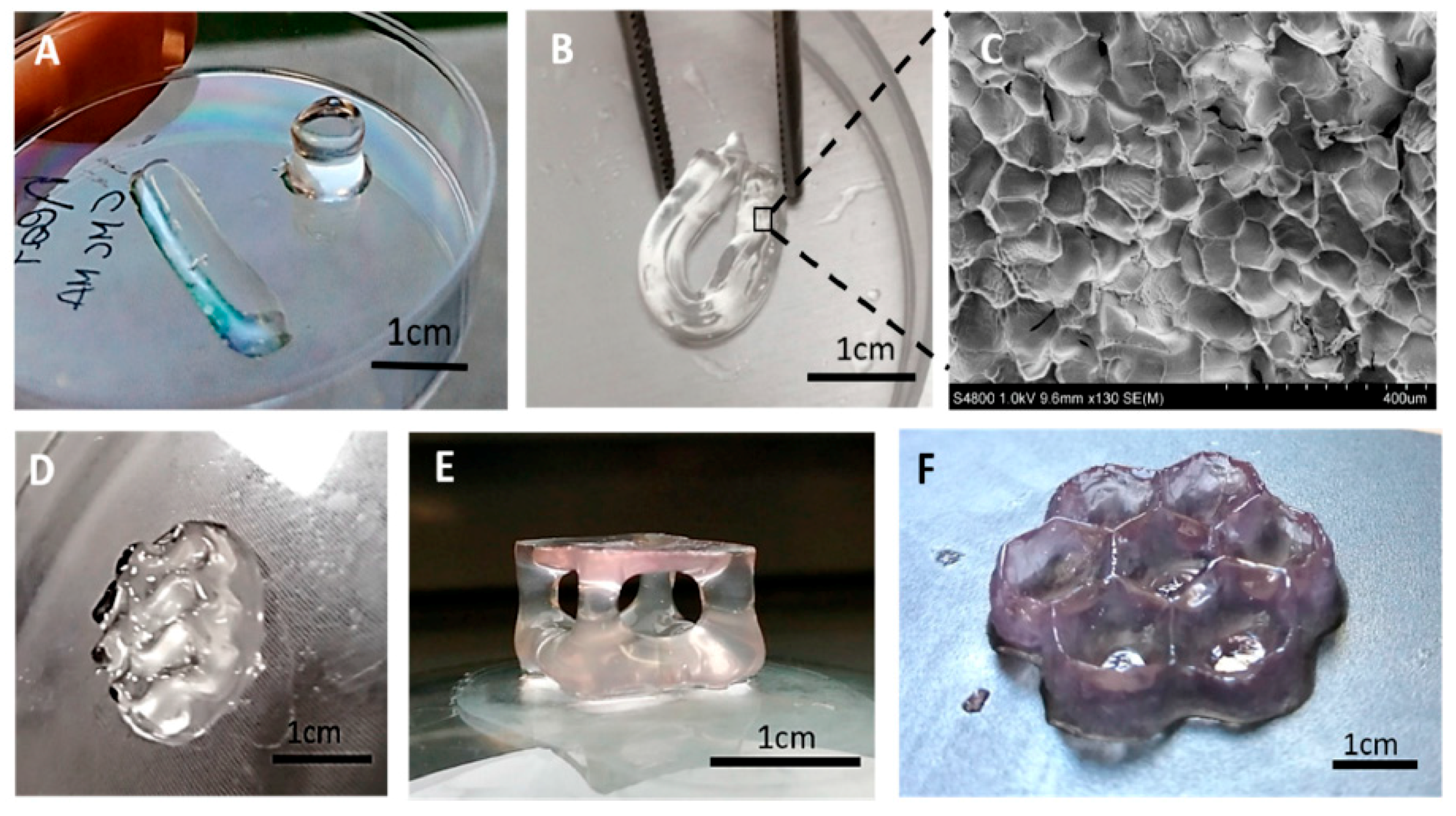
3.3. Vat Photopolymerization
3.4. Liquid Deposition Modeling
3.5. Applications
3.5.1. Biomedical Engineering
3.5.2. Electronic Engineering Applications
3.5.3. Other Applications
4. Cellulose-Based Polymers in 4D Printing Technology
4.1. Cellulose-Based Responsive Materials
4.1.1. Heat Responsive
4.1.2. Moisture Responsive
4.1.3. Light Responsive
4.1.4. Magnetic Responsive
4.1.5. Electrical Responsive
4.1.6. PH Responsive
4.2. 4D Printing of Cellulose-Based Responsive Materials
5. Summary, Conclusions, and Future Trends
Author Contributions
Funding
Conflicts of Interest
References
- Wadley, D. The City of Grace: An Urban Manifesto; Springer Nature Singapore Pte Ltd.: Gateway East, Singapore, 2020; pp. 1–12. [Google Scholar]
- Corolleur, F.; Matt, M.; Perez, S. Innovation potentials triggered by glycoscience research. Carbohydr. Polym. 2020, 233, 115833. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.H.; Qi, P.; Rostagno, M.; Feteha, A.; Miller, S.A. The quest for high glass transition temperature bioplastics. J. Mater. Chem. A 2018, 6, 9298–9331. [Google Scholar] [CrossRef]
- Neufeld, L.; Stassen, F.; Sheppard, R.; Gilman, T. The New Plastics Economy: Rethinking the Future of Plastics; World Economic Forum: Cologne, Switzerland, 2016; Volume 7, pp. 1–36. [Google Scholar]
- Iacovidou, E.; Velenturf, A.P.M.; Purnell, P. Quality of resources: A typology for supporting transitions towards resource efficiency using the single-use plastic bottle as an example. Sci. Total Environ. 2019, 647, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Dijkstra, P.; Loos, K. The recent developments in biobased polymers toward general and engineering applications: Polymers that are upgraded from biodegradable polymers, analogous to petroleum-derived polymers, and newly developed. Polymers 2017, 9, 523. [Google Scholar] [CrossRef]
- O’Dea, R.M.; Willie, J.A.; Epps III, T.H. 100th Anniversary of Macromolecular Science Viewpoint: Polymers from Lignocellulosic Biomass. Current Challenges and Future Opportunities. ACS Macro. Lett. 2020, 9, 476–493. [Google Scholar] [CrossRef]
- Lucia, L.A. Lignocellulosic biomass: A potential feedstock to replace petroleum. BioResources 2008, 3, 981–982. [Google Scholar]
- Pasangulapati, V.; Ramachandriya, K.D.; Kumar, A.; Wilkins, M.R.; Jones, C.L.; Huhnke, R.L. Effects of cellulose, hemicellulose and lignin on thermochemical conversion characteristics of the selected biomass. Bioresour. Technol. 2012, 114, 663–669. [Google Scholar] [CrossRef]
- Ibrahim, F.; Mohan, D.; Sajab, M.S.; Bakarudin, S.B.; Kaco, H. Evaluation of the Compatibility of Organosolv Lignin-Graphene Nanoplatelets with Photo-Curable Polyurethane in Stereolithography 3D Printing. Polymers 2019, 11, 1544. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, Y.C.; Hu, H.Q.; Xie, F.J.; Wei, X.Y.; Fan, X. Structural characterization of lignin and its degradation products with spectroscopic methods. J. Spectrosc. 2017, 2017, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Awalludin, M.F.; Sulaiman, O.; Hashim, R.; Nadhari, W.N.A.W. An overview of the oil palm industry in Malaysia and its waste utilization through thermochemical conversion, specifically via liquefaction. Renew. Sustain. Energy Rev. 2015, 50, 1469–1484. [Google Scholar] [CrossRef]
- Charreau, H.; Cavallo, E.; Foresti, M.L. Patents involving nanocellulose: Analysis of their evolution since 2010. Carbohydr. Polym. 2020, 237, 116039. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Kharkar, P.S.; Pethe, A.M. Biomass and waste materials as potential sources of nanocrystalline cellulose: Comparative review of preparation methods (2016 – Till date). Carbohydr. Polym. 2019, 207, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Salimi, S.; Sotudeh-gharebagh, R.; Zarghami, R.; Chan, S.Y.; Yuen, K.H. Production of Nanocellulose and Its Applications in Drug Delivery: A Critical Review. ACS Sustain. Chem. Eng. 2019, 7, 15800–15827. [Google Scholar] [CrossRef]
- Mohan, D.; Sajab, M.S.; Kaco, H.; Bakarudin, S.B.; Mohamed Noor, A. 3D Printing of UV-Curable Polyurethane Incorporated with Surface-Grafted Nanocellulose. Nanomaterials 2019, 9, 1726. [Google Scholar] [CrossRef] [Green Version]
- Parandoush, P.; Lin, D. A review on additive manufacturing of polymer-fiber composites. Compos. Struct. 2017, 182, 36–53. [Google Scholar] [CrossRef]
- Mazzanti, V.; Malagutti, L.; Mollica, F. FDM 3D printing of polymers containing natural fillers: A review of their mechanical properties. Polymers 2019, 11, 1094. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Mathur, T.; Pandian, N.K.R.; Selahi, A. Organ-on-a-chip and 3D printing as preclinical models for medical research and practice. In Precision Medicine for Investigators, Practitioners and Providers; Elsevier Ltd.: Duxford, UK, 2020; Chapter 9; pp. 83–95. [Google Scholar]
- Durfee, K.; Iaizzo, P.A. Medical Applications of 3D Printing. In Engineering in Medicine; Elsevier Ltd.: Duxford, UK, 2019; Chapter 21; pp. 495–509. [Google Scholar]
- Visser, C.W.; Pohl, R.; Sun, C.; Romer, G.W.; Huis in’t Veld, B.; Lohse, D. Toward 3D Printing of Pure Metals by Laser-Induced Forward Transfer. Adv. Mater. 2015, 27, 4087–4092. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Li, Z.; Li, J.; Liu, C.; Lao, C.; Fu, Y.; Liu, C.; Li, Y.; Wang, P.; He, Y. 3D printing of ceramics: A review. J. Eur. Ceram. Soc. 2019, 39, 661–687. [Google Scholar] [CrossRef]
- Zhang, D.; Jonhson, W.; Herng, T.S.; Ang, Y.Q.; Yang, L.; Tan, S.C.; Peng, E.; He, H.; Ding, J. A 3D-printing method of fabrication for metals, ceramics, and multi-materials using a universal self-curable technique for robocasting. Mater. Horizons 2020, 7, 1083–1090. [Google Scholar] [CrossRef]
- Potter, P.M.; Al-Abed, S.R.; Lay, D.; Lomnicki, S.M. VOC Emissions and Formation Mechanisms from Carbon Nanotube Composites During 3D Printing. Environ. Sci. Technol. 2019, 53, 4364–4370. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.; Jiang, L.; Wang, Y.; Gang, F.; Xu, N.; Li, T.; Liu, Z.; Chi, Y.; Wang, X.; Zhao, L.; et al. 3D Printing of Conductive Tissue Engineering Scaffolds Containing Polypyrrole Nanoparticles with Different Morphologies and Concentrations. Materials 2019, 12, 2491. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Tao, O.; Kort-Mascort, J.; Lin, Y.; Pham, H.M.; Charbonneau, A.M.; ElKashty, O.A.; Kinsella, J.M.; Tran, S.D. The Applications of 3D Printing for Craniofacial Tissue Engineering. Micromachines 2019, 10, 480. [Google Scholar] [CrossRef] [Green Version]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Maire, M.; Lerouge, S.; Therriault, D.; Heuzey, M.-C. 3D Printing of Microstructured and Stretchable Chitosan Hydrogel for Guided Cell Growth. Adv. Biosyst. 2017, 1, 1700058. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. C 2017, 83, 195–201. [Google Scholar] [CrossRef]
- Shahrubudin, N.; Lee, T.; Ramlan, R. An Overview on 3D Printing Technology: Technological, Materials, and Applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Redwood, B. Additive Manufacturing Technologies: An Overview. Available online: https://www.3dhubs.com/knowledge-base/additive-manufacturing-technologies-overview/ (accessed on 10 August 2020).
