The Thermal Properties and Degradability of Chiral Polyester-Imides Based on Several l/d-Amino Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Characterization
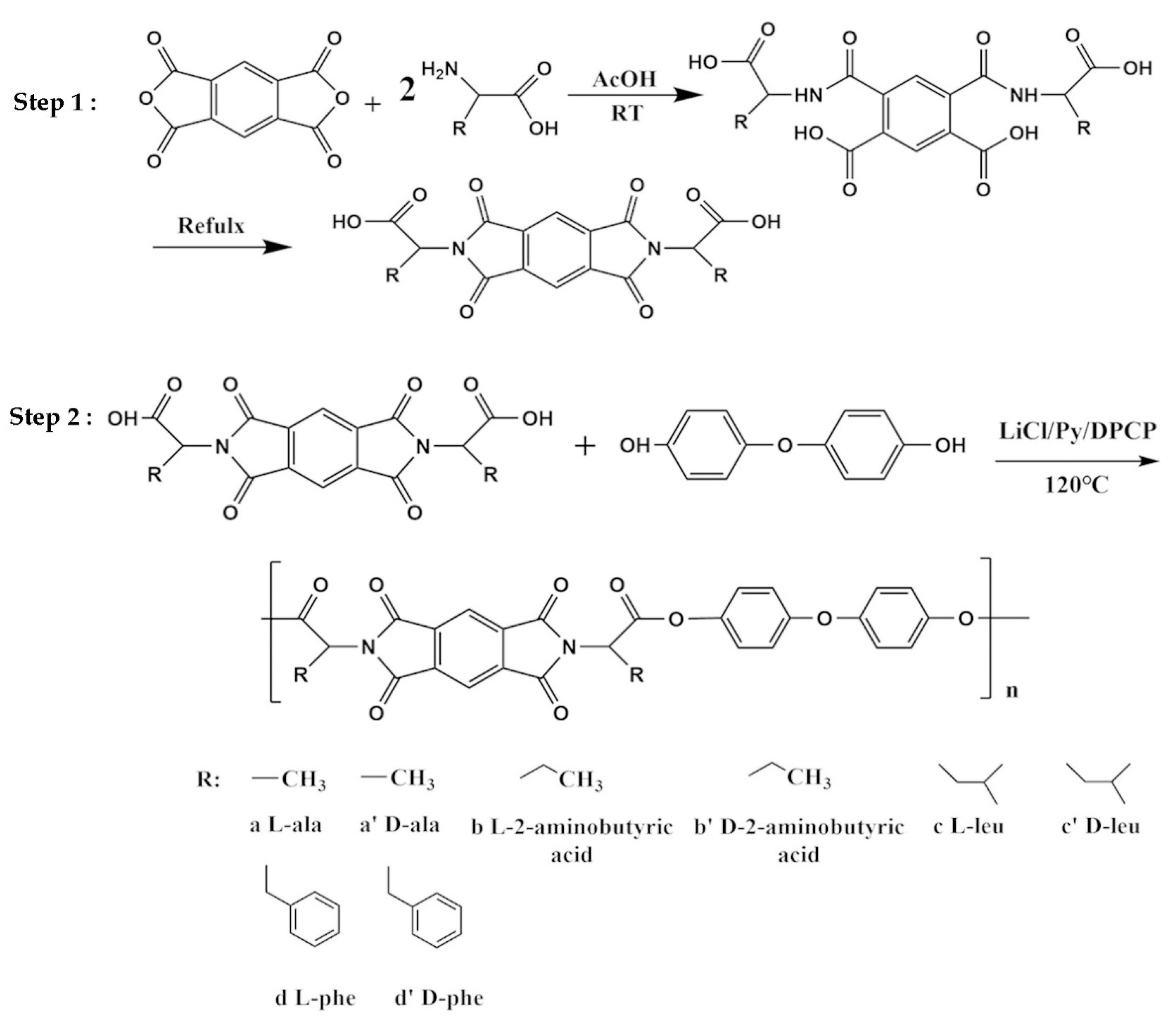
2.3. Synthesis of Chiral Diacid Monomers
2.4. Synthesis of Chiral Polyester-Imides (PEI3a–PEI3d′)
2.5. Preparation of PEI Films
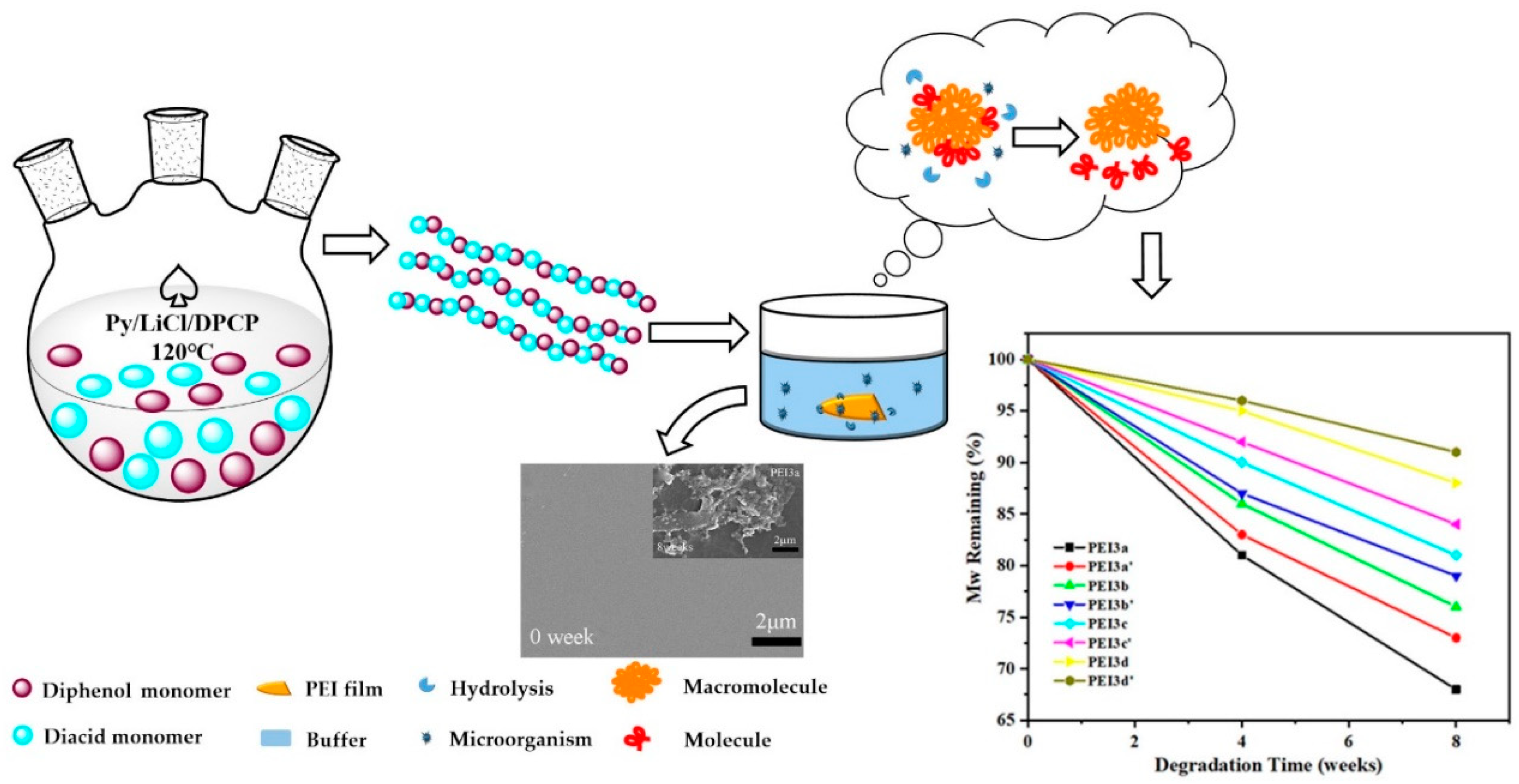
2.6. Degradation of PEI Films in Buffer
3. Results and Discussion
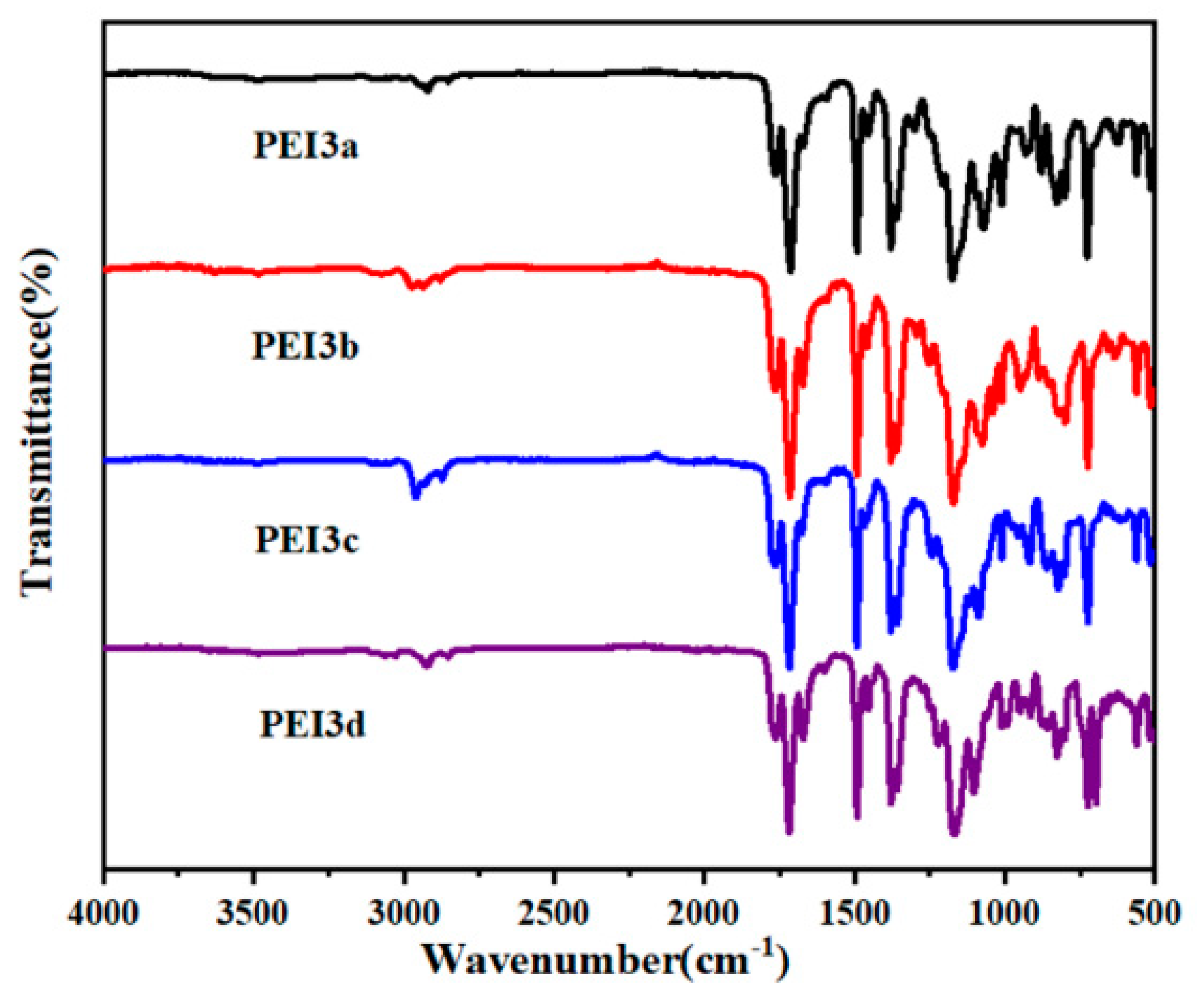
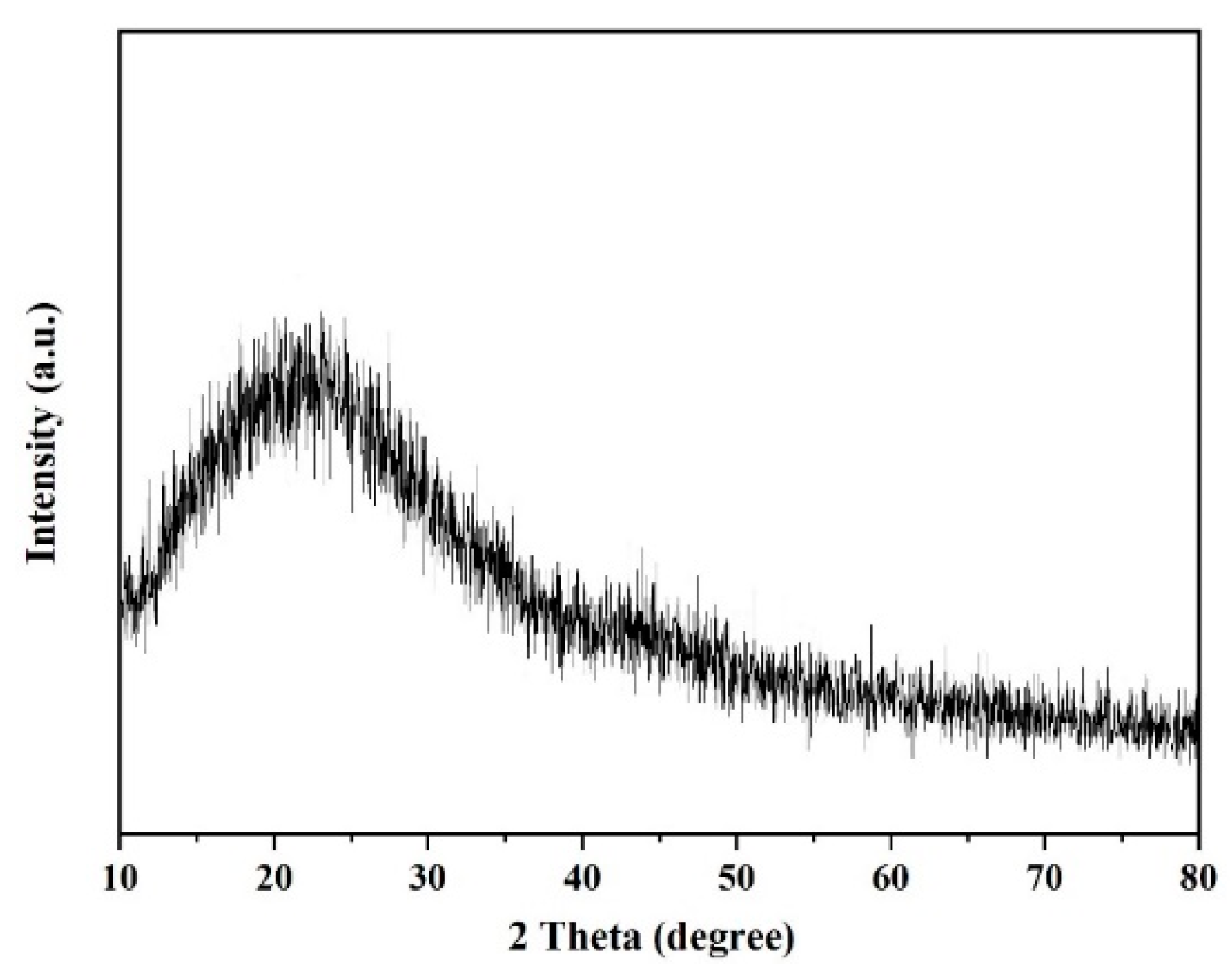
3.1. Characterization on Polymers
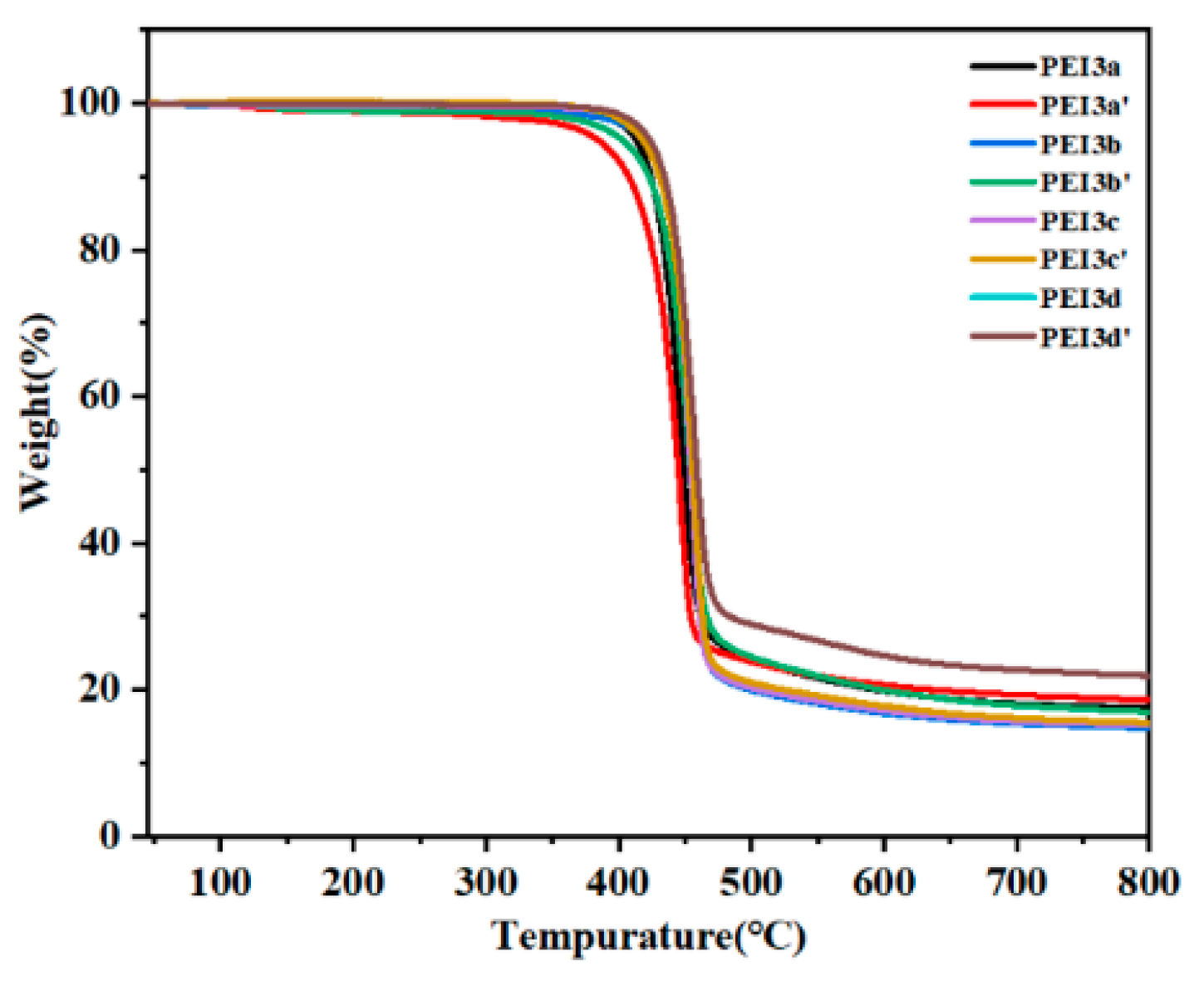
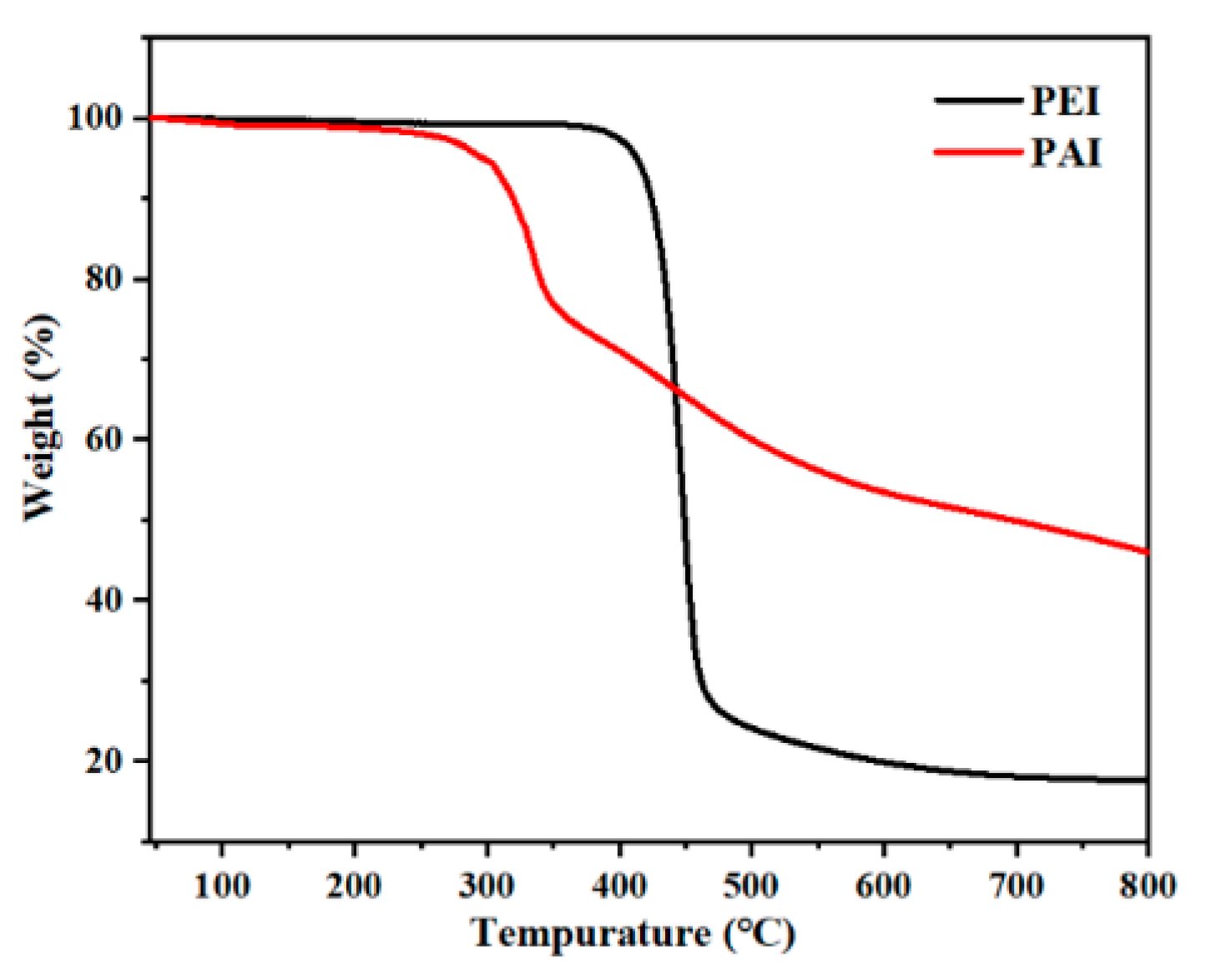
3.2. Thermal Properties
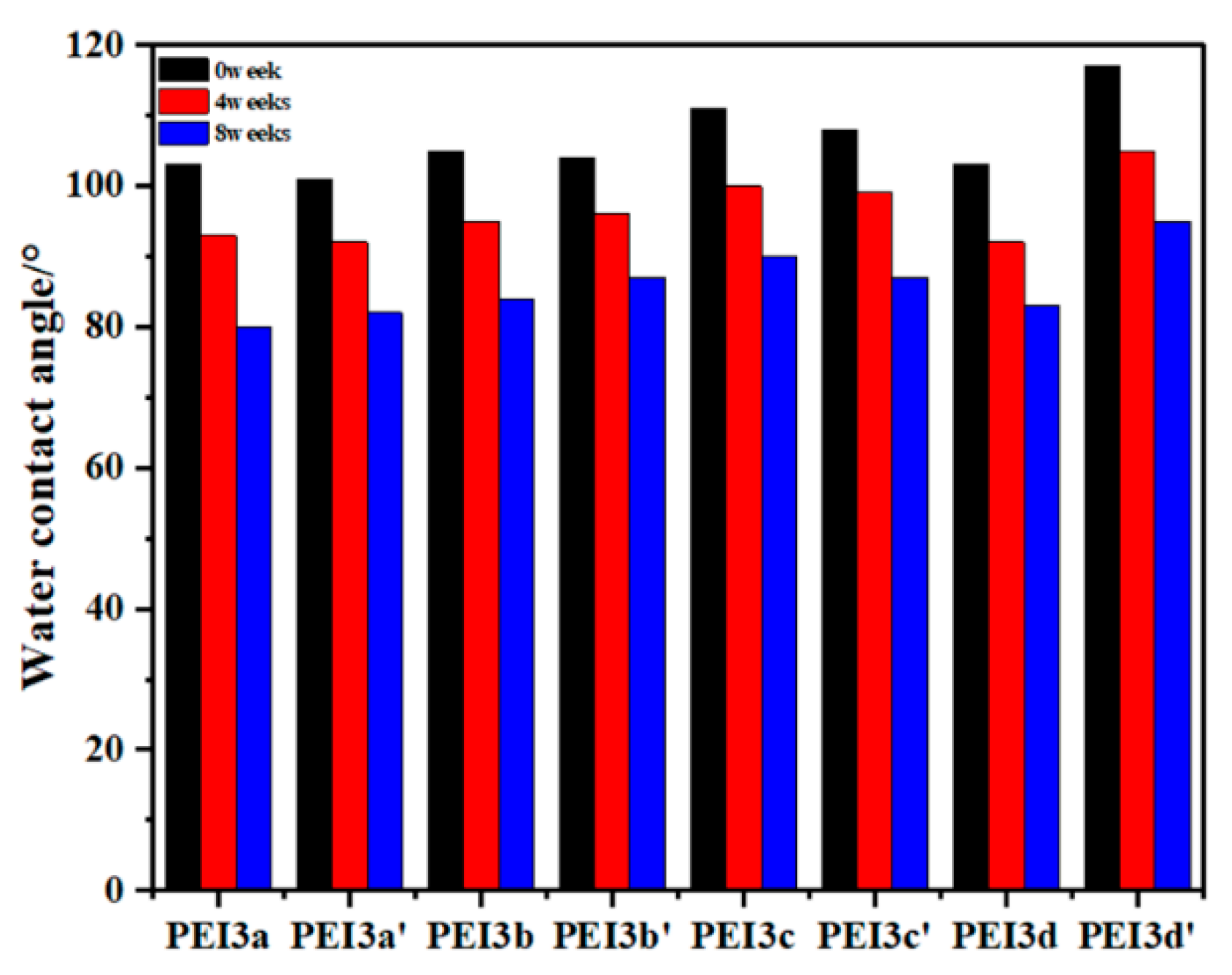
3.3. Solubility
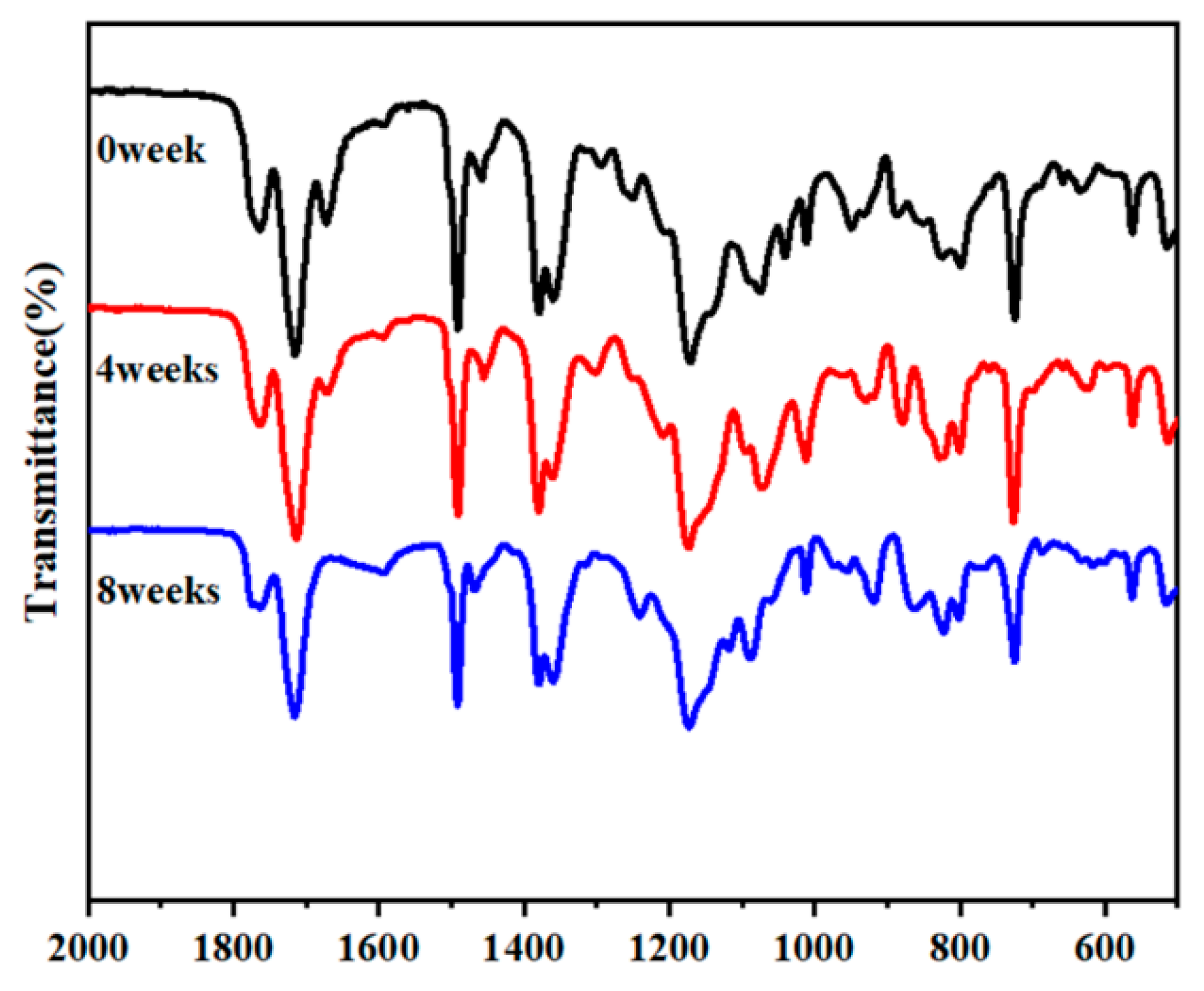
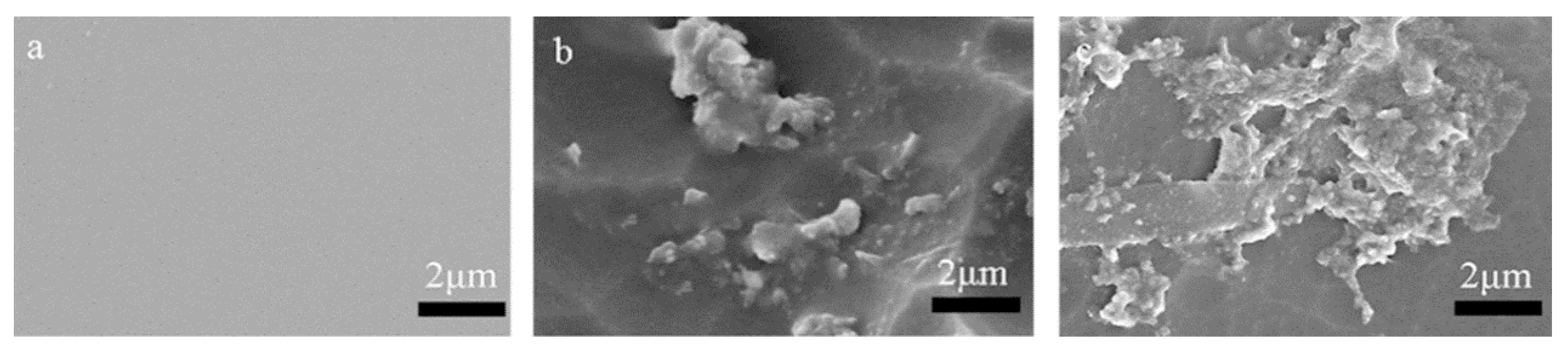
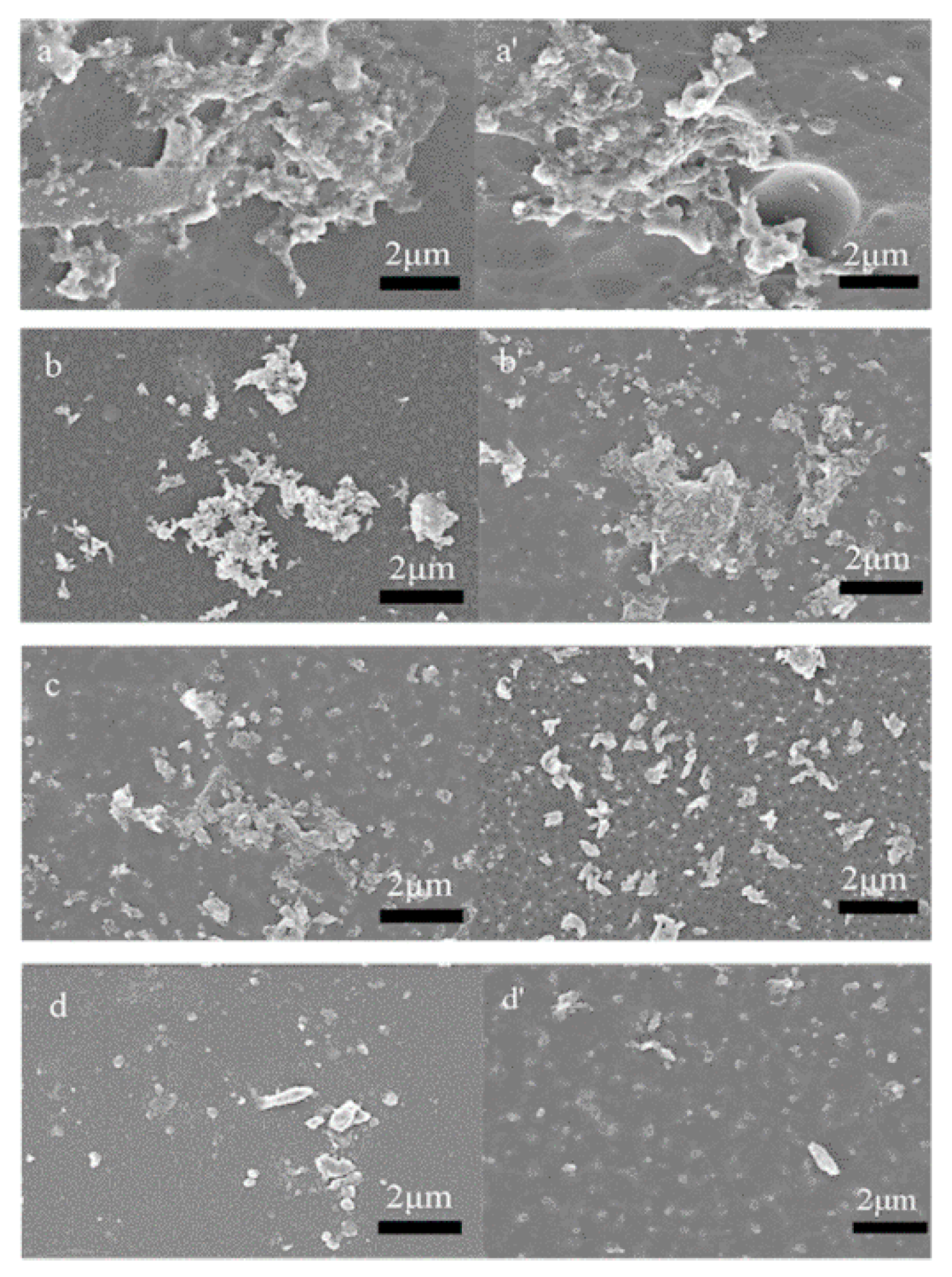
3.4. Degradability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Baillargeon, A.L.; Mequanint, K. Biodegradable Polyphosphazene Biomaterials for Tissue Engineering and Delivery of Therapeutics. Biomed Res. Int. 2014, 2014, 16. [Google Scholar] [CrossRef] [PubMed]
- Li, C.T.; Zhang, M.; Qin, J.X.; Zhang, Y.; Qiu, J.H. Study on molecular modeling and the difference of PC lipase-catalyzed degradation of poly (butylene succinate) copolymers modified by linear monomers. Polym. Degrad. Stabil. 2015, 116, 75–80. [Google Scholar] [CrossRef]
- Mallakpour, S.; Banihassan, K.; Sabzalian, M.R. Novel Bioactive Chiral Poly(amide–imide)s Containing Different Amino Acids Linkages: Studies on Synthesis, Characterization and Biodegradability. J. Polym. Environ. 2013, 21, 568–574. [Google Scholar] [CrossRef]
- Wang, W.S.; Guo, Y.L.; Otaigbe, J.U. Synthesis and characterization of novel biodegradable and biocompatible poly(ester-urethane) thin films prepared by homogeneous solution polymerization. Polymer 2008, 49, 4393–4398. [Google Scholar] [CrossRef]
