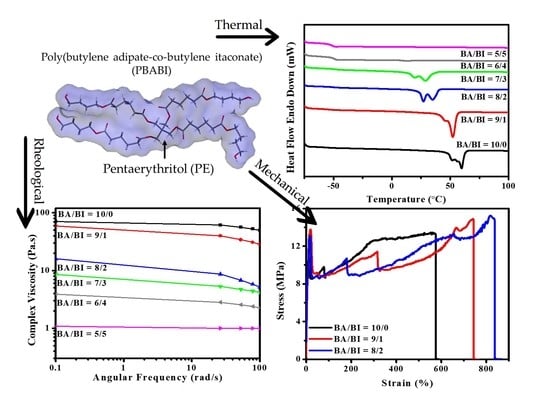
Synthesis of Bio-Based Poly(Butylene Adipate-co-Butylene Itaconate) Copolyesters with Pentaerythritol: A Thermal, Mechanical, Rheological, and Molecular Dynamics Simulation Study
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation
2.3. Identification of PBABI Copolyesters
2.4. Thermal Analysis of PBABI Copolyesters
2.5. Mechanical Properties of PBABI Copolyesters
2.6. Rheological Test and X-ray Diffraction (XRD) of PBABI Copolyesters
2.7. All-Atom Molecular Dynamics Simulation Procedures
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Díaz, A.; Katsarava, R.; Puiggalí, J. Synthesis, properties and applications of biodegradable polymers derived from diols and dicarboxylic acids: From polyesters to poly(ester amide)s. Int. J. Mol. Sci. 2014, 15, 7064–7123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douka, A.; Vouyiouka, S.; Papaspyridi, L.-M.; Papaspyrides, C.D. A review on enzymatic polymerization to produce polycondensation polymers: The case of aliphatic polyesters, polyamides and polyesteramides. Prog. Polym. Sci. 2018, 79, 1–25. [Google Scholar] [CrossRef]
- Simpson, J.M.; Mallon, P.E.; McLeary, D.J.B. Synthesis and Characterization of Unsaturated Polyesters for Use in Multi-Vesiculated Particles (MVPs). Master’s Thesis, University of Stellenbosch, Stellenbosch, South Africa, 2010. [Google Scholar]
- Vert, M. Aliphatic polyesters: Great degradable polymers that cannot do everything †. Biomacromolecules 2005, 6, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Zia, K.M.; Noreen, A.; Zuber, M.; Tabasum, S.; Mujahid, M. Recent developments and future prospects on bio-based polyesters derived from renewable resources: A review. Int. J. Biol. Macromol. 2016, 82, 1028–1040. [Google Scholar] [CrossRef]
- Chan, H.; Cho, C.; Hsu, K.; He, C.; Kuo, C.; Chu, C.; Chen, Y.; Chen, C.; Rwei, S. Smart wearable textiles with breathable properties and repeatable shaping in in vitro orthopedic support from a novel biomass thermoplastic copolyester. Macromol. Mater. Eng. 2019, 1900103. [Google Scholar] [CrossRef]
- Hsu, K.-H.; Chen, C.-W.; Wang, L.-Y.; Chan, H.-W.; He, C.-L.; Cho, C.-J.; Rwei, S.-P.; Kuo, C.-C. Bio-based thermoplastic poly(butylene succinate-co-propylene succinate) copolyesters: Effect of glycerol on thermal and mechanical properties. Soft Matter 2019, 15, 9710–9720. [Google Scholar] [CrossRef]
- Chen, C.-W.; Hsu, T.-S.; Rwei, S.-P. Effect of ethylenediaminetetraacetic acid on unsaturated poly(Butylene adipate-co-butylene itaconate) Copolyester with low-melting point and controllable hardness. Polymers 2019, 11, 611. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-W.; Hsu, T.-S.; Huang, K.-W.; Rwei, S.-P. Effect of 1,2,4,5-benzenetetracarboxylic acid on unsaturated poly(butylene adipate-co-butylene itaconate) copolyesters: Synthesis, non-isothermal crystallization kinetics, thermal and mechanical properties. Polymers 2020, 12, 1160. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Ha, U.; Okorie, N.C. Renewable polymers: Synthesis and characterization of poly(levulinic acid-pentaerythritol). J. Polym. Sci. Part Polym. Chem. 2018, 56, 955–958. [Google Scholar] [CrossRef] [Green Version]