- Harikrishnan, U.; Soundarapandian, S. Fused Deposition Modelling based Printing of Full Complement Bearings. Procedia Manuf. 2018, 26, 818–825. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.W.; Stucker, B. Vat Photopolymerization Processes. In Additive Manufacturing Technologies; Springer: New York, NY, USA, 2010; Chapter 11; pp. 299–332. [Google Scholar]
- Kunchala, P.; Kappagantula, K. 3D printing high density ceramics using binder jetting with nanoparticle densifiers. Mater. Des. 2018, 155, 443–450. [Google Scholar] [CrossRef]
- Hossain, M.S.; Gonzalez, J.A.; Hernandez, R.M.; Shuvo, M.A.I.; Mireles, J.; Choudhuri, A.; Lin, Y.; Wicker, R.B. Fabrication of smart parts using powder bed fusion additive manufacturing technology. Addit. Manuf. 2016, 10, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Yap, Y.L.; Wang, C.; Sing, S.L.; Dikshit, V.; Yeong, W.Y.; Wei, J. Material jetting additive manufacturing: An experimental study using designed metrological benchmarks. Precis. Eng. 2017, 50, 275–285. [Google Scholar] [CrossRef]
- Rahman, N.U.; Capuano, L.; Cabeza, S.; Feinaeugle, M.; Garcia-Junceda, A.; De Rooij, M.; Matthews, D.; Walmag, G.; Gibson, I.; Römer, G. Directed energy deposition and characterization of high-carbon high speed steels. Addit. Manuf. 2019, 30, 100838. [Google Scholar] [CrossRef]
- Diegel, O.; Nordin, A.; Motte, D. A Practical Guide to Design for Additive Manufacturing; Springer Nature Singapore Pte Ltd.: Gateway East, Singapore, 2020; Chapter 2; pp. 19–39. [Google Scholar]
- Dizon, J.R.C.; Espera, A.H.; Chen, Q.; Advincula, R.C. Mechanical characterization of 3D-printed polymers. Addit. Manuf. 2018, 20, 44–67. [Google Scholar] [CrossRef]
- Wohlers, T.T. 3D Printing and Additive Manufacturing State of the Industry; Wohlers Report; WOHLERS Associates: Fort Collins, CO, USA, 2017. [Google Scholar]
- Dong, M.; Zhang, S.; Gao, D.; Chou, B. The study on polypropylene applied in fused deposition modeling. In Proceedings of the AIP Conference Proceedings, Taipei, Taiwan, 24 May 2018. [Google Scholar]
- Drzyzga, O.; Prieto, A. Plastic waste management, a matter for the ‘community’. Microb. Biotechnol. 2019, 12, 66–68. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.M.; Moxon, S.; Morris, G.A. Biopolymers as wound healing materials. In Wound Healing Biomaterials; Woodhead Publishing Ltd.: Cambridge, UK, 2016; Volume 2, Chapter 13; pp. 261–287. [Google Scholar]
- Laycock, B.; Nikolić, M.; Colwell, J.M.; Gauthier, E.; Halley, P.; Bottle, S.; George, G. Lifetime prediction of biodegradable polymers. Prog. Polym. Sci. 2017, 71, 144–189. [Google Scholar] [CrossRef] [Green Version]
- Glaser, J.A. Biological degradation of polymers in the environment. In Plastics in the Environment; Gomiero, A., Ed.; Intechopen: London, UK, 2019; Chapter 5; p. 675. [Google Scholar]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef]
- Zhao, C.Q.; Liu, W.G.; Xu, Z.Y.; Li, J.G.; Huang, T.T.; Lu, Y.J.; Huang, H.G.; Lin, J.X. Chitosan ducts fabricated by extrusion-based 3D printing for soft-tissue engineering. Carbohydr. Polym. 2020, 236, 116058. [Google Scholar] [CrossRef]
- Wei, L.; Wu, S.; Kuss, M.; Jiang, X.; Sun, R.; Reid, P.; Qin, X.; Duan, B. 3D printing of silk fibroin-based hybrid scaffold treated with platelet rich plasma for bone tissue engineering. Bioact. Mater. 2019, 4, 256–260. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.; Liu, S. Rapid prototyping of continuous carbon fiber reinforced polylactic acid composites by 3D printing. J. Mater. Process. Technol. 2016, 238, 218–225. [Google Scholar] [CrossRef]
- Heidari-Rarani, M.; Rafiee-Afarani, M.; Zahedi, A.M. Mechanical characterization of FDM 3D printing of continuous carbon fiber reinforced PLA composites. Compos. Part B Eng. 2019, 175, 107147. [Google Scholar] [CrossRef]
- Prashantha, K.; Roger, F. Multifunctional properties of 3D printed poly(lactic acid)/graphene nanocomposites by fused deposition modeling. J. Macromol. Sci. A 2017, 54, 24–29. [Google Scholar] [CrossRef]
- Wu, C.S.; Tsou, C.H. Fabrication, characterization, and application of biocomposites from poly(lactic acid) with renewable rice husk as reinforcement. J. Polym. Res. 2019, 26, 44. [Google Scholar] [CrossRef]
- Liu, Z.; Lei, Q.; Xing, S. Mechanical characteristics of wood, ceramic, metal and carbon fiber-based PLA composites fabricated by FDM. J. Mater. Res. Technol. 2019, 8, 3741–3751. [Google Scholar] [CrossRef]
- Zhao, X.; Tekinalp, H.; Meng, X.; Ker, D.; Benson, B.; Pu, Y.; Ragauskas, A.J.; Wang, Y.; Li, K.; Webb, E.; et al. Poplar as Biofiber Reinforcement in Composites for Large-Scale 3D Printing. ACS Appl. Bio Mater. 2019, 2, 4557–4570. [Google Scholar] [CrossRef]
- Varga, P.; Lorinczy, D.; Toth, L.; Pentek, A.; Nyitrai, M.; Maroti, P. Novel PLA-CaCO3 composites in additive manufacturing of upper limb casts and orthotics-a feasibility study. Mater. Res. Express 2019, 6. [Google Scholar] [CrossRef]
- Tanase-Opedal, M.; Espinosa, E.; Rodríguez, A.; Chinga-Carrasco, G. Lignin: A biopolymer from forestry biomass for biocomposites and 3D printing. Materials 2019, 12, 3006. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Milentis, J.; Pramanik, A. Additive manufacturing of mechanical testing samples based on virgin poly (lactic acid) (PLA) and PLA/wood fibre composites. Adv. Manuf. 2018, 6, 71–82. [Google Scholar] [CrossRef]
- Daver, F.; Lee, K.P.M.; Brandt, M.; Shanks, R. Cork–PLA composite filaments for fused deposition modelling. Compos. Sci. Technol. 2018, 168, 230–237. [Google Scholar] [CrossRef]
- Kavitha, A.A.; Paul, K.T.; Anilkumar, P. Cellulose-derived materials for drug delivery applications. In Sustainable Nanocellulose and Nanohydrogels from Natural Sources; Elsevier Ltd.: Duxford, UK, 2020; Chapter 18; pp. 367–390. [Google Scholar]
- Tosh, B. Synthesis and Sustainable Applications of Cellulose Esters and Ethers : A Review. Int. J. Energy, Sustain. Environ. Eng. 2014, 1, 2. [Google Scholar]
- Ioelovich, M.; Leykin, A.; Haemek, M. Formation Nano-Structure of Microcrystalline Cellulose. Cell. Chem. Technol. 2006, 40, 313–317. [Google Scholar]