- Jang, J.-C.; Shin, P.-K.; Yoon, J.-S.; Lee, I.-M.; Lee, H.-S.; Kim, M.-N. Glucose effect on the biodegradation of plastics by compost from food garbage. Polym. Degrad. Stabil. 2002, 76, 155–159. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Medeiros Garcia Alcântara, J.; Distante, F.; Storti, G.; Moscatelli, D.; Morbidelli, M.; Sponchioni, M. Current trends in the production of biodegradable bioplastics: The case of polyhydroxyalkanoates. Biotechnol. Adv. 2020, 42, 107582. [Google Scholar] [CrossRef]
- Wu, T.T.; Ding, M.Z.; Shi, C.P.; Qiao, Y.Q.; Wang, P.P.; Qiao, R.R.; Wang, X.C.; Zhong, J. Resorbable polymer electrospun nanofibers: History, shapes and application for tissue engineering. Chin. Chem. Lett. 2020, 31, 617–625. [Google Scholar] [CrossRef]
- Ma, W.J.; Ding, Y.C.; Zhang, M.J.; Gao, S.T.; Li, Y.S.; Huang, C.B.; Fu, G.D. Nature-inspired chemistry toward hierarchical superhydrophobic, antibacterial and biocompatible nanofibrous membranes for effective UV-shielding, self-cleaning and oil-water separation. J. Hazard. Mater. 2020, 384, 13. [Google Scholar] [CrossRef]
- Yang, C.Y.; Xu, W.X.; Nan, Y.; Wang, Y.G.; Hu, Y.X.; Gao, C.J.; Chen, X.H. Fabrication and characterization of a high performance polyimide ultrafiltration membrane for dye removal. J. Colloid Interface Sci. 2020, 562, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Dodda, J.M.; Belsky, P. Progress in designing poly(amide imide)s (PAI) in terms of chemical structure, preparation methods and processability. Eur. Polym. J. 2016, 84, 514–537. [Google Scholar] [CrossRef]
- Garcia, J.M.; Garcia, F.C.; Serna, F.; de la Pena, J.L. High-performance aromatic polyamides. Prog. Polym. Sci. 2010, 35, 623–686. [Google Scholar] [CrossRef]