- Brannigan, R.P.; Walder, A.; Dove, A.P. Application of functional diols derived from pentaerythritol as chain extenders in the synthesis of novel thermoplastic polyester-urethane elastomers. Polym. Chem. 2019, 10, 5236–5241. [Google Scholar] [CrossRef] [Green Version]
- Duan, K.; He, Y.; Li, Y.; Liu, J.; Zhang, J.; Hu, Y.; Lin, R.; Wang, X.; Deng, W.; Li, L. Machine-learning assisted coarse-grained model for epoxies over wide ranges of temperatures and cross-linking degrees. Mater. Des. 2019, 183, 108130. [Google Scholar] [CrossRef]
- Hacker, M.C.; Klouda, L.; Ma, B.B.; Kretlow, J.D.; Mikos, A.G. Synthesis and characterization of injectable, thermally and chemically gelable, amphiphilic poly(N-isopropylacrylamide)-based macromers. Biomacromolecules 2008, 9, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Kricheldorf, H.R.; Behnken, G. Biodegradable hyperbranched aliphatic polyesters derived from pentaerythritol. Macromolecules 2008, 41, 5651–5657. [Google Scholar] [CrossRef]
- Liu, C.; Qian, Z.; Gu, Y.; Fan, L.; Li, J.; Chao, G.; Jia, W.; Tu, M. Synthesis, characterization, and thermal properties of biodegradable aliphatic copolyester based on ε-caprolactone, adipic acid, and 1,6-hexanediol. Mater. Lett. 2006, 60, 31–38. [Google Scholar] [CrossRef]
- Liu, G.-C.; Zhang, W.-Q.; Zhou, S.-L.; Wang, X.-L.; Wang, Y.-Z. Improving crystallization and processability of PBS via slight cross-linking. RSC Adv. 2016, 6, 68942–68951. [Google Scholar] [CrossRef]
- Lu, J.; Wu, L.; Li, B.-G. Long chain branched poly(butylene succinate-co-terephthalate) copolyesters using pentaerythritol as branching agent: Synthesis, thermo-mechanical, and rheological properties. J. Appl. Polym. Sci. 2017, 134, 44544. [Google Scholar] [CrossRef]
- Mahmud, H.A.; Salih, N.; Salimon, J. Oleic acid based polyesters of trimethylolpropane and pentaerythritol for biolubricant application. Malays. J. Anal. Sci. 2015, 19, 9. [Google Scholar]
- Murillo, E.A.; Vallejo, P.P.; López, B.L. Characterization of hydroxylated hyperbranched polyesters of fourth and fifth generation. E-Polym. 2010, 10, 1–12. [Google Scholar] [CrossRef]
- Nagata, M.; Ibuki, H.; Sakai, W.; Tsutsumi, N. Synthesis, characterization, and enzymatic degradation of novel regular network aliphatic polyesters based on pentaerythritol. Macromolecules 1997, 30, 6525–6530. [Google Scholar] [CrossRef]
- Park, S.Y.; Chun, J.; Jeon, J.Y.; Lee, P.C.; Hwang, Y.; Song, B.G.; Ramos, R.; Ryu, C.Y.; Lee, B.Y. Branched poly(1,4-butylene carbonate-co-terephthalate)s: LDPE-like semicrystalline thermoplastics. J. Polym. Sci. Part Polym. Chem. 2015, 53, 914–923. [Google Scholar] [CrossRef]
- Shen, J.; Lin, X.; Liu, J.; Li, X. Effects of cross-link density and distribution on static and dynamic properties of chemically cross-linked polymers. Macromolecules 2019, 52, 121–134. [Google Scholar] [CrossRef]
