Imprinted Contact Lenses for Ocular Administration of Antiviral Drugs
Abstract
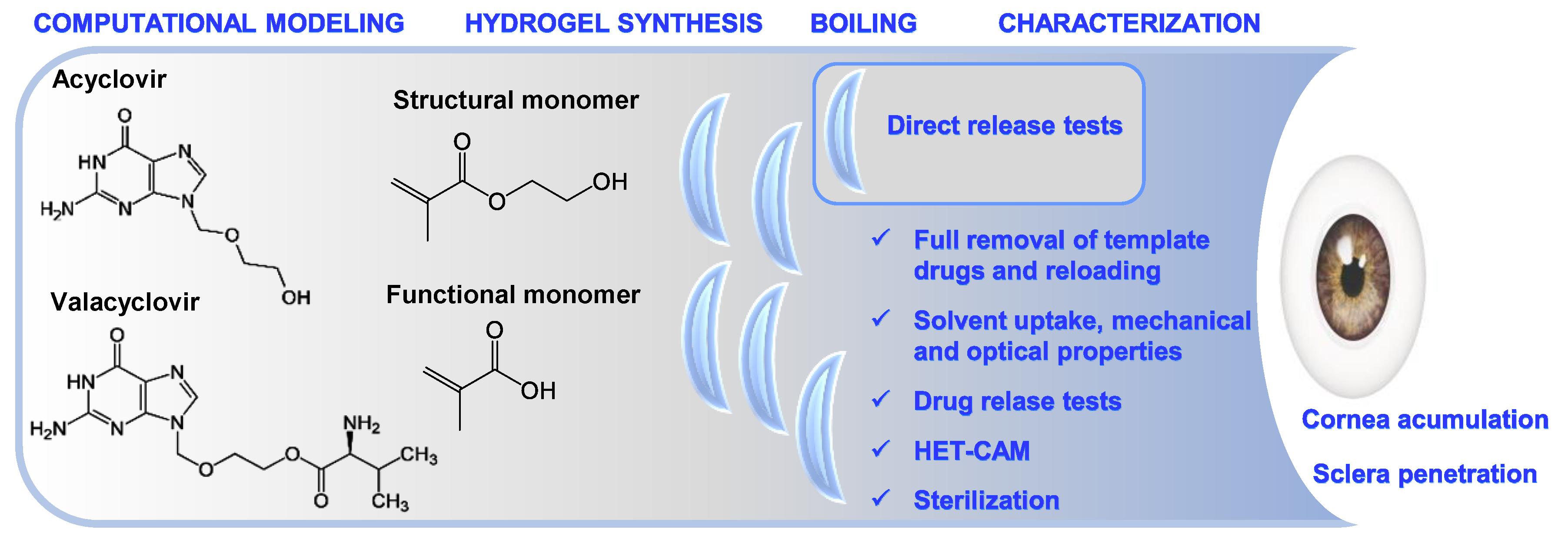
:1. Introduction
2. Materials and Methods
2.1. Materials
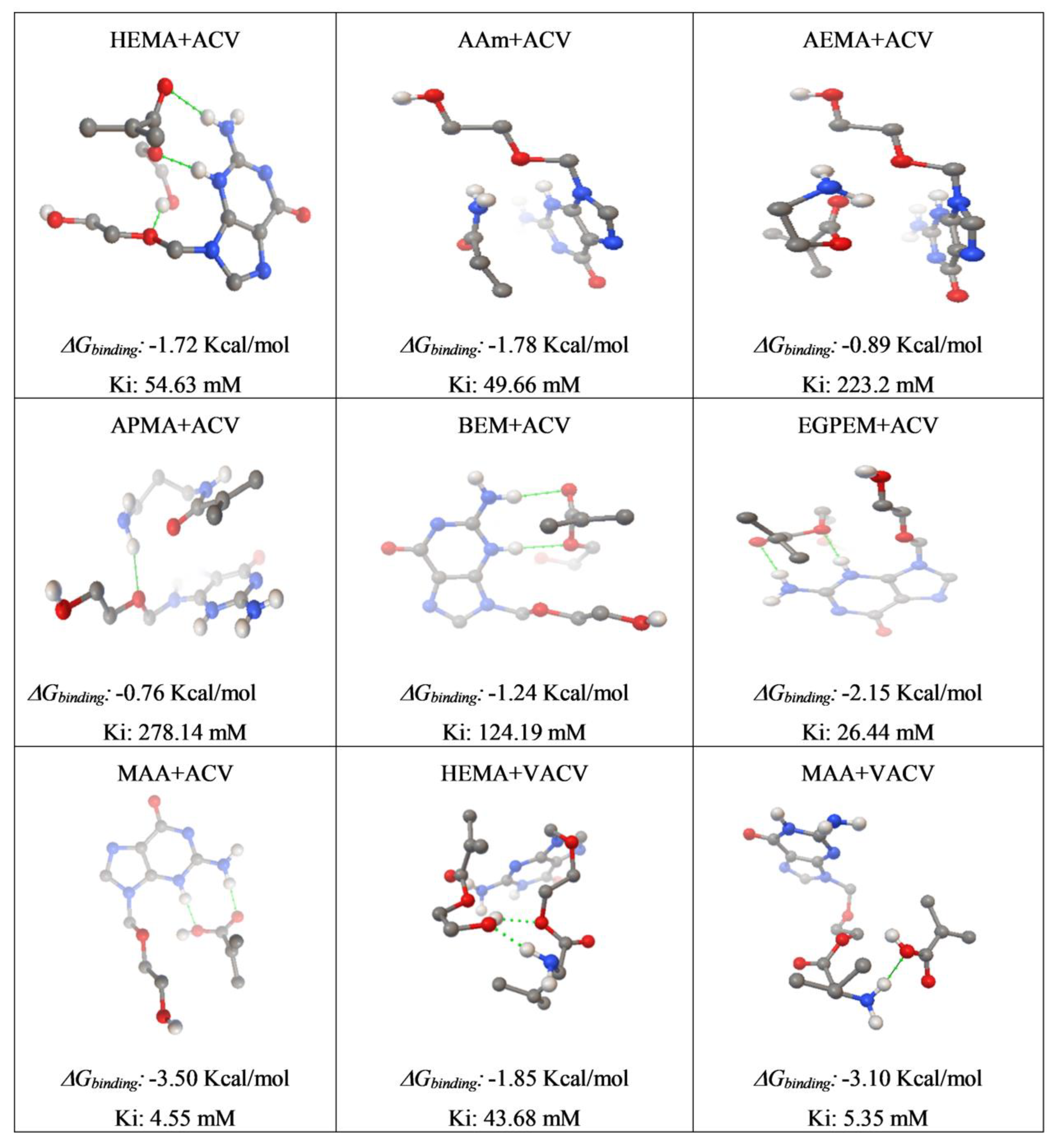
2.2. Computational Modeling
2.3. Synthesis of Imprinted and Non-Imprinted Hydrogels
2.4. Drug Removal
2.5. Direct Drug Release Test from Boiled Hydrogels
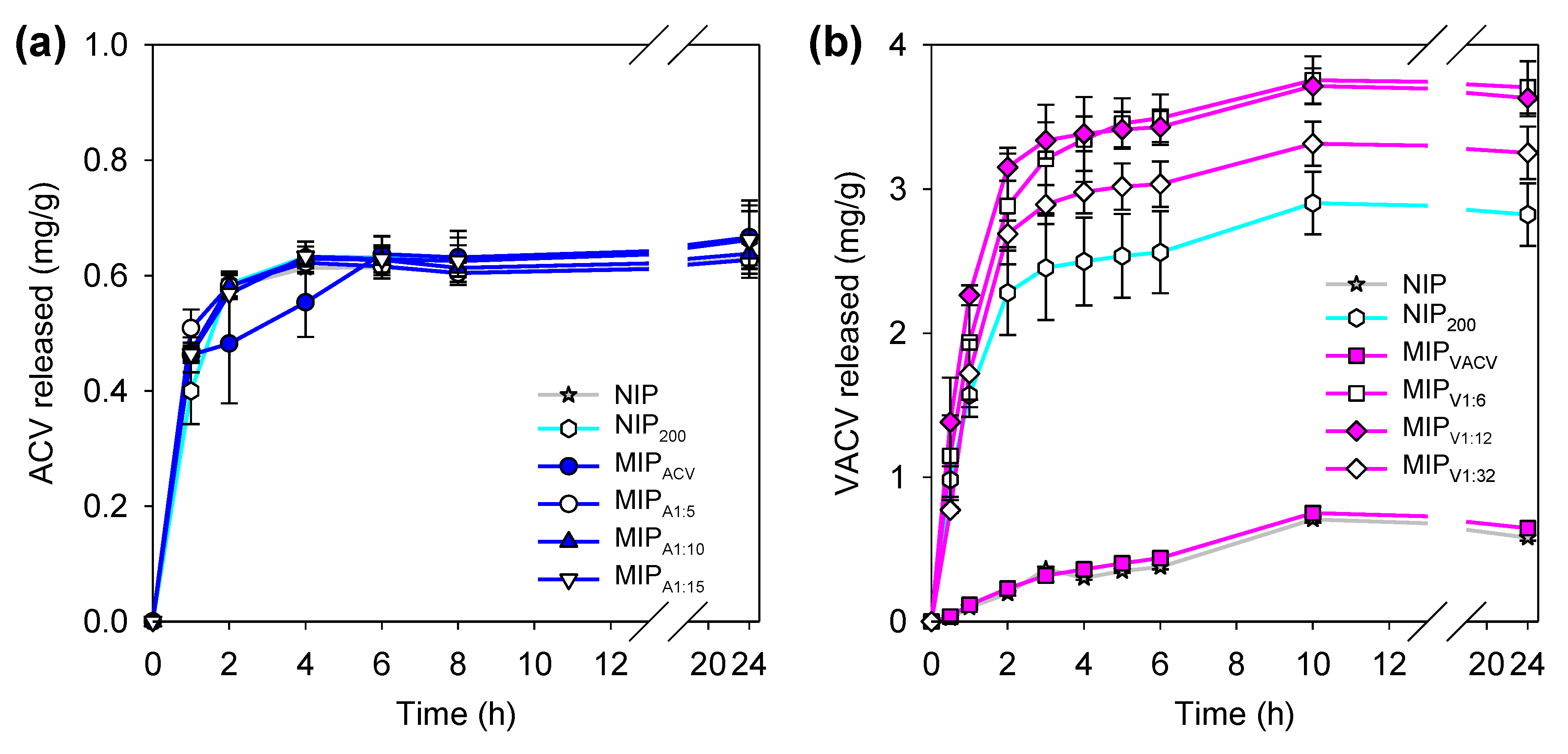
2.6. Drug Loading and Release
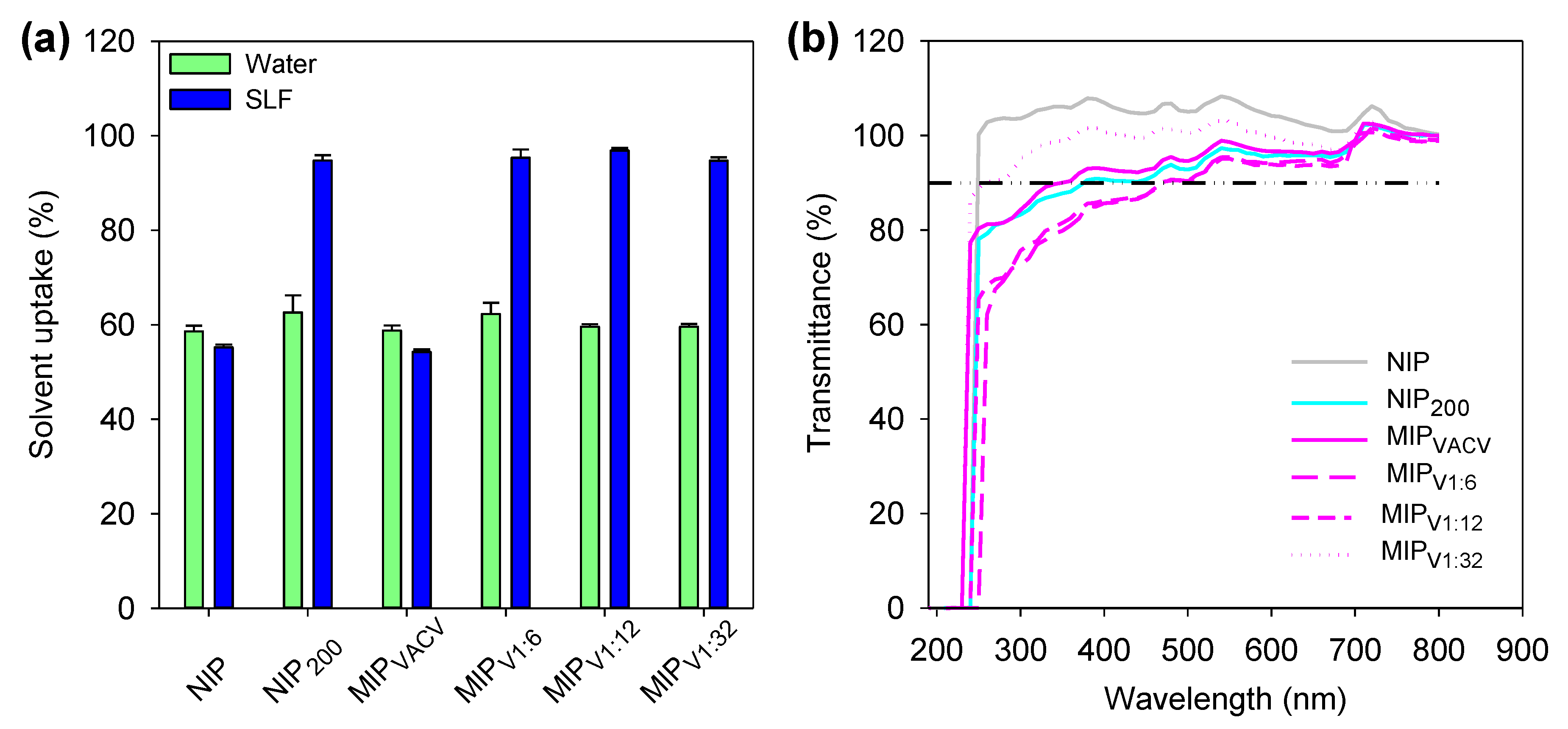
2.7. Solvent Uptake
2.8. Light Transmission
2.9. Mechanical Properties
2.10. HET-CAM Test
2.11. Cornea and Sclera Permeability Tests
2.12. Statistical Analysis
3. Results and Discussion
3.1. Computational Modeling
3.2. Synthesis of Hydrogels and Drug Removal
3.3. Direct Drug Release Test from Boiled Hydrogels
3.4. Drug Loading
3.5. Drug Release
3.6. Hydrogels Characterization
3.7. Sterilization Tests
3.8. HET-CAM Test
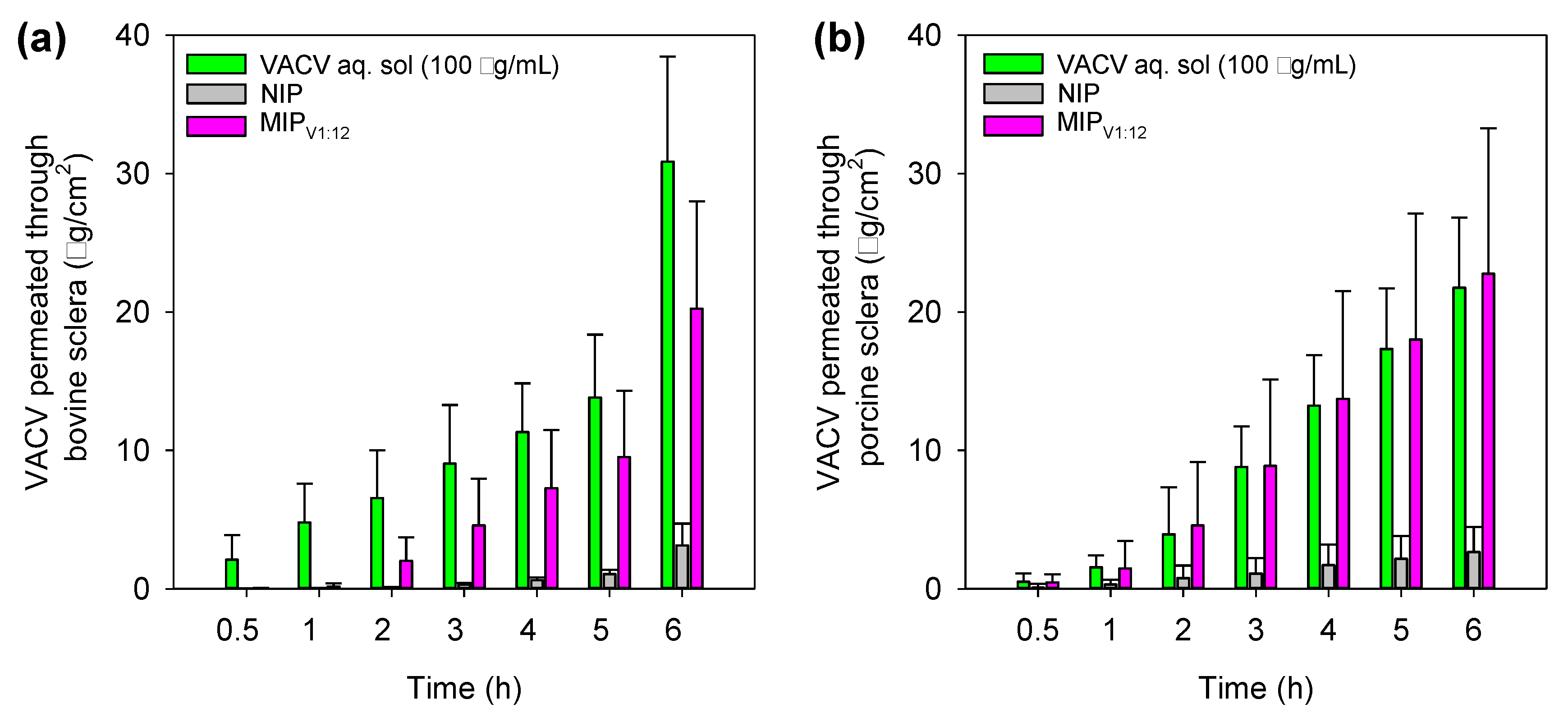
3.9. Corneal and Scleral Permeability Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Whitley, R.J.; Roizman, B. Herpes simplex virus infections. Lancet 2001, 357, 1513–1518. [Google Scholar] [CrossRef]
- Lloyd, J.; Copaciu, R.; Yahyabeik, A.; DeWit, C.; Cummings, K.; Lacey, M.; Su, Q. Characterization of polyclonal antibodies to herpes simplex virus types 1 and 2. J. Histotechnol. 2019, 42, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Fatahzadeh, M.; Schwartz, R.A. Human herpes simplex virus infections: Epidemiology, pathogenesis, symptomatology, diagnosis, and management. J. Am. Acad. Dermatol. 2007, 57, 737–763; quiz 764–766. [Google Scholar] [CrossRef] [PubMed]