- Zomorodian, A.; Garcia, M.P.; Silva, T.M.E.; Fernandes, J.C.S.; Fernandes, M.H.; Montemor, M.F. Corrosion resistance of a composite polymeric coating applied on biodegradable AZ31 magnesium alloy. Acta Biomater. 2013, 9, 8660–8670. [Google Scholar] [CrossRef]
- Nampoothiri, K.M.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Preziosi, R.; Robson, G.D. Abiotic and biotic environmental degradation of the bioplastic polymer poly(lactic acid): A review. Polym. Degrad. Stabil. 2017, 137, 122–130. [Google Scholar] [CrossRef] [Green Version]
- Mallakpour, S.; Soltanian, S. A facile approach towards functionalization of MWCNTs with vitamin B2 for reinforcing of biodegradable and chiral poly(ester-imide) having l-phenylalanine linkages: Morphological and thermal investigations. J. Polym. Res. 2015, 22, 9. [Google Scholar] [CrossRef]
- Mallakpour, S.; Soltanian, S. Environmentally friendly functionalization of multiwalled carbon nanotube using ascorbic acid and efficient dispersion in chiral poly(ester-imide) containing 4,4′-thiobis(2-tert-butyl-5-methylphenol) moiety: Thermal and morphological studies. Colloid Polym. Sci. 2015, 293, 1141–1149. [Google Scholar] [CrossRef]
- Li, X.R.; Su, Y.L.; Chen, Q.Y.; Lin, Y.; Tong, Y.J.; Li, Y.S. Synthesis and characterization of biodegradable hyperbranched poly(ester-amide)s based on natural material. Biomacromolecules 2005, 6, 3181–3188. [Google Scholar] [CrossRef]
- Li, P.; He, F.Y.; Yang, Z.Z.; Yang, W.K.; Yao, J.S. The degradability and thermal properties of chiral polyamide-imides synthesized from several l-amino acids: Side group effects. Polym. Degrad. Stabil. 2018, 147, 267–273. [Google Scholar] [CrossRef]
- Wu, Q.X.; Yang, Z.Z.; Yao, J.S.; Yu, D.H. Synthesis and biodegradation studies of optically active poly(amide-imide)s based on N,N′-(pyromellitoyl)-bis-l-amino acid. High Perform. Polym. 2016, 28, 34–46. [Google Scholar] [CrossRef]