- Hua, Y.L.; Harun, S.; Sajab, M.S.; Jahim, J.M.; Shah, S.S.M. Extraction of Cellulose and Microcrystalline Cellulose from Kenaf. Jurnal Kejuruteraan 2020, 32, 205–213. [Google Scholar]
- Xian, X.; Wang, X.; Zhu, Y.; Guo, Y.; Tian, Y. Effects of MCC Content on the Structure and Performance of PLA/MCC Biocomposites. J. Polym. Environ. 2018, 26, 3484–3492. [Google Scholar] [CrossRef]
- Long, W.J.; Tao, J.L.; Lin, C.; Gu, Y.C.; Mei, L.; Duan, H.B.; Xing, F. Rheology and buildability of sustainable cement-based composites containing micro-crystalline cellulose for 3D-printing. J. Clean. Prod. 2019, 239, 118054. [Google Scholar] [CrossRef]
- Nsor-Atindana, J.; Chen, M.; Goff, H.D.; Zhong, F.; Sharif, H.R.; Li, Y. Functionality and nutritional aspects of microcrystalline cellulose in food. Carbohydr. Polym. 2017, 172, 159–174. [Google Scholar] [CrossRef]
- Saputra, A.H.; Qadhayna, L.; Pitaloka, A.B. Synthesis and Characterization of Carboxymethyl Cellulose (CMC) from Water Hyacinth Using Ethanol-Isobutyl Alcohol Mixture as the Solvents. Int. J. Chem. Eng. Appl. 2014, 5, 36. [Google Scholar] [CrossRef] [Green Version]
- Abdulkhani, A.; Najd, A.; Sahab, M.; Yahya, H. Preparation of xylan bio - composite films reinforced with oxidized carboxymethyl cellulose and nanocellulose. Polym. Bull. 2019, 1–13. [Google Scholar] [CrossRef]
- Dürig, T.; Karan, K. Binders in Wet Granulation. In Handbook of Pharmaceutical Wet Granulation; Academic Press: Cambridge, MA, USA, 2019; Chapter 9; pp. 317–349. [Google Scholar]
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Natural and Synthetic Biomedical Polymers; Elsevier Inc.: Amsterdam, The Netherlands, 2014; Chapter 4; pp. 67–89. [Google Scholar]
- Chen, L.; Wang, Q.; Hirth, K.; Baez, C.; Agarwal, U.P.; Zhu, J.Y. Tailoring the yield and characteristics of wood cellulose nanocrystals (CNC) using concentrated acid hydrolysis. Cellulose 2015, 22, 1753–1762. [Google Scholar] [CrossRef]
- Xie, H.; Du, H.; Yang, X.; Si, C. Recent Strategies in Preparation of Cellulose Nanocrystals and Cellulose Nanofibrils Derived from Raw Cellulose Materials. Int. J. Polym. Sci. 2018, 2018, 1–25. [Google Scholar] [CrossRef]
- Shankaran, D.R. Cellulose Nanocrystals for Healthcare Applications. In Application of Nanomaterials; Elsevier Ltd.: Duxford, UK, 2018; Chapter 14; pp. 415–459. [Google Scholar]
- Khanjanzadeh, H.; Behrooz, R.; Bahramifar, N.; Pinkl, S.; Gindl-Altmutter, W. Application of surface chemical functionalized cellulose nanocrystals to improve the performance of UF adhesives used in wood based composites—MDF type. Carbohydr. Polym. 2019, 206, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sajab, M.S.; Mohan, D.; Santanaraj, J.; Chia, C.H.; Kaco, H.; Harun, S.; Kamarudin, N.H.N. Telescopic synthesis of cellulose nanofibrils with a stable dispersion of Fe(0) nanoparticles for synergistic removal of 5-fluorouracil. Sci. Rep. 2019, 9, 11703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, C.H.; Chia, C.H.; Zakaria, S.; Sajab, M.S.; Chin, S.X. Cellulose nanofibrils: A rapid adsorbent for the removal of methylene blue. RSC Adv. 2015, 5, 18204–18212. [Google Scholar] [CrossRef]
- Mariani, L.M.; Johnson, W.R.; Considine, J.M.; Turner, K.T. Printing and mechanical characterization of cellulose nanofibril materials. Cellulose 2019, 26, 2639–2651. [Google Scholar] [CrossRef]
- Wang, X.; Chang, C.H.; Jiang, J.; Liu, Q.; Liao, Y.P.; Lu, J.; Li, L.; Liu, X.; Kim, J.; Ahmed, A.; et al. The Crystallinity and Aspect Ratio of Cellulose Nanomaterials Determine Their Pro-Inflammatory and Immune Adjuvant Effects in Vitro and In Vivo. Small 2019, 15, 1901642. [Google Scholar] [CrossRef]
- Abudula, T.; Saeed, U.; Memic, A.; Gauthaman, K.; Hussain, M.A.; Al-Turaif, H. Electrospun cellulose Nano fibril reinforced PLA/PBS composite scaffold for vascular tissue engineering. J. Polym. Res. 2019, 26, 110. [Google Scholar] [CrossRef]
- Costa, A.F.S.; Almeida, F.C.G.; Vinhas, G.M.; Sarubbo, L.A. Production of bacterial cellulose by Gluconacetobacter hansenii using corn steep liquor as nutrient sources. Front. Microbiol. 2017, 8, 2027. [Google Scholar] [CrossRef]
- Eslahi, N.; Mahmoodi, A.; Mahmoudi, N.; Zandi, N.; Simchi, A. Processing and Properties of Nanofibrous Bacterial Cellulose-Containing Polymer Composites: A Review of Recent Advances for Biomedical Applications. Polym. Rev. 2020, 60, 144–170. [Google Scholar] [CrossRef]
- Ramírez, J.A.Á.; Bovi, J.; Bernal, C.; Errea, M.I.; Foresti, M.L. Development of Poly(lactic acid) Nanocomposites Reinforced with Hydrophobized Bacterial Cellulose. J. Polym. Environ. 2020, 28, 61–73. [Google Scholar] [CrossRef]
- Kaya, M.; Demir, A.; Akçay, H.T. A Novel Highly Porous Cellulosic Aerogel Regenerated by Solvent Exchange Mechanism. J. Polym. Environ. 2019, 27, 1801–1806. [Google Scholar] [CrossRef]
- Felgueiras, H.P.; Teixeira, M.A.; Tavares, T.D.; Amorim, M.T.P. New method to produce poly(vinyl alcohol)/cellulose acetate films with improved antibacterial action. Mater. Today Proc. 2020. [Google Scholar] [CrossRef] [Green Version]
- Murphy, C.A.; Collins, M.N. Microcrystalline cellulose reinforced polylactic acid biocomposite filaments for 3D printing. Polym. Compos. 2018, 39, 1311–1320. [Google Scholar] [CrossRef]
- Wang, Q.; Ji, C.; Sun, J.; Yao, Q.; Liu, J.; Saeed, R.M.Y.; Zhu, Q. Kinetic thermal behavior of nanocellulose filled polylactic acid filament for fused filament fabrication 3D printing. J. Appl. Polym. Sci. 2019, 137, 48374. [Google Scholar] [CrossRef]
- Dong, J.; Li, M.; Zhou, L.; Lee, S.; Mei, C.; Xu, X.; Wu, Q. The influence of grafted cellulose nanofibers and postextrusion annealing treatment on selected properties of poly(lactic acid) filaments for 3D printing. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 847–855. [Google Scholar] [CrossRef]
- Frone, A.N.; Batalu, D.; Chiulan, I.; Oprea, M.; Gabor, A.R.; Nicolae, C.-A.; Raditoiu, V.; Trusca, R.; Panaitescu, D.M. Morpho-Structural, Thermal and Mechanical Properties of PLA/PHB/Cellulose Biodegradable Nanocomposites Obtained by Compression Molding, Extrusion, and 3D Printing. Nanomaterials 2020, 10, 51. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Chen, Y.; Yu, T.; Wang, N.; Wang, C.; Wang, H. Preparation of polylactic acid/TEMPO-oxidized bacterial cellulose nanocomposites for 3D printing via Pickering emulsion approach. Compos. Commun. 2019, 16, 162–167. [Google Scholar] [CrossRef]