- Shim, Y.S.; Chun, B.C.; Chung, Y.-C. Thermomechanical properties and shape memory effect of PET-PEG copolymers cross-linked with pentaerythritol. Fibers Polym. 2006, 7, 328–332. [Google Scholar] [CrossRef]
- Soccio, M.; Finelli, L.; Lotti, N.; Marchese, P.; Siracusa, V.; Munari, A. A novel hyperbranched polyester based on 2,2-bis(hydro xylmethyl)butyric acid: Synthesis and characterization. E-Polym. 2007, 7, 1–14. [Google Scholar] [CrossRef]
- Tieghi, G.; Levi, M.; Fallini, A. Characterization of crosslinked polyester resins by dynamic mechanical properties. Polymer 1992, 33, 3748–3750. [Google Scholar] [CrossRef]
- Tow, G.M.; Maginn, E.J. Fully atomistic molecular dynamics simulations of hydroxyl-terminated polybutadiene with insights into hydroxyl aggregation. Macromolecules 2020, 53, 2594–2605. [Google Scholar] [CrossRef]
- Uto, K.; Yamamoto, K.; Hirase, S.; Aoyagi, T. Temperature-responsive cross-linked poly(ε-caprolactone) membrane that functions near body temperature. J. Control. Release 2006, 110, 408–413. [Google Scholar] [CrossRef]
- Waig Fang, S.; De Caro, P.; Pennarun, P.-Y.; Vaca-Garcia, C.; Thiebaud-Roux, S. Synthesis and characterization of new polyesters based on renewable resources. Ind. Crops Prod. 2013, 43, 398–404. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Wu, Z.-H.; Yang, W.; Yang, M.-B. Thermal and mechanical properties of chemical crosslinked polylactide (PLA). Polym. Test. 2008, 27, 957–963. [Google Scholar] [CrossRef]
- Žagar, E.; Žigon, M. Aliphatic hyperbranched polyesters based on 2,2-bis(methylol)propionic acid—Determination of structure, solution and bulk properties. Prog. Polym. Sci. 2011, 36, 53–88. [Google Scholar] [CrossRef]
- Chen, C.-W.; Huang, C.-I. Effects of intra/inter-molecular potential parameters, length and grafting density of side-chains on the self-assembling behavior of poly(3′-alkylthiophene)s in the ordered state. Polymer 2015, 77, 189–198. [Google Scholar] [CrossRef]
- Sun, H.; Ren, P.; Fried, J.R. The COMPASS force field: Parameterization and validation for phosphazenes. Comput. Theor. Polym. Sci. 1998, 8, 229–246. [Google Scholar] [CrossRef]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Tang, T.; Moyori, T.; Takasu, A. Isomerization-free polycondensations of cyclic anhydrides with diols and preparation of polyester gels containing Cis or Trans carbon double bonds via photo-cross-linking and isomerization in the gels. Macromolecules 2013, 46, 5464–5472. [Google Scholar] [CrossRef]
- Brännström, S.; Finnveden, M.; Johansson, M.; Martinelle, M.; Malmström, E. Itaconate based polyesters: Selectivity and performance of esterification catalysts. Eur. Polym. J. 2018, 103, 370–377. [Google Scholar] [CrossRef]
- Tang, T.; Takasu, A. Facile synthesis of unsaturated polyester-based double-network gels via chemoselective cross-linking using Michael addition and subsequent UV-initiated radical polymerization. RSC Adv. 2015, 5, 819–829. [Google Scholar] [CrossRef]