- Liu, W.P.; He, F.Y.; Yang, W.K.; Yang, Z.Z.; Yao, J.S.; Zhao, H. Study on the Thermal Properties and Enzymatic Degradability of Chiral Polyamide-Imides Films Based on Amino Acids. Appl. Sci. Basel 2019, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Mallakpour, S.; Tirgir, F.; Sabzalian, M.R. Synthesis, characterization and in vitro antimicrobial and biodegradability study of pseudo-poly(amino acid)s derived from N,N′-(pyromellitoyl)-bis-l-tyrosine dimethyl ester as a chiral bioactive diphenolic monomer. Amino Acids 2011, 40, 611–621. [Google Scholar] [CrossRef]
- Mallakpour, S.; Dinari, M. Progress in Synthetic Polymers Based on Natural Amino Acids. J. Macromol. Sci. Part A Pure Appl. Chem. 2011, 48, 644–679. [Google Scholar] [CrossRef]
- Guan, Q.B.; Norder, B.; Dingemans, T.J. Flexible all-aromatic polyesterimide films with high glass transition temperatures. J. Appl. Polym. Sci. 2017, 134, 14. [Google Scholar] [CrossRef]
- Guan, Q.B.; Norder, B.; Chug, L.Y.; Besseling, N.A.M.; Picken, S.J.; Dingemans, T.J. All-Aromatic (AB)(n)-Multiblock Copolymers via Simple One-Step Melt Condensation Chemistry. Macromolecules 2016, 49, 8549–8562. [Google Scholar] [CrossRef]
- Huang, T.; Guan, Q.B.; Yuan, L.; Liang, G.Z.; Gu, A.J. Facilely Synthesizing Ethynyl Terminated All-Aromatic Liquid Crystalline Poly(esterimide)s with Good Processability and Thermal Resistance under Medium-Low Temperature via Direct Esterification. Ind. Eng. Chem. Res. 2018, 57, 7090–7098. [Google Scholar] [CrossRef]
- Lin, R.H.; Wang, W.M.; Chen, Y.H.; Ho, T.H. Preparation and characterization of biodegradable condensation polyimide. Polym. Degrad. Stabil. 2012, 97, 1534–1544. [Google Scholar] [CrossRef]