- Jiang, G.; Yang, T.; Xu, J.; Tao, D.; Luo, C.; Wang, C.; Dong, Q.; Wang, Y. Investigation into hydroxypropyl-methylcellulose-reinforced polylactide composites for fused deposition modelling. Ind. Crops Prod. 2020, 146, 112174. [Google Scholar] [CrossRef]
- Paggi, R.A.; Salmoria, G.V.; Ghizoni, G.B.; de Medeiros Back, H.; de Mello Gindri, I. Structure and mechanical properties of 3D-printed cellulose tablets by fused deposition modeling. Int. J. Adv. Manuf. Technol. 2019, 100, 2767–2774. [Google Scholar] [CrossRef]
- Dong, J.; Mei, C.; Han, J.; Lee, S.; Wu, Q. 3D printed poly(lactic acid) composites with grafted cellulose nanofibers: Effect of nanofiber and post-fabrication annealing treatment on composite flexural properties. Addit. Manuf. 2019, 28, 621–628. [Google Scholar] [CrossRef]
- Feng, X.; Yang, Z.; Rostom, S.S.H.; Dadmun, M.; Wang, S.; Wang, Q.; Xie, Y. Reinforcing 3D printed acrylonitrile butadiene styrene by impregnation of methacrylate resin and cellulose nanocrystal mixture: Structural effects and homogeneous properties. Mater. Des. 2018, 138, 62–70. [Google Scholar] [CrossRef]
- Cisneros-López, E.O.; Pal, A.K.; Rodriguez, A.U.; Wu, F.; Misra, M.; Mielewski, D.F.; Kiziltas, A.; Mohanty, A.K. Recycled poly(lactic acid)-based 3D printed sustainable biocomposites: A comparative study with injection molding. Mater. Today Sustain. 2020, 8, 1–12. [Google Scholar] [CrossRef]
- Coppola, B.; Garofalo, E.; Di Maio, L.; Scarfato, P.; Incarnato, L. Investigation on the use of PLA/hemp composites for the fused deposition modelling (FDM) 3D printing. In Proceedings of the AIP Conference Proceedings, Bali, Indonesia, 11 July 2018; Volume 1981, p. 020086. [Google Scholar]
- Huang, B.; He, H.; Meng, S.; Jia, Y. Optimizing 3D printing performance of acrylonitrile-butadiene-styrene composites with cellulose nanocrystals/silica nanohybrids. Polym. Int. 2019, 68, 1351–1360. [Google Scholar] [CrossRef]
- Alemán-Domínguez, M.E.; Giusto, E.; Ortega, Z.; Tamaddon, M.; Benítez, A.N.; Liu, C. Three-dimensional printed polycaprolactone-microcrystalline cellulose scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 521–528. [Google Scholar] [CrossRef]
- Zander, N.E.; Park, J.H.; Boelter, Z.R.; Gillan, M.A. Recycled Cellulose Polypropylene Composite Feedstocks for Material Extrusion Additive Manufacturing. ACS Omega 2019, 4, 13879–13888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, M.N.; Wahid, M.K.; Maidin, N.A.; Ab Rahman, M.H.; Osman, M.H.; Alis, I.F. Mechanical characteristics of oil palm fiber reinforced thermoplastics as filament for fused deposition modeling (FDM). Adv. Manuf. 2020, 8, 72–81. [Google Scholar] [CrossRef]
- Cataldi, A.; Rigotti, D.; Nguyen, V.D.H.; Pegoretti, A. Polyvinyl alcohol reinforced with crystalline nanocellulose for 3D printing application. Mater. Today Commun. 2018, 15, 236–244. [Google Scholar] [CrossRef]
- Feng, X.; Yang, Z.; Rostom, S.S.H.; Dadmun, M.; Xie, Y.; Wang, S. Structural, mechanical, and thermal properties of 3D printed L-CNC / acrylonitrile butadiene styrene nanocomposites. J. Appl. Polym. Sci. 2017, 134, 45082. [Google Scholar] [CrossRef]
- Tekinalp, H.L.; Meng, X.; Lu, Y.; Kunc, V.; Love, L.J.; Peter, W.H.; Ozcan, S. High modulus biocomposites via additive manufacturing: Cellulose nanofibril networks as “microsponges”. Compos. Part. B Eng. 2019, 173, 106817. [Google Scholar] [CrossRef]
- Xiao, X.; Chevali, V.S.; Song, P.; He, D.; Wang, H. Polylactide/hemp hurd biocomposites as sustainable 3D printing feedstock. Compos. Sci. Technol. 2019, 184, 107887. [Google Scholar] [CrossRef]
- Tran, T.N.; Bayer, I.S.; Heredia-Guerrero, J.A.; Frugone, M.; Lagomarsino, M.; Maggio, F.; Athanassiou, A. Cocoa shell waste biofilaments for 3D printing applications. Macromol. Mater. Eng. 2017, 302, 1700219. [Google Scholar] [CrossRef]
- Palaganas, N.B.; Mangadlao, J.D.; de Leon, A.C.C.; Palaganas, J.O.; Pangilinan, K.D.; Lee, Y.J.; Advincula, R.C. 3D Printing of Photocurable Cellulose Nanocrystal Composite for Fabrication of Complex Architectures via Stereolithography. ACS Appl. Mater. Interf. 2017, 9, 34314–34324. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Yang, Z.; Chmely, S.; Wang, Q.; Wang, S.; Xie, Y. Lignin-coated cellulose nanocrystal filled methacrylate composites prepared via 3D stereolithography printing: Mechanical reinforcement and thermal stabilization. Carbohydr. Polym. 2017, 169, 272–281. [Google Scholar] [CrossRef]
- Melilli, G.; Carmagnola, I.; Tonda-Turo, C.; Pirri, F.; Ciardelli, G.; Sangermano, M.; Hakkarainen, M.; Chiappone, A. DLP 3D Printing Meets Lignocellulosic Biopolymers: Carboxymethyl Cellulose Inks for 3D Biocompatible Hydrogels. Polymers 2020, 12, 1655. [Google Scholar] [CrossRef]
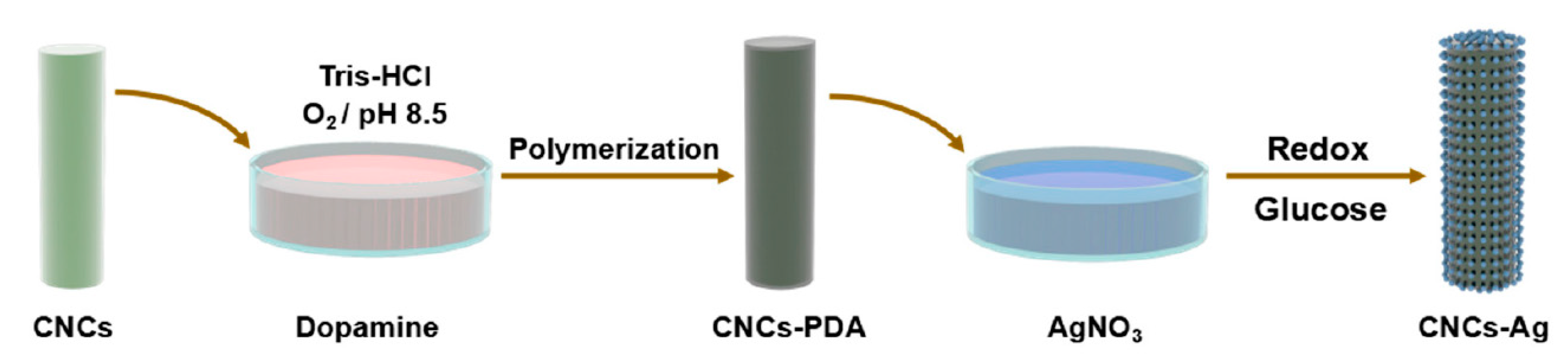
- Chen, S.; Yang, J.; Jia, Y.-G.; Lu, B.; Ren, L. A Study of 3D-Printable Reinforced Composite Resin: PMMA Modified with Silver Nanoparticles Loaded Cellulose Nanocrystal. Materials 2018, 11, 2444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Wu, G.; Wang, S. Dynamic postpolymerization of 3D-printed photopolymer nanocomposites: Effect of cellulose nanocrystal and postcure temperature. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 935–946. [Google Scholar] [CrossRef]
- Lu, C.; Wang, C.; Yu, J.; Wang, J.; Chu, F. Two-Step 3 D-Printing Approach toward Sustainable, Repairable, Fluorescent Shape-Memory Thermosets Derived from Cellulose and Rosin. ChemSusChem 2020, 13, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Li, V.C.F.; Kuang, X.; Mulyadi, A.; Hamel, C.M.; Deng, Y.; Qi, H.J. 3D printed cellulose nanocrystal composites through digital light processing. Cellulose 2019, 26, 3973–3985. [Google Scholar] [CrossRef]
- Gatenholm, P.; Martinez, H.; Karabulut, E.; Amoroso, M.; Kölby, L.; Markstedt, K.; Gatenholm, E.; Henriksson, I. Development of Nanocellulose-Based Bioinks for 3D Bioprinting of Soft Tissue. In 3D Printing and Biofabrication; Springer: Cham, Switzerland, 2018; pp. 331–352. [Google Scholar]