- Brady, R.C.; Bernstein, D.I. Treatment of herpes simplex virus infections. Antiviral Res. 2004, 61, 73–81. [Google Scholar] [CrossRef]
- Kaye, S.; Choudhary, A. Herpes simplex keratitis. Prog. Retin. Eye Res. 2006, 25, 355–380. [Google Scholar] [CrossRef]
- Tsatsos, M.; MacGregor, C.; Athanasiadis, I.; Moschos, M.M.; Hossain, P.; Anderson, D. Herpes simplex virus keratitis: An update of the pathogenesis and current treatment with oral and topical antiviral agents. Clin. Exp. Ophthalmol. 2016, 44, 824–837. [Google Scholar] [CrossRef]
- Wilhelmus, K.R. Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst. Rev. 2015, 1, CD002898. [Google Scholar] [CrossRef]
- Karsten, E.; Watson, S.L.; Foster, L.J. Diversity of microbial species implicated in keratitis: A review. Open Ophthalmol. J. 2012, 6, 110–124. [Google Scholar] [CrossRef]
- Esmann, J. The many challenges of facial herpes simplex virus infection. J. Antimicrob. Chemother. 2001, 47 (Suppl. S1), 17–27. [Google Scholar] [CrossRef]
- Koganti, R.; Yadavalli, T.; Shukla, D. Current and emerging therapies for ocular herpes simplex virus type-1 infections. Microorganisms 2019, 7, 429. [Google Scholar] [CrossRef] [Green Version]
- Austin, A.; Lietman, T.; Rose-Nussbaumer, J. Update on the management of infectious keratitis. Ophthalmology 2017, 124, 1678–1689. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, D.M.; Castillo, E.; Duarte, L.F.; Arriagada, J.; Corrales, N.; Farias, M.A.; Henriquez, A.; Agurto-Munoz, C.; Gonzalez, P.A. Current antivirals and novel botanical molecules interfering with herpes simplex virus infection. Front. Microbiol. 2020, 11, 139. [Google Scholar] [CrossRef] [PubMed]