- Kuhire, S.S.; Sharma, P.; Chakrabarty, S.; Wadgaonkar, P.P. Partially Bio-based Poly(amide imide)s by Polycondensation of Aromatic Diacylhydrazides Based on Lignin-Derived Phenolic Acids and Aromatic Dianhydrides: Synthesis, Characterization, and Computational Studies. J. Polym. Sci. Pol. Chem. 2017, 55, 3636–3645. [Google Scholar] [CrossRef]
- Arnold, F.; Bruno, K.; Shen, D.; Eashoo, M.; Lee, C.; Harris, F.; Cheng, S. Origin of β relaxations in segmented rigid-rod polyimide and copolyimide films. Polym. Eng. Sci. 1993, 33, 1373–1380. [Google Scholar] [CrossRef]
- Xiao, W.M.; Zhang, Y.; Cheng, K.L.; Liu, S.W.; Chi, Z.G.; Xu, J.R. Structure and property studies of liquid crystalline poly(ester imide)/pet blends. Acta Polym. Sin. 2011, 4, 402–408. [Google Scholar] [CrossRef]
- Zhuang, Y.B.; Gu, Y. Poly(benzoxazole-amide-imide) copolymers for interlevel dielectrics: Interchain hydrogen bonding, molecular arrangement and properties. J. Polym. Res. 2013, 20, 8. [Google Scholar] [CrossRef]
- Mays, J.W.; Siakali-Kioulafa, E.; Hadjichristidis, N. Glass transition temperatures of polymethacrylates with alicyclic side groups. Macromolecules 1990, 23, 3530–3531. [Google Scholar] [CrossRef]
- Tagle, L.H.; Terraza, C.A.; Tundidor-Camba, A.; Coll, D. Silicon-containing halogenated poly(amides) derived from diacids with aminoacidic and imidic moieties in the side chain: Synthesis, characterization, optical studies and thermal properties. Polym. Bull. 2017, 74, 263–281. [Google Scholar] [CrossRef]
- Tagle, L.H.; Terraza, C.A.; Leiva, A.; Coll, D. Silicon-containing oligomeric poly(amides) with phthalimidyl-amide side groups: Synthesis, characterization and thermal studies. High Perform. Polym. 2012, 24, 77–84. [Google Scholar] [CrossRef]