- Markstedt, K.; Sundberg, J.; Gatenholm, P. 3D bioprinting of cellulose structures from an ionic liquid. 3D Print. Addit. Manuf. 2014, 1, 115–121. [Google Scholar] [CrossRef]
- Pattinson, S.W.; Hart, A.J. Additive Manufacturing of Cellulosic Materials with Robust Mechanics and Antimicrobial Functionality. Adv. Mater. Technol. 2017, 2, 1600084. [Google Scholar] [CrossRef]
- Li, V.C.F.; Dunn, C.K.; Zhang, Z.; Deng, Y.; Qi, H.J. Direct Ink Write (DIW) 3D Printed Cellulose Nanocrystal Aerogel Structures. Sci. Rep. 2017, 7, 8018. [Google Scholar] [CrossRef]
- Brandley, A.; Hollfelder, R.; Nesaei, S.; Vanwie, B.; Abu-Lail, N.; Gozen, B.A. Direct-Ink-Writing of Degradable Carboxymethylcellulose. Procedia Manuf. 2018, 26, 993–1002. [Google Scholar] [CrossRef]
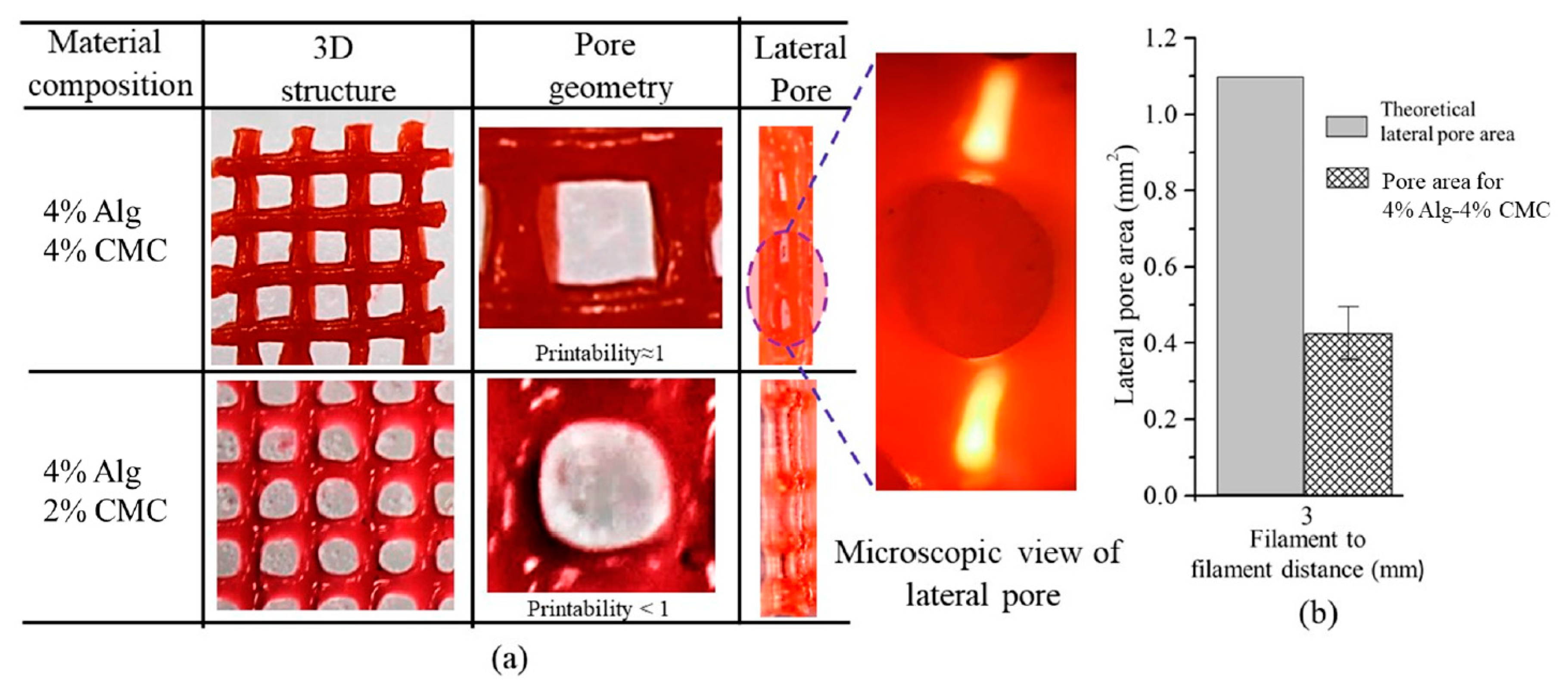
- Habib, A.; Sathish, V.; Mallik, S.; Khoda, B. 3D Printability of Alginate-Carboxymethyl Cellulose Hydrogel. Materials 2018, 11, 454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kam, D.; Chasnitsky, M.; Nowogrodski, C.; Braslavsky, I.; Abitbol, T.; Magdassi, S.; Shoseyov, O. Direct Cryo Writing of Aerogels Via 3D Printing of Aligned Cellulose Nanocrystals Inspired by the Plant Cell Wall. Colloids Interfaces 2019, 3, 46. [Google Scholar] [CrossRef] [Green Version]
- Zuo, M.; Pan, N.; Liu, Q.; Ren, X.; Liu, Y.; Huang, T.S. Three-dimensionally printed polylactic acid/cellulose acetate scaffolds with antimicrobial effect. RSC Adv. 2020, 10, 2952–2958. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, E.; Burdiles, P.A.; Quero, F.; Palma, P.; Olate-Moya, F.; Palza, H. 3D Printing of Antimicrobial Alginate/Bacterial-Cellulose Composite Hydrogels by Incorporating Copper Nanostructures. ACS Biomater. Sci. Eng. 2019, 5, 6290–6299. [Google Scholar] [CrossRef]
- Wei, J.; Wang, B.; Li, Z.; Wu, Z.; Zhang, M.; Sheng, N.; Liang, Q.; Wang, H.; Chen, S. A 3D-Printable TEMPO-Oxidized Bacterial Cellulose/Alginate Hydrogel with Enhanced Stability via Nanoclay Incorporation. Carbohydr. Polym. 2020, 238, 116207. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, J.; Yang, Z.; Liu, D.; Xv, X.; Zhao, G.; Shi, H.; Zhang, Q. Dialdehyde cellulose nanocrystal/gelatin hydrogel optimized for 3D printing applications. J. Mater. Sci. 2018, 53, 11883–11900. [Google Scholar] [CrossRef]
- Heggset, E.B.; Strand, B.L.; Sundby, K.W.; Simon, S.; Chinga-Carrasco, G.; Syverud, K. Viscoelastic properties of nanocellulose based inks for 3D printing and mechanical properties of CNF/alginate biocomposite gels. Cellulose 2019, 26, 581–595. [Google Scholar] [CrossRef]
- Huang, L.; Du, X.; Fan, S.; Yang, G.; Shao, H.; Li, D.; Caod, C.; Zhu, Y.; Zhua, M.; Zhang, Y. Bacterial cellulose nanofibers promote stress and fidelity of 3D-printed silk based hydrogel scaffold with hierarchical pores. Carbohydr. Polym. 2019, 221, 146–156. [Google Scholar] [CrossRef]
- Markstedt, K.; Escalante, A.; Toriz, G.; Gatenholm, P. Biomimetic Inks Based on Cellulose Nanofibrils and Cross-Linkable Xylans for 3D Printing. ACS Appl. Mater. Interfaces 2017, 9, 40878–40886. [Google Scholar] [CrossRef]
- Mohan, T.; Dobaj Štiglic, A.; Beaumont, M.; Konnerth, J.; Gürer, F.; Makuc, D.; Maver, U.; Gradišnik, L.; Plavec, J.; Kargl, R.; et al. Generic Method for Designing Self-Standing and Dual Porous 3D Bioscaffolds from Cellulosic Nanomaterials for Tissue Engineering Applications. ACS Appl. Bio Mater. 2020, 3, 1197–1209. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.Y.; Zhu, H.L.; Wang, Y.B.; Ray, U.; Zhu, S.Z.; Dai, J.Q.; Chen, C.J.; Fu, K.; Jang, S.; Henderson, D.; et al. Cellulose-nanofiber-enabled 3d printing of a carbon-nanotube microfiber network. Small Methods 2017, 1, 1700222. [Google Scholar] [CrossRef]
- Li, V.C.F.; Mulyadi, A.; Dunn, C.K.; Deng, Y.; Qi, H.J. Direct Ink Write 3D Printed Cellulose Nanofiber Aerogel Structures with Highly Deformable, Shape Recoverable, and Functionalizable Properties. ACS Sustain. Chem. Eng. 2018, 6, 2011–2022. [Google Scholar] [CrossRef]
- Li, V.C.F.; Kuang, X.; Hamel, C.M.; Roach, D.; Deng, Y.; Qi, H.J. Cellulose nanocrystals support material for 3D printing complexly shaped structures via multi-materials-multi-methods printing. Addit. Manuf. 2019, 28, 14–22. [Google Scholar] [CrossRef]
- Sultan, S.; Mathew, A.P. 3D printed porous cellulose nanocomposite hydrogel scaffolds. J. Vis. Exp. 2019, 146, 59401. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, T.; Kim, W.S. Conductive Cellulose Composites with Low Percolation Threshold for 3D Printed Electronics. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Siqueira, G.; Kokkinis, D.; Libanori, R.; Hausmann, M.K.; Gladman, A.S.; Neels, A.; Tingaut, P.; Zimmermann, T.; Lewis, J.A.; Studart, A.R. Cellulose Nanocrystal Inks for 3D Printing of Textured Cellular Architectures. Adv. Funct. Mater. 2017, 27, 1604619. [Google Scholar] [CrossRef]
- Li, L.; Zhu, Y.; Yang, J. 3D bioprinting of cellulose with controlled porous structures from NMMO. Mater. Lett. 2018, 210, 136–138. [Google Scholar] [CrossRef]
- Thibaut, C.; Denneulin, A.; Rolland du Roscoat, S.; Beneventi, D.; Orgéas, L.; Chaussy, D. A fibrous cellulose paste formulation to manufacture structural parts using 3D printing by extrusion. Carbohydr. Polym. 2019, 212, 119–128. [Google Scholar] [CrossRef]
- Kuzmenko, V.; Karabulut, E.; Pernevik, E.; Enoksson, P.; Gatenholm, P. Tailor-made conductive inks from cellulose nanofibrils for 3D printing of neural guidelines. Carbohydr. Polym. 2018, 189, 22–30. [Google Scholar] [CrossRef]
- Mohan, D.; Khairullah, N.F.; How, Y.P.; Sajab, M.S.; Kaco, H. 3D Printed Laminated CaCO3-Nanocellulose Films as Controlled-Release 5-Fluorouracil. Polymers 2020, 12, 986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borujeni, S.H.; Mirdamadian, S.Z.; Varshosaz, J.; Taheri, A. Three-dimensional (3D) printed tablets using ethyl cellulose and hydroxypropyl cellulose to achieve zero order sustained release profile. Cellulose 2020, 27, 1573–1589. [Google Scholar] [CrossRef]