- Kalezic, T.; Mazen, M.; Kuklinski, E.; Asbell, P. Herpetic eye disease study: Lessons learned. Curr. Opin. Ophthalmol. 2018, 29, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Rajasagi, N.K.; Rouse, B.T. Application of our understanding of pathogenesis of herpetic stromal keratitis for novel therapy. Microbes Infect. 2018, 20, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Kapanigowda, U.G.; Nagaraja, S.H.; Ramaiah, B.; Boggarapu, P.R.; Subramanian, R. Enhanced trans-corneal permeability of valacyclovir by polymethacrylic acid copolymers based ocular microspheres: In vivo evaluation of estimated pharmacokinetic/pharmacodynamic indices and simulation of aqueous humor drug concentration-time profile. J. Pharm. Innov. 2016, 11, 82–91. [Google Scholar] [CrossRef]
- Kumar, M.; Kaufman, H.E.; Clement, C.; Bhattacharjee, P.S.; Huq, T.S.; Varnell, E.D.; Thompson, H.W.; Hill, J.M. Effect of high versus low oral doses of valacyclovir on herpes simplex virus-1 DNA shedding into tears of latently infected rabbits. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4703–4706. [Google Scholar] [CrossRef] [Green Version]
- Kang-Mieler, J.J.; Osswald, C.R.; Mieler, W.F. Advances in ocular drug delivery: Emphasis on the posterior segment. Expert Opin. Drug Deliv. 2014, 11, 1647–1660. [Google Scholar] [CrossRef]