- Tagle, L.H.; Terraza, C.A.; Tundidor-Camba, A.; Ortiz, P.A. Silicon-containing oligomeric poly(imido-amides) with amino moieties. Synthesis, characterization and thermal studies. RSC Adv. 2014, 4, 30197–30210. [Google Scholar] [CrossRef]
- Feng, J.H.; He, F.Y.; Yang, Z.Z.; Yao, J.S. Differential study of the biological degradation of polyamide-imides based on the amino acids. Polym. Degrad. Stabil. 2016, 129, 231–238. [Google Scholar] [CrossRef]
- Jia, X.; Ma, Z.Y.; Zhang, G.X.; Hu, J.M.; Liu, Z.Y.; Wang, H.Y.; Zhou, F. Polydopamine Film Coated Controlled-Release Multielement Compound Fertilizer Based on Mussel-Inspired Chemistry. J. Agric. Food Chem. 2013, 61, 2919–2924. [Google Scholar] [CrossRef]
- Kumar, A.; Capua, E.; Kesharwani, M.K.; Martin, J.M.L.; Sitbon, E.; Waldeck, D.H.; Naaman, R. Chirality-induced spin polarization places symmetry constraints on biomolecular interactions. Proc. Natl. Acad. Sci. USA 2017, 114, 2474–2478. [Google Scholar] [CrossRef] [Green Version]
- Dehaut, A.; Cassone, A.L.; Frere, L.; Hermabessiere, L.; Himber, C.; Rinnert, E.; Riviere, G.; Lambert, C.; Soudant, P.; Huvet, A.; et al. Microplastics in seafood: Benchmark protocol for their extraction and characterization. Environ. Pollut. 2016, 215, 223–233. [Google Scholar] [CrossRef] [Green Version]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Diacids (M) | Yield (%) | Melting Points (°C) | Formula (Mw) | Elemental Analysis (%) | ||||
|---|---|---|---|---|---|---|---|---|
| C | H | N | ||||||
| 2a | 96 | 274 | −60.20 | C16H12N2O8 (360.28) | Calcd | 53.29 | 3.33 | 7.78 |
| Found | 53.22 | 3.29 | 7.74 | |||||
| 2a′ | 95 | 272 | 68.85 | C16H12N2O8 (360.28) | Calcd | 53.29 | 3.33 | 7.78 |
| Found | 53.27 | 3.32 | 7.76 | |||||
| 2b | 93 | 231 | −60.02 | C18H16N2O8 (388.33) | Calcd | 55.62 | 4.12 | 7.21 |
| Found | 55.66 | 4.20 | 7.28 | |||||
| 2b′ | 89 | 230 | 61.71 | C18H16N2O8 (388.33) | Calcd | 55.62 | 4.12 | 7.21 |