- Chen, R.-D.; Huang, C.-F.; Hsu, S.-H. Composites of waterborne polyurethane and cellulose nanofibers for 3D printing and bioapplications. Carbohydr. Polym. 2019, 212, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Altun, E.; Ekren, N.; Kuruca, S.E.; Gunduz, O. Cell studies on electrohydrodynamic (ehd)-3d-bioprinted bacterial cellulose\polycaprolactone scaffolds for tissue engineering. Mater. Lett. 2019, 234, 163–167. [Google Scholar] [CrossRef]
- Espinosa, E.; Filgueira, D.; Rodríguez, A.; Chinga-Carrasco, G. Nanocellulose-Based Inks—Effect of Alginate Content on the Water Absorption of 3D Printed Constructs. Bioengineering 2019, 6, 65. [Google Scholar] [CrossRef] [Green Version]
- Kanjou, M.M.; Abdulhakim, H.; de Olyveira, G.M.; Basmaji, P. 3-D Print Celulose Nanoskin: Future Diabetic Wound Healing. J. Biomater. Nanobiotechnol. 2019, 10, 190–195. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Dean, D. 3-D printed porous cellulose acetate tissue scaffolds for additive manufacturing. Addit. Manuf. 2020, 31, 100927. [Google Scholar] [CrossRef]
- Meng, X.; Yang, J.; Liu, Z.; Lu, W.; Sun, Y.; Dai, Y. Non-contact, fibrous cellulose acetate/aluminum flexible electronic-sensor for humidity detecting. Compos. Commun. 2020, 20, 100347. [Google Scholar] [CrossRef]
- Cao, D.; Cing, Y.; Tantratian, K.; Wang, X.; Ma, Y.; Mukhopadhyay, A.; Cheng, Z.; Zhang, Q.; Jiao, Y.; Chen, L.; et al. 3D printed high-performance lithium metal microbatteries enabled by nanocellulose. Adv. Mater. 2019, 31, 1807313. [Google Scholar] [CrossRef]
- Françon, H.; Wang, Z.; Marais, A.; Mystek, K.; Piper, A.; Granberg, H.; Malti, A.; Gatenholm, P.; Larsson, P.A.; Wågberg, L. Ambient-Dried, 3D-Printable and Electrically Conducting Cellulose Nanofiber Aerogels by Inclusion of Functional Polymers. Adv. Funct. Mater. 2020, 30, 1909383. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Bao, C.; Hausmann, M.; Siqueira, G.; Zimmermann, T.; Kim, W.S. 3D printed disposable wireless ion sensors with biocompatible cellulose composites. Adv. Electron. Mater. 2019, 5, 1800778. [Google Scholar] [CrossRef]
- Lille, M.; Nurmela, A.; Nordlund, E.; Metsä-Kortelainen, S.; Sozer, N. Applicability of protein and fiber-rich food materials in extrusion-based 3D printing. J. Food Eng. 2017, 220, 20–27. [Google Scholar] [CrossRef]
- Holland, S.; Foster, T.; MacNaughtan, W.; Tuck, C. Design and characterisation of food grade powders and inks for microstructure control using 3D printing. J. Food Eng. 2018, 220, 12–19. [Google Scholar] [CrossRef]
- Tenhunen, T.M.; Moslemian, O.; Kammiovirta, K.; Harlin, A.; Kääriäinen, P.; Österberg, M.; Tammelin, T.; Orelma, H. Surface tailoring and design-driven prototyping of fabrics with 3D-printing: An all-cellulose approach. Mater. Des. 2018, 140, 409–419. [Google Scholar] [CrossRef] [Green Version]
- Nechyporchuk, O.; Yu, J.; Nierstrasz, V.A.; Bordes, R. Cellulose Nanofibril-Based Coatings of Woven Cotton Fabrics for Improved Inkjet Printing with a Potential in E-Textile Manufacturing. ACS Sustain. Chem. Eng. 2017, 5, 4793–4801. [Google Scholar] [CrossRef]
- Wang, J.; Gardner, D.J.; Stark, N.M.; Bousfield, D.W.; Tajvidi, M.; Cai, Z. Moisture and Oxygen Barrier Properties of Cellulose Nanomaterial-Based Films. ACS Sustain. Chem. Eng. 2018, 6, 49–70. [Google Scholar] [CrossRef]
- Nicharat, A.; Shirole, A.; Foster, E.J.; Weder, C. Thermally activated shape memory behavior of melt-mixed polyurethane/cellulose nanocrystal composites. J. Appl. Polym. Sci. 2017, 134, 45033. [Google Scholar] [CrossRef]
- Cudjoe, E.; Khani, S.; Way, A.E.; Hore, M.J.A.; Maia, J.; Rowan, S.J. Biomimetic Reversible Heat-Stiffening Polymer Nanocomposites. ACS Cent. Sci. 2017, 3, 886–894. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Tian, X.; Ras, R.H.A.; Ikkala, O. Sensitive Humidity-Driven Reversible and Bidirectional Bending of Nanocellulose Thin Films as Bio-Inspired Actuation. Adv. Mater. Interfaces 2015, 2, 1500080. [Google Scholar] [CrossRef]
- Kuang, Y.; Chen, C.; Cheng, J.; Pastel, G.; Li, T.; Song, J.; Jiang, F.; Li, Y.; Zhang, Y.; Jang, S.-H.; et al. Selectively aligned cellulose nanofibers towards high-performance soft actuators. Extrem. Mech. Lett. 2019, 29, 100463. [Google Scholar] [CrossRef]
- Mredha, M.T.I.; Le, H.H.; Tran, V.T.; Trtik, P.; Cui, J.X.; Jeon, I. Anisotropic tough multilayer hydrogels with programmable orientation. Mater. Horiz. 2019, 6, 1504–1511. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Kwon, S.; Lee, W.; Ko, S. Optical response of photonic cellulose nanocrystal film for a novel humidity indicator. Int. J. Biol. Macromol. 2019, 140, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Khiabani, P.S.; Soeriyadi, A.H.; Reece, P.J.; Gooding, J.J. Paper-Based Sensor for Monitoring Sun Exposure. ACS Sens. 2016, 1, 775–780. [Google Scholar] [CrossRef]
- Koga, H.; Nogi, M.; Isogai, A. Ionic Liquid Mediated Dispersion and Support of Functional Molecules on Cellulose Fibers for Stimuli-Responsive Chromic Paper Devices. ACS Appl. Mater. Interfaces 2017, 9, 40914–40920. [Google Scholar] [CrossRef]
- Nau, M.; Seelinger, D.; Biesalski, M. Independent Two Way Switching of the Wetting Behavior of Cellulose-Derived Nanoparticle Surface Coatings by Light and by Temperature. Adv. Mater. Interfaces 2019, 6, 1900378. [Google Scholar] [CrossRef]
- Liu, X.; Li, M.; Zheng, X.; Retulainen, E.; Fu, S. Dual Light- and pH-Responsive Composite of Polyazo-Derivative Grafted Cellulose Nanocrystals. Materials 2018, 11, 1725. [Google Scholar] [CrossRef] [Green Version]
- Ortega, G.; Pérez-Rodríguez, S.; Reguera, E. Magnetic paper–based ELISA for IgM-dengue detection. RSC Adv. 2017, 7, 4921–4932. [Google Scholar] [CrossRef] [Green Version]
- Mo, Z.; Guo, H.; Wang, Y.; Yin, H.; Yang, C. Surface modification of graphene oxide sheets on magnetic particles for magnetic paper. J. Alloys Compd. 2017, 695, 2525–2531. [Google Scholar] [CrossRef]
- Rajala, S.; Siponkoski, T.; Sarlin, E.; Mettänen, M.; Vuoriluoto, M.; Pammo, A.; Juuti, J.; Rojas, O.J.; Franssila, S.; Tuukkanen, S. Cellulose nanofibril film as a piezoelectric sensor material. ACS Appl. Mater. Interfaces 2016, 8, 15607–15614. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.; Zhang, H.; Mi, H.; Cai, Z.; Ma, Z.; Gong, S. High-performance flexible piezoelectric nanogenerators consisting of porous cellulose nanofibril (CNF)/poly(dimethylsiloxane) (PDMS) aerogel films. Nano Energy 2016, 26, 504–512. [Google Scholar] [CrossRef]