- Kumar, M.; Hill, J.M.; Clement, C.; Varnell, E.D.; Thompson, H.W.; Kaufman, H.E. A double-blind placebo-controlled study to evaluate valacyclovir alone and with aspirin for asymptomatic HSV-1 DNA shedding in human tears and saliva. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5601–5608. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Sinha, V.R. Lipid nanocarrier: An efficient approach towards ocular delivery of hydrophilic drug (valacyclovir). AAPS PharmSciTech 2017, 18, 884–894. [Google Scholar] [CrossRef]
- Anand, B.S.; Mitra, A.K. Mechanism of corneal permeation of L-valyl ester of acyclovir: Targeting the oligopeptide transporter on the rabbit cornea. Pharm. Res. 2002, 19, 1194–1202. [Google Scholar] [CrossRef]
- Hatanaka, T.; Haramura, M.; Fei, Y.J.; Miyauchi, S.; Bridges, C.C.; Ganapathy, P.S.; Smith, S.B.; Ganapathy, V.; Ganapathy, M.E. Transport of amino acid-based prodrugs by the Na+- and Cl- -coupled amino acid transporter ATB0,+ and expression of the transporter in tissues amenable for drug delivery. J. Pharmacol. Exp. Ther. 2004, 308, 1138–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiratani, H.; Fujiwara, A.; Tamiya, Y.; Mizutani, Y.; Alvarez-Lorenzo, C. Ocular release of timolol from molecularly imprinted soft contact lenses. Biomaterials 2005, 26, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- White, C.J.; Byrne, M.E. Molecularly imprinted therapeutic contact lenses. Expert Opin. Drug Deliv. 2010, 7, 765–780. [Google Scholar] [CrossRef] [PubMed]