- Woo, E.M.; Wu, M.C. Thermal and X-ray analysis of polymorphic crystals, melting, and crystalline transformation in poly(butylene adipate). J. Polym. Sci. Part B Polym. Phys. 2005, 43, 1662–1672. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Z.; Yang, X.; Liu, K.; Zhang, M.; Qiang, X.; Wang, X. Epitaxial crystallization behavior of poly(butylene adipate) on orientated POLY(butylene succinate) substrate. Polymers 2018, 10, 110. [Google Scholar] [CrossRef] [Green Version]
- Gan, Z.; Abe, H.; Doi, Y. Temperature-induced polymorphic crystals of poly(butylene adipate). Macromol. Chem. Phys. 2002, 203, 2369–2374. [Google Scholar] [CrossRef]
- Hou, C.; Li, H.; Sun, X.; Yan, S.; Wang, Y.; Chen, S. The dependence of the β-to-α phase transition behavior of poly(1,4-butylene adipate) on phase separated morphology in its blends with poly(vinylidene fluoride). Phys. Chem. Chem. Phys. 2018, 20, 15718–15724. [Google Scholar] [CrossRef]
- Minke, R.; Blackwell, J. Polymorphic structures of poly(tetramethylene adipate). J. Macromol. Sci. Part B 1979, 16, 407–417. [Google Scholar] [CrossRef]
- Noguchi, K.; Kondo, H.; Ichikawa, Y.; Okuyama, K.; Washiyama, J. Molecular and crystal structure of poly(tetramethylene adipate) α form based on synchrotron X-ray fiber diffraction. Polymer 2005, 46, 10823–10830. [Google Scholar] [CrossRef]
- Chen, C.-W.; Hsu, T.-S.; Rwei, S.-P. Isothermal kinetics of poly(butylene adipate-co-butylene itaconate) copolyesters with ethylenediaminetetraacetic acid. ACS Omega 2020, 5, 3080–3089. [Google Scholar] [CrossRef] [PubMed]













| Sample | IV (dL g−1) | Tg (°C) | Tc (°C) | ΔHc (J g−1) | Tm (°C) | ΔHm (J g−1) | Td−5% (°C) |
|---|---|---|---|---|---|---|---|
| BA/BI = 10/0 | 0.68 | −54.6 | 28.2 | −48.3 | 51.5, 59.5 | 42.3 | 344.7 |
| BA/BI = 9/1 | 0.61 | −53.3 | 23.6 | −50.2 | 45.5, 52.4 | 41.3 | 338.0 |
| BA/BI = 8/2 | 0.62 | −48.1 | 1.3 | −35.6 | 26.9, 34.8 | 33.2 | 318.7 |
| BA/BI = 7/3 | 0.57 | −50.1 (DSC) | −9.1 | −30.8 | 19.5, 28.4 | 27.1 | 312.6 |
| BA/BI = 6/4 | 0.52 | −50.3 (DSC) | n/a | n/a | n/a | n/a | 301.9 |
| BA/BI = 5/5 | 0.38 | −52.9 (DSC) | n/a | n/a | n/a | n/a | 261.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-W.; Mao, H.-I.; Yang, Z.-Y.; Huang, K.-W.; Yan, H.-C.; Rwei, S.-P. Synthesis of Bio-Based Poly(Butylene Adipate-co-Butylene Itaconate) Copolyesters with Pentaerythritol: A Thermal, Mechanical, Rheological, and Molecular Dynamics Simulation Study. Polymers 2020, 12, 2006. https://doi.org/10.3390/polym12092006
Chen C-W, Mao H-I, Yang Z-Y, Huang K-W, Yan H-C, Rwei S-P. Synthesis of Bio-Based Poly(Butylene Adipate-co-Butylene Itaconate) Copolyesters with Pentaerythritol: A Thermal, Mechanical, Rheological, and Molecular Dynamics Simulation Study. Polymers. 2020; 12(9):2006. https://doi.org/10.3390/polym12092006
Chicago/Turabian StyleChen, Chin-Wen, Hsu-I Mao, Zhi-Yu Yang, Kuan-Wei Huang, Hao-Chen Yan, and Syang-Peng Rwei. 2020. "Synthesis of Bio-Based Poly(Butylene Adipate-co-Butylene Itaconate) Copolyesters with Pentaerythritol: A Thermal, Mechanical, Rheological, and Molecular Dynamics Simulation Study" Polymers 12, no. 9: 2006. https://doi.org/10.3390/polym12092006