- Alam, M.M.; Mandal, D. Native cellulose microfiber-based hybrid piezoelectric generator for mechanical energy harvesting utility. ACS Appl. Mater. Interfaces 2016, 8, 1555–1558. [Google Scholar] [CrossRef]
- Yang, H.; Choi, S.E.; Kim, D.; Park, D.; Lee, D.; Choi, S.; Nam, Y.S.; Kim, J.W. Color-spectrum-broadened ductile cellulose films for vapor-pH -responsive colorimetric sensors. J. Ind. Eng. Chem. 2019, 80, 590–596. [Google Scholar] [CrossRef]
- Gladman, A.S.; Matsumoto, E.A.; Nuzzo, R.G.; Mahadevan, L.; Lewis, J.A. Biomimetic 4D printing. Nat. Mater. 2016, 15, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Mulakkal, M.C.; Trask, R.S.; Ting, V.P.; Seddon, A.M. Responsive cellulose-hydrogel composite ink for 4D printing. Mater. Des. 2018, 160, 108–118. [Google Scholar] [CrossRef]
- Giachini, P.A.G.S.; Gupta, S.S.; Wang, W.; Wood, D.; Yunusa, M.; Baharlou, E.; Sitti, M.; Menges, A. Additive manufacturing of cellulose-based materials with continuous, multidirectional stiffness gradients. Sci. Adv. 2020, 6, eaay0929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolz, B.; Mülhaupt, R. Cellular, Mineralized, and Programmable Cellulose Composites Fabricated by 3D Printing of Aqueous Pastes Derived from Paper Wastes and Microfibrillated Cellulose. Macromol. Mater. Eng. 2020, 305, 1900740. [Google Scholar] [CrossRef]
- Uchida, T.; Onoe, H. 4D Printing of Multi-Hydrogels Using Direct Ink Writing in a Supporting Viscous Liquid. Micromachines 2019, 10, 433. [Google Scholar] [CrossRef] [Green Version]
- Oladapo, B.I.; Adebiyi, A.V.; Elemure, E.I. Microstructural 4D printing investigation of ultra-sonication biocomposite polymer. J. King Saud Univ.-Eng. Sci. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Oshin, E.A.; Olawumi, A.M. Nanostructural computation of 4D printing carboxymethylcellulose (CMC) composite. Nanostruct. Nanoobjects 2020, 21, 100423. [Google Scholar] [CrossRef]



| Technology | Material | Printing Resolution (mm) | Advantages | Applications | Ref. |
|---|---|---|---|---|---|
| Material Extrusion (ME) | Thermoplastic polymers | 0.05–0.6 | Ease of use, low cost, wide availability of printers | Rapid prototyping, soft robotics, tissue engineering | [35] |
| Vat Polymerization (VP) | Photocurable resins | 0.025–0.1 | Low cost, high resolution, smooth printing surface | Tissue engineering, dental products, electronic insulators | [36] |
| Binder Jetting (BJ) | Ceramics, metals, Biomaterials | 0.3–0.5 | Fast printing, bigger build volume | Prototypes, casting patterns | [37] |
| Powder bed fusion (PBF) | Metal, polymers | 0.08–0.25 | High resolution, good quality | Electronics, aerospace, biomedical engineering | [38] |
| Material Jetting (MJ) | Photocurable polymers | 0.016–0.060 | Very high resolution, multicolor printing | Rapid prototyping, casting patterns | [39] |
| Directed Energy Deposition (DED) | Metal powder, polymer composite | 0.25–0.3 | Low manufacturing cost, high strength product | Aerospace, manufacturing | [40] |
| Sheet Lamination (SL) | Polymer, metal, ceramics | 0.1 | Low cost, wide available materials | Electronics, paper production | [41] |
| Filler | Filler Fraction (%) | Composite Tensile Strength (MPa) | Difference (%) | Ref |
|---|---|---|---|---|
| Modified carbon fiber | 34 | 91.0 | +225.0 | [52] |
| Carbon fiber | 28 | 61.4 | +36.8 | [53] |
| Graphene nanoplatelets | 10 | 40.2 | +27.2 | [54] |
| Rice husk | 20 | 53.0 | +18.3 | [55] |
| Ceramics | 40 | 43.2 | +1.9 | [56] |
| Poplar fibers | 20 | 54.0 | 0.0 | [57] |
| Copper | 40 | 40.3 | −4.9 | [56] |
| Aluminum | 40 | 40.2 | −5.1 | [56] |
| Calcium carbonate | 20 | 37.0 | −17.8 | [58] |
| Lignin | 40 | 45.65 | −21.9 | [59] |
| Wood fiber | 60 | 13.49 | −63.9 | [60] |
| Cork granules | 50 | 10.4 | −82.6 | [61] |
| Polymer/Cellulose Composition (wt%) | Extrusion Technique | FDM Printer | Nozzle Temp. (°C) | Nozzle Diameter (mm) | Improvements. | Ref. |
|---|---|---|---|---|---|---|
| 73.5% PLA, 24.5% PHB, 1% CNC, 1% dicumyl peroxide | Twin-screw | WASP Delta 2040 Turbo 2 | 200 | 0.4 | Mechanical properties and thermal stability | [90] |
| 93% PLA, 7% Hydroxypropyl methylcellulose | Single-screw | Ender-3S | 200 | 0.4 | Thermal properties and contact angle | [92] |
| 70% PLA, 25% recycled PLA, 5% MCC, 0.5 phr epoxy-based chain extender | Twin-screw | LulzBot TAZ 6 | 200 | 0.5 | Tensile strength, modulus and Izod impact strength | [96] |
| 95% PLA, 5% Hemp powder | Single-screw | Ultimaker 3 | 180 | 0.4 | Elastic modulus, tensile strength | [97] |
| 95% ABS, 5% CNC/Silica nanohybdrids | Twin-screw | S1 Architect 3D | 235 | 0.3 | Reduced warping, tensile strength, and layer adhesion | [98] |
| 90% Polycaprolactone, (PCL), 10% MCC | Single-screw | Prusa i3 | 210 | 0.4 | Mechanical strength and cell proliferation | [99] |
| 80% Polypropylene (PP), 20% cellulose waste materials | Twin-screw | Lulzot Taz 6 MEAM | 220 | 0.8 | Storage and elastic modulus | [100] |
| 95% ABS, 5% Oil palm fibre | Single-screw | UP Plus 2 | N/A | 0.4 | Tensile strength and elastic modulus | [101] |
| 80% PVA, 20% CNC | Single-screw | Sharebot Next Generation | 230 | 0.35 | Tensile strength and thermal properties | [102] |
| 90% ABS, 10% Lignin coated CNC | Single-screw | Solidoodle 3 | 210 | 0.35 | Mechanical and thermal properties | [103] |
| 70% PLA, 30% CNF | Single-screw | Solidoodle 3 | 180 | 0.35 | Tensile strength and elastic modulus | [104] |
| 87% PLA, 13% polybutylene adipate terephthalate (PBAT), 40 phr Hemp hurd | Twin-screw | 3D da Vinci 1.0 | 200 | 0.4 | Flexural modulus and dimensional accuracy | [105] |
| 50% Poly (ε-caprolactone), 50% cocoa shell waste | Single-screw | Prusa i3 Hephestos | 120 | 0.3 | Mechanical and thermal properties | [106] |
| Cellulose Composition | 3D Printer | Printing Parameter | Solidification Method | Potential Application | Ref. |
|---|---|---|---|---|---|