- González-Chomón, C.; Concheiro, A.; Alvarez-Lorenzo, C. Soft contact lenses for controlled ocular delivery: 50 years in the making. Ther. Deliv. 2013, 4, 1141–1161. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Lorenzo, C.; Anguiano-Igea, S.; Varela-Garcia, A.; Vivero-Lopez, M.; Concheiro, A. Bioinspired hydrogels for drug-eluting contact lenses. Acta Biomater. 2019, 84, 49–62. [Google Scholar] [CrossRef]
- Ribeiro, A.; Veiga, F.; Santos, D.; Torres-Labandeira, J.J.; Concheiro, A.; Alvarez-Lorenzo, C. Bioinspired imprinted PHEMA-hydrogels for ocular delivery of carbonic anhydrase inhibitor drugs. Biomacromolecules 2011, 12, 701–709. [Google Scholar] [CrossRef]
- Tieppo, A.; White, C.J.; Paine, A.C.; Voyles, M.L.; McBride, M.K.; Byrne, M.E. Sustained in vivo release from imprinted therapeutic contact lenses. J. Control. Release 2012, 157, 391–397. [Google Scholar] [CrossRef]
- González-Chomón, C.; Silva, M.; Concheiro, A.; Alvarez-Lorenzo, C. Biomimetic contact lenses eluting olopatadine for allergic conjunctivitis. Acta Biomater. 2016, 41, 302–311. [Google Scholar] [CrossRef]
- Malakooti, N.; Alexander, C.; Alvarez-Lorenzo, C. Imprinted contact lenses for sustained release of polymyxin b and related antimicrobial peptides. J. Pharm. Sci. 2015, 104, 3386–3394. [Google Scholar] [CrossRef]
- Hui, A.; Willcox, M.; Jones, L. In vitro and in vivo evaluation of novel ciprofloxacin-releasing silicone hydrogel contact lenses. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4896–4904. [Google Scholar] [CrossRef]