| Found | 55.57 | 4.08 | 7.20 | |||||
| 2c | 97 | 288 | −52.78 | C22H24N2O8 (444.48) | Calcd | 59.46 | 5.41 | 6.31 |
| Found | 59.50 | 5.42 | 6.33 | |||||
| 2c′ | 94 | 286 | 52.70 | C22H24N2O8 (444.48) | Calcd | 59.46 | 5.41 | 6.31 |
| Found | 59.44 | 5.43 | 6.28 | |||||
| 2d | 92 | 290 | −176.41 | C28H20N2O8 (512.47) | Calcd | 65.56 | 3.90 | 5.46 |
| Found | 65.62 | 3.95 | 5.50 | |||||
| 2d′ | 91 | 290 | 169.89 | C28H20N2O8 (512.47) | Calcd | 65.56 | 3.90 | 5.46 |
| Found | 65.61 | 3.87 | 5.42 | |||||
| PEIs (P) | Yield (%) | Mw × 104 (g/mol) | Formula (Mw) | Elemental Analysis (%) | ||||
|---|---|---|---|---|---|---|---|---|
| C | H | N | ||||||
| PEI3a | 94 | 5.423 | −13.53 | (C28H18N2O9)n (556.53)n | Calcd | 60.04 | 3.23 | 5.03 |
| Found | 59.77 | 3.20 | 4.96 | |||||
| PEI3a′ | 91 | 5.278 | 14.85 | (C28H18N2O9)n (556.53)n | Calcd | 60.04 | 3.23 | 5.03 |
| Found | 59.73 | 3.15 | 4.92 | |||||
| PEI3b | 87 | 4.908 | −10.02 | (C30H22N2O9)n (584.58)n | Calcd | 61.64 | 3.76 | 4.79 |
| Found | 60.99 | 3.73 | 4.75 | |||||
| PEI3b′ | 89 | 4.762 | 12.21 | (C30H22N2O9)n (584.58)n | Calcd | 61.64 | 3.76 | 4.79 |
| Found | 69.97 | 3.70 | 4.73 | |||||
| PEI3c | 93 | 5.953 | −15.45 | (C34H30N2O9)n (610.45)n | Calcd | 66.88 | 4.91 | 4.59 |
| Found | 66.77 | 4.94 | 4.53 | |||||
| PEI3c′ | 90 | 5.087 | 12.45 | (C34H30N2O9)n (610.45)n | Calcd | 66.88 | 4.91 | 4.59 |
| Found | 66.85 | 4.89 | 4.55 | |||||
| PEI3d′ | 87 | 6.712 | −25.12 | (C40H26N2O9)n (708.72)n | Calcd | 67.79 | 3.67 | 3.95 |
| Found | 67.74 | 3.65 | 3.88 | |||||
| PEI3d′ | 92 | 6.458 | 28.27 | (C40H26N2O9)n (708.72)n | Calcd | 67.79 | 3.67 | 3.95 |
| Found | 67.77 | 3.64 | 3.93 | |||||
| Polymer | E′ (MPa) a × 103 | Tg (°C) | T5 (°C) b | T10 (°C) c | Char Yield (%) d |
|---|---|---|---|---|---|
| PEI3a | 2.389 | 225 | 413 | 424 | 17.56 |