| Polyurethane aryclate, CNF-rGO, CNF-PEG | Wanhao Duplicator D7 Plus | UV light of wavelength 405 nm | UV curing | Bio based resin | [17] |
| Polymethyl methacrylate (PMMA), CNC-Silver Nanoparticles (CNC-AgNPs) | Envision TEC | Layer thickness 100 μm, 4.4 s exposure time, UV intensity 2500 μm/cm2 | UV curing | Dental restoration material | [110] |
| CNC, methacrylate resin | Form 1+ | N/A | Photocuring and heating | Electronic, engineering and tissue engineering | [111] |
| Ethyl cellulose macromonomerm resin-based monomer | Creality, LD 001, | N/A | Photocuring | Flexible electronic materials | [112] |
| CNC, PEGDA, 1,3-diglycerolate diacrylate (DiGlyDA) | DLP 3D printer | Layer thickness 100 μm, 4.0 s exposure time, UV intensity 18 mW/cm2 | UV curing | Biomedical application | [113] |
| Cellulose Composition | 3D Printer | Printing Parameter | Solidification Method | Potential Application | Ref. |
|---|---|---|---|---|---|
| Dialdehyde CNC, gelatin | Bio-Architect | Nozzle 0.21 mm, Extrusion pressure 100–250 kPa, Print speed 10–40 mm/s | Crosslinking with Ca2+ | Tissue engineering | [124] |
| CNF, Alginate | Regemat3D Designer | Nozzle 0.58 mm, Flow speed 3.0 mm/s | Crosslinking with CaCl2 | Tissue engineering | [125] |
| Bacterial CNF, silk fibroin (SF)/gelatin composite | 3D Bioplotter | Nozzle 0.41 mm, Extrusion pressure 1–2 bar, Print speed 3.0 | Crosslinking with genipin | Biomedical applications | [126] |
| CNF, xylan-tyramine | 3D bioprinter, RegenHU, Switzerland | Nozzle 0.42 mm, print speed 40 mm/s, layer height 0.4 mm | Crosslinking with H2O2 | Clothes, packaging, health care products, furniture | [127] |
| CNF, CMC | Bioscaffolder 3.1 | Nozzle 0.25 mm, Extrusion pressure 260 kPa, print speed 15 mm/s | Crosslinking with dehyrothermal treatment (DHT) | Bone tissue engineering | [128] |
| CNF, carbon nanotubes, | Fisnar F4200n, | Nozzle pressure 345 kPa, Print speed 10 mm/s | Solvent exchange with ethanol | Biomedical application | [129] |
| CNF, 2,2,6,6-Tetramethyl-1-piperidinyloxy | Custom built LDM printer | N/A | Oven treatment | Oil-in-water separation, electronic related application | [130] |
| CNC (20 wt%) | Custom built multi-material-multi-method 3D printer | Nozzle 0.4 mm Extrusion pressure kPa, Print speed 40 mm/s | None | Support during 3D printing | [131] |
| CNC, sodium alginate, gelatin | Ultimaker with Discov3ry Complete | Print speed 30 mm/s, printing temperature 26 °C | Crosslinking with CaCl2 | Cartilage regeneration application | [132] |
| CMC, silver nanowire, lithium iron phosphate/lithium titanate | Delta FDM 3D printer integrated with Discov3ry Extruder | Nozzle 0.84–1.54 mm, print speed 0.7–1.2 mm/s, Extrusion flow rate 0.0002–0.00083 mL/s | Drying | Conductive nanocomposites | [133] |
| CNC | ABL 900010, | Nozzle 0.41 mm, extrusion pressure 200–400 kPa, print speed 10–20 mm/s | Drying | Cellular architectures | [134] |
| CNC, 2-hydroxyethyl methacrylate monomer, polyether urethane acrylate oligomer, photoinitiator | 3D Discovery, RegenHU Ltd., Switzerland | Nozzle 0.41 mm, extrusion pressure 300–400 kPa, print speed 10 mm/s | Photocuring | Cellular architectures | [134] |
| Cellulose, N-methylmorpholine-N-oxide (NMMO) | 3D Bioplotter | Printing temperature 70 °C | Coagulation in water (NMMO removal), freeze drying | Tissue engineering | [135] |
| Cellulose fibers, CMC | Prusa i3 with WASP extruder | Nozzle 0.7 mm, print speed 10–20 mm/s | Solvent exchange, Drying | Lightweight 3D printed material | [136] |
| CNF, carbon nanotubes, NaOH | RegenHU 3D Discov3ry | Nozzle 0.3 mm, Extrusion pressure 65 kPA, Print speed 10 mm/s | Drying (ambient condition) | Neural tissue engineering | [137] |
| Cellulose Composition | 3D Printer | Printing Parameter | Solidification Method | Stimulus | Potential Application | Ref. |
|---|---|---|---|---|---|---|
| CNF, clay, N-isopropylacrylamide | ABG 10000, Aerotech | Nozzle 0.15–1.5 mm | UV Curing | Water | Tissue engineering and soft robotics applications | [170] |
| CMC, cellulose fibers, HEC, clay | Prusa MK2 | Nozzle 0.8 mm, layer height 0.6 mm | Crosslinked with citric acid | Water | Tissue engineering applications | [171] |
| HEC, MFC, citric acid/hydrochloric acid, lignin | Modified TEVO Tarantula i3 | Nozzle 0.55–4 mm | Crosslinking using citric acid/hydrochloric acid | Water | Biomedical application | [172] |
| MFC, PVA | 3D Bioplotter, EnvisionTEC | Extrusion pressure 5.0 bar, print speed xy 400 mm/min, print speed Z 350 mm/min, layer thickness 0.67 mm | Crosslink using glyoxal solution | Heat and Water | Tissue engineering applications | [173] |
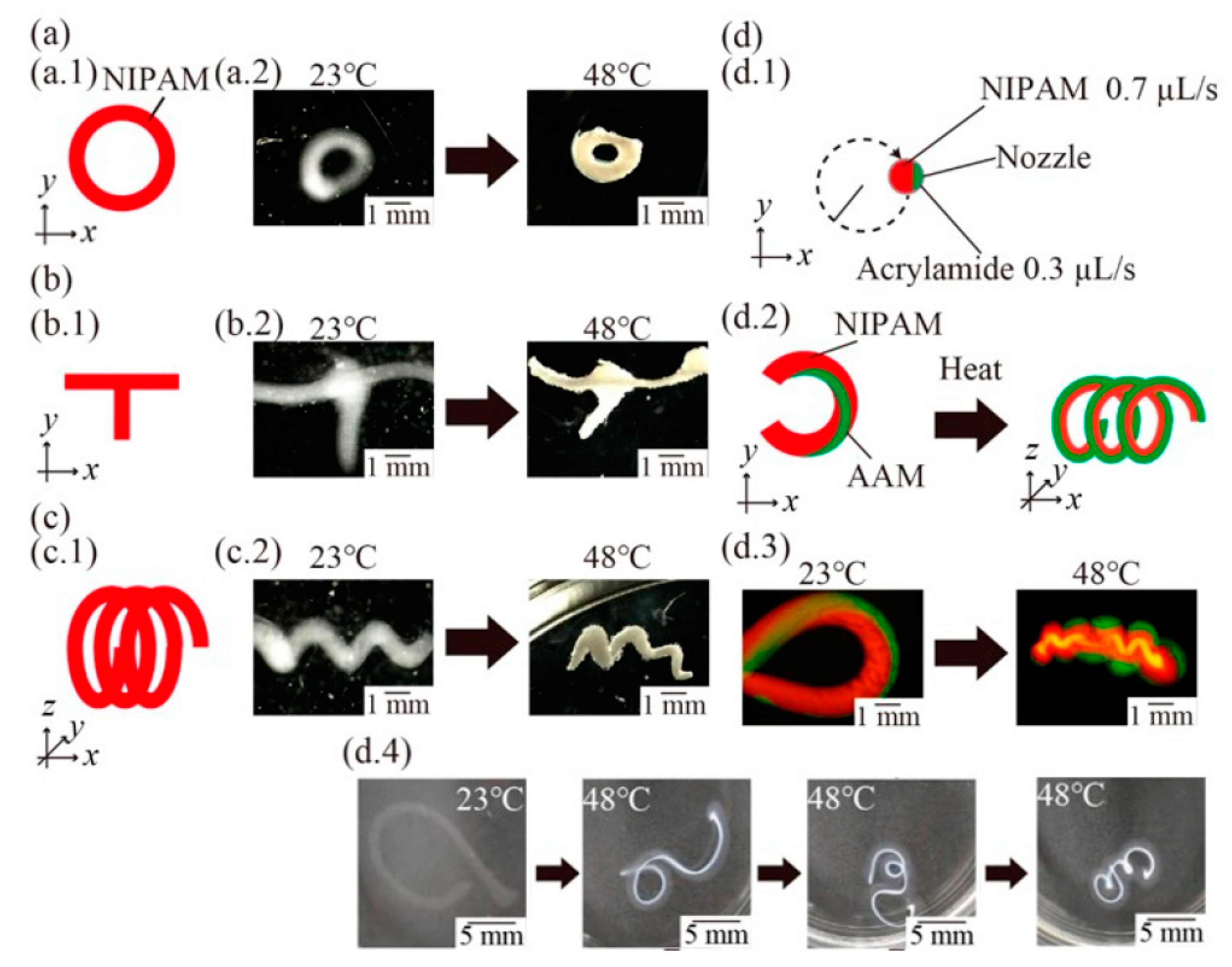
| N-isopropylacrylamide, CMC, sodium alginate, acrylamide | Custom built printer | Extrusion flow rate 1.0 μL/s, print speed 1.0 mm/s | Irradiation with UV light, then soaked in water | Heat | Environmental monitoring and medical applications | [174] |
| CMC-Na, clay, HEC | N/A | N/A | Crosslinking with citric acid | Water | Tissue engineering applications | [175] |
| CMC-Na, montmorillonite clay | N/A | N/A | Crosslinking with citric acid | Water | Tissue engineering applications | [176] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohan, D.; Teong, Z.K.; Bakir, A.N.; Sajab, M.S.; Kaco, H. Extending Cellulose-Based Polymers Application in Additive Manufacturing Technology: A Review of Recent Approaches. Polymers 2020, 12, 1876. https://doi.org/10.3390/polym12091876
Mohan D, Teong ZK, Bakir AN, Sajab MS, Kaco H. Extending Cellulose-Based Polymers Application in Additive Manufacturing Technology: A Review of Recent Approaches. Polymers. 2020; 12(9):1876. https://doi.org/10.3390/polym12091876
Chicago/Turabian StyleMohan, Denesh, Zee Khai Teong, Afifah Nabilah Bakir, Mohd Shaiful Sajab, and Hatika Kaco. 2020. "Extending Cellulose-Based Polymers Application in Additive Manufacturing Technology: A Review of Recent Approaches" Polymers 12, no. 9: 1876. https://doi.org/10.3390/polym12091876
APA StyleMohan, D., Teong, Z. K., Bakir, A. N., Sajab, M. S., & Kaco, H. (2020). Extending Cellulose-Based Polymers Application in Additive Manufacturing Technology: A Review of Recent Approaches. Polymers, 12(9), 1876. https://doi.org/10.3390/polym12091876