- White, C.J.; DiPasquale, S.A.; Byrne, M.E. Controlled release of multiple therapeutics from silicone hydrogel contact lenses. Optom. Vis. Sci. 2016, 93, 377–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Rivera, F.; Concheiro, A.; Alvarez-Lorenzo, C. Epalrestat-loaded silicone hydrogels as contact lenses to address diabetic-eye complications. Eur. J. Pharm. Biopharm. 2018, 122, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rivera, F.; Serro, A.P.; Silva, D.; Concheiro, A.; Alvarez-Lorenzo, C. Hydrogels for diabetic eyes: Naltrexone loading, release profiles and cornea penetration. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 105, 110092. [Google Scholar] [CrossRef] [PubMed]
- Marc, M.; Kupka, T.; Wieczorek, P.P.; Namiesnik, J. Computational modeling of molecularly imprinted polymers as a green approach to the development of novel analytical sorbents. Trac-Trends Anal. Chem. 2018, 98, 64–78. [Google Scholar] [CrossRef]
- Varela-Garcia, A.; Concheiro, A.; Alvarez-Lorenzo, C. Soluplus micelles for acyclovir ocular delivery: Formulation and cornea and sclera permeability. Int. J. Pharm. 2018, 552, 39–47. [Google Scholar] [CrossRef] [PubMed]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Valacyclovir (accessed on 24 August 2020).
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem substance and compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- O´Boyle, N.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Available online: http://autodock.scripps.edu/faqs-help/manual/autodock-4-2-user-guide/AutoDock4.2.6_UserGuide.pdf (accessed on 30 June 2020).