| PEI3a′ | 1.565 | 223 | 384 | 407 | 18.66 |
| PEI3b | 2.652 | 220 | 418 | 431 | 14.88 |
| PEI3b′ | 1.520 | 219 | 402 | 422 | 17.03 |
| PEI3c | 2.148 | 215 | 421 | 433 | 15.21 |
| PEI3c′ | 1.975 | 216 | 417 | 430 | 15.53 |
| PEI3d | 2.051 | 197 | 424 | 436 | 17.55 |
| PEI3d′ | 2.034 | 193 | 423 | 434 | 21.97 |
| Solvent | DMSO | DMF | DMAc | NMP | THF | MeOH | EtOH |
|---|---|---|---|---|---|---|---|
| PEI3a | + | ++ | ++ | ++ | ++ | − | − |
| PEI3a′ | + | ++ | ++ | ++ | ++ | − | − |
| PEI3b | + | ++ | ++ | ++ | ++ | − | − |
| PEI3b′ | + | ++ | ++ | ++ | ++ | − | − |
| PEI3c | + | ++ | ++ | ++ | ++ | − | − |
| PEI3c′ | + | ++ | ++ | ++ | ++ | − | − |
| PEI3d | + | ++ | ++ | ++ | ++ | − | − |
| PEI3d′ | + | ++ | ++ | ++ | ++ | − | − |
| PAI | +− | + | + | + | − | − | − |
| Degradation Time | PEI3a | PEI3a′ | PEI3b | PEI3b′ | PEI3c | PEI3c′ | PEI3d | PEI3d′ |
|---|---|---|---|---|---|---|---|---|
| 0 week | 5.423 | 5.278 | 4.908 | 4.762 | 5.953 | 5.087 | 6.712 | 6.458 |
| 4 weeks | 4.392 | 4.381 | 4.221 | 4.142 | 5.357 | 4.680 | 6.376 | 6.199 |
| 8 weeks | 3.687 | 3.852 | 3.730 | 3.762 | 4.822 | 4.273 | 5.906 | 5.876 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, C.; Yang, W.; He, F.; Yao, J. The Thermal Properties and Degradability of Chiral Polyester-Imides Based on Several l/d-Amino Acids. Polymers 2020, 12, 2053. https://doi.org/10.3390/polym12092053
Qi C, Yang W, He F, Yao J. The Thermal Properties and Degradability of Chiral Polyester-Imides Based on Several l/d-Amino Acids. Polymers. 2020; 12(9):2053. https://doi.org/10.3390/polym12092053
Chicago/Turabian StyleQi, Chen, Wenke Yang, Fuyan He, and Jinshui Yao. 2020. "The Thermal Properties and Degradability of Chiral Polyester-Imides Based on Several l/d-Amino Acids" Polymers 12, no. 9: 2053. https://doi.org/10.3390/polym12092053