- Tranoudis, I.; Efron, N. Tensile properties of soft contact lens materials. Cont. Lens Anterior Eye 2004, 27, 177–191. [Google Scholar] [CrossRef]
- Bhamra, T.S.; Tighe, B.J. Mechanical properties of contact lenses: The contribution of measurement techniques and clinical feedback to 50 years of materials development. Cont. Lens Anterior Eye 2017, 40, 70–81. [Google Scholar] [CrossRef] [Green Version]
- OECD. Test Guideline No. 437. Bovine Corneal Opacity and Permeability Test Method for Identifying Ocular Corrosives and Severe Irritants. 2020. Available online: https://www.oecd-ilibrary.org/environment/test-no-437-bovine-corneal-opacity-and-permeability-test-method-for-identifying-i-chemicals-inducing-serious-eye-damage-and-ii-chemicals-not-requiring-classification-for-eye-irritation-or-serious-eye-damage_9789264203846-en (accessed on 24 June 2020). [CrossRef]
- Palacios, M.L.; Demasi, G.; Pizzorno, M.T.; Segall, A.I. Validation of an HPLC method for the determination of valacyclovir in pharmaceutical dosage. J. Liq. Chromatogr. Rel. Technol. 2005, 28, 751–762. [Google Scholar] [CrossRef]
- Al-Ghabeish, M.; Xu, X.; Krishnaiah, Y.S.; Rahman, Z.; Yang, Y.; Khan, M.A. Influence of drug loading and type of ointment base on the in vitro performance of acyclovir ophthalmic ointment. Int. J. Pharm. 2015, 495, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Volpato, N.M.; Santi, P.; Laureri, C.; Colombo, P. Assay of acyclovir in human skin layers by high-performance liquid chromatography. J. Pharm. Biomed. Anal. 1997, 16, 515–520. [Google Scholar] [CrossRef]
- Yañez, F.; Chianella, I.; Piletsky, S.A.; Concheiro, A.; Alvarez-Lorenzo, C. Computational modeling and molecular imprinting for the development of acrylic polymers with high affinity for bile salts. Anal. Chim. Acta 2010, 659, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Dorado, R.; Carro, A.M.; Chianella, I.; Karim, K.; Concheiro, A.; Lorenzo, R.A.; Piletsky, S.; Alvarez-Lorenzo, C. Oxytetracycline recovery from aqueous media using computationally designed molecularly imprinted polymers. Anal. Bioanal. Chem. 2016, 408, 6845–6856. [Google Scholar] [CrossRef] [Green Version]
- Eroglu, B.; Dalgakiran, D.; Inan, T.; Kurkcuoglu, O.; Güner, F.S. A computational and experimental approach to develop minocycline-imprinted hydrogels and determination of their drug delivery performances. J. Polym. Res. 2018, 25, 258. [Google Scholar] [CrossRef]
- Kiani, F.; Rostami, A.A.; Sharifi, S.; Bahadori, A.; Chaichi, M.J. Determination of acidic dissociation constants of glycine, valine, phenylalanine, glycylvaline, and glycylphenylalanine in water using ab initio methods. J. Chem. Eng. Data 2010, 55, 2732–2740. [Google Scholar] [CrossRef]
- Hiratani, H.; Mizutani, Y.; Alvarez-Lorenzo, C. Controlling drug release from imprinted hydrogels by modifying the characteristics of the imprinted cavities. Macromol. Biosci. 2005, 5, 728–733. [Google Scholar] [CrossRef]
- Rodriguez-Tenreiro, C.; Alvarez-Lorenzo, C.; Rodriguez-Perez, A.; Concheiro, A.; Torres-Labandeira, J.J. New cyclodextrin hydrogels cross-linked with diglycidylethers with a high drug loading and controlled release ability. Pharm. Res. 2006, 23, 121–130. [Google Scholar] [CrossRef]
- Brezani, V.; Lelakova, V.; Hassan, S.T.S.; Berchova-Bimova, K.; Novy, P.; Kloucek, P.; Marsik, P.; Dall’Acqua, S.; Hosek, J.; Smejkal, K. Anti-infectivity against Herpes Simplex Virus and selected microbes and anti-inflammatory activities of compounds isolated from Eucalyptus globulus Labill. Viruses 2018, 10, 360. [Google Scholar] [CrossRef] [Green Version]
- Mayo, M.; Cachafeiro-Andrade, N.; Alvarez-Lorenzo, C.; Martinez-Pacheco, R.; Concheiro, A. In situ photopolymerization-coated pellets for pH-dependent drug delivery. Eur. Polym. J. 2008, 44, 2629–2638. [Google Scholar] [CrossRef]
- Chou, L.Y.; Blanch, H.W.; Prausnitz, J.M.; Siegel, R.A. Buffer effects on aqueous swelling kinetics of polyelectrolyte gels. J. Appl. Polym. Sci. 1992, 45, 1411–1423. [Google Scholar] [CrossRef] [Green Version]
- González-Meijome, J.M.; Lira, M.; López-Alemany, A.; Almeida, J.B.; Parafita, M.A.; Refojo, M.F. Refractive index and equilibrium water content of conventional and silicone hydrogel contact lenses. Ophthal. Physiol. Opt. 2006, 26, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Derayea, S.M.; Mostafa, I.M.; Omar, M.A. Spectrofluorimetric and TLC-densitometric methods for a stability indicating assay of valacyclovir hydrochloride in the presence of its degradation product. RSC Adv. 2014, 4, 42308. [Google Scholar] [CrossRef]
- ICCVAM. ICCVAM-Recommended Test Method Protocol: Hen’s Egg Test—Chorioallantoic Membrane (HET-CAM) Test Method. 2010. Available online: https://ntp.niehs.nih.gov/iccvam/docs/protocols/ivocular-hetcam.pdf (accessed on 30 June 2020).
- Schrage, A.; Kolle, S.N.; Rey Moreno, M.C.; Norman, K.; Raabe, H.; Curren, R.; van Ravenzwaay, B.; Landsiedel, R. The bovine corneal opacity and permeability test in routine ocular irritation testing and its improvement within the limits of OECD Test Guideline 437. Altern. Lab. Anim. 2011, 39, 37–53. [Google Scholar] [CrossRef]
- Loch, C.; Zakelj, S.; Kristl, A.; Nagel, S.; Guthoff, R.; Weitschies, W.; Seidlitz, A. Determination of permeability coefficients of ophthalmic drugs through different layers of porcine, rabbit and bovine eyes. Eur. J. Pharm. Sci. 2012, 47, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Alambiaga-Caravaca, A.M.; Calatayud-Pascual, M.A.; Rodilla, V.; Concheiro, A.; López-Castellano, A.; Alvarez-Lorenzo, C. Micelles of progesterone for topical eye administration: Interspecies and intertissues differences in ex vivo ocular permeability. Pharmaceutics 2020, 12, 702. [Google Scholar] [CrossRef]




| Hydrogel | HEMA (mL) | EGDMA (µL) | MAA (mL) | ACV (mg) | VACV (mg) | AIBN (mg) |
|---|---|---|---|---|---|---|
| NIP | 5 | 7.55 | 0 | 0 | 0 | 8.21 |
| NIP200 | 5 | 7.55 | 0.084 | 0 | 0 | 8.21 |
| MIPACV | 5 | 7.55 | 0 | 45 | 0 | 8.21 |
| MIPA1:5 | 5 | 7.55 | 0.084 | 45 | 0 | 8.21 |
| MIPA1:10 | 5 | 7.55 | 0.084 | 23 | 0 | 8.21 |
| MIPA1:15 | 5 | 7.55 | 0.084 | 15 | 0 | 8.21 |
| MIPVACV | 5 | 7.55 | 0 | 0 | 25 | 8.21 |
| MIPV1:6 | 5 | 7.55 | 0.084 | 0 | 50 | 8.21 |
| MIPV1:12 | 5 | 7.55 | 0.084 | 0 | 25 | 8.21 |
| MIPV1:32 | 5 | 7.55 | 0.084 | 0 | 10 | 8.21 |
| Hydrogel | Young’s Modulus (MPa) |
|---|---|
| NIP | 0.547 |
| NIP200 | 0.358 |
| MIPVACV | 0.536 |
| MIPV1:6 | 0.623 |
| MIPV1:12 | 0.538 |
| MIPV1:32 | 0.548 |
| Formulation | Bovine Sclera | Porcine Sclera | ||||
|---|---|---|---|---|---|---|
| J (μg/(cm2·h)) | Papp (× 106) (cm/s) | VACV Accumulated (μg/cm2) | J (μg/(cm2·h)) | Papp (× 106) (cm/s) | VACV Accumulated (μg/cm2) | |
| VACV sol. | 2.28 (0.35) | 8.79 (1.37) | 0.58 (0.41) | 4.09 (0.72) | 13.82 (2.46) | 1.13 (0.51) |
| NIP | 0.27 (0.06) | 8.78 (2.13) | n.d. | 0.42 (0.32) | 9.75 (7.70) | 0.12 (0.13) |
| MIPV1:12 | 2.40 (1.15) | 12.91 (6.23) | 0.17 (0.02) | 4.23 (1.74) | 11.37 (4.67) | 1.40 (0.42) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varela-Garcia, A.; Gomez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C. Imprinted Contact Lenses for Ocular Administration of Antiviral Drugs. Polymers 2020, 12, 2026. https://doi.org/10.3390/polym12092026
Varela-Garcia A, Gomez-Amoza JL, Concheiro A, Alvarez-Lorenzo C. Imprinted Contact Lenses for Ocular Administration of Antiviral Drugs. Polymers. 2020; 12(9):2026. https://doi.org/10.3390/polym12092026
Chicago/Turabian StyleVarela-Garcia, Angela, José Luis Gomez-Amoza, Angel Concheiro, and Carmen Alvarez-Lorenzo. 2020. "Imprinted Contact Lenses for Ocular Administration of Antiviral Drugs" Polymers 12, no. 9: 2026. https://doi.org/10.3390/polym12092026
APA StyleVarela-Garcia, A., Gomez-Amoza, J. L., Concheiro, A., & Alvarez-Lorenzo, C. (2020). Imprinted Contact Lenses for Ocular Administration of Antiviral Drugs. Polymers, 12(9), 2026. https://doi.org/10.3390/polym12092026







