Mechanical and Optical Properties of Thermochromic Reversible Waterborne Primer Film on Tilia europaea with 1,2-Benzo-6-diethylaminofluorane Based Microcapsules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Fabrication of Coatings
2.3. Testing and Characterization
2.3.1. Temperature Test
2.3.2. The Hardness Test
2.3.3. The Adhesion Test
2.3.4. The Impact Resistance Test
2.3.5. The Liquid Resistance Test
2.3.6. Microstructure Test
2.3.7. Infrared Spectrum Test
3. Results and Discussion
3.1. Analysis of Morphology and Properties of Thermochromic Microcapsules
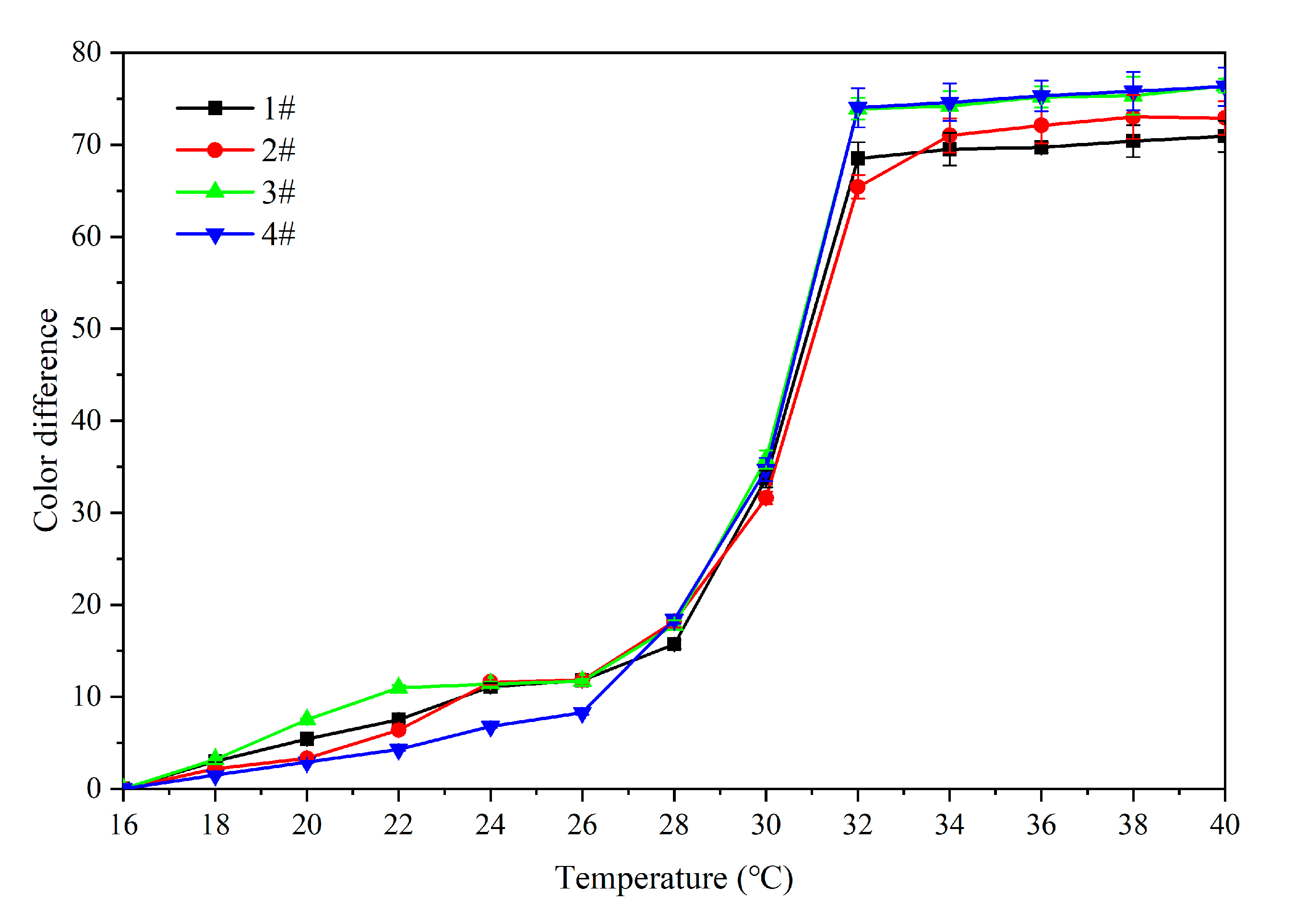
3.2. Orthogonal Experiment Analysis
3.3. Optimization of Experimental Analysis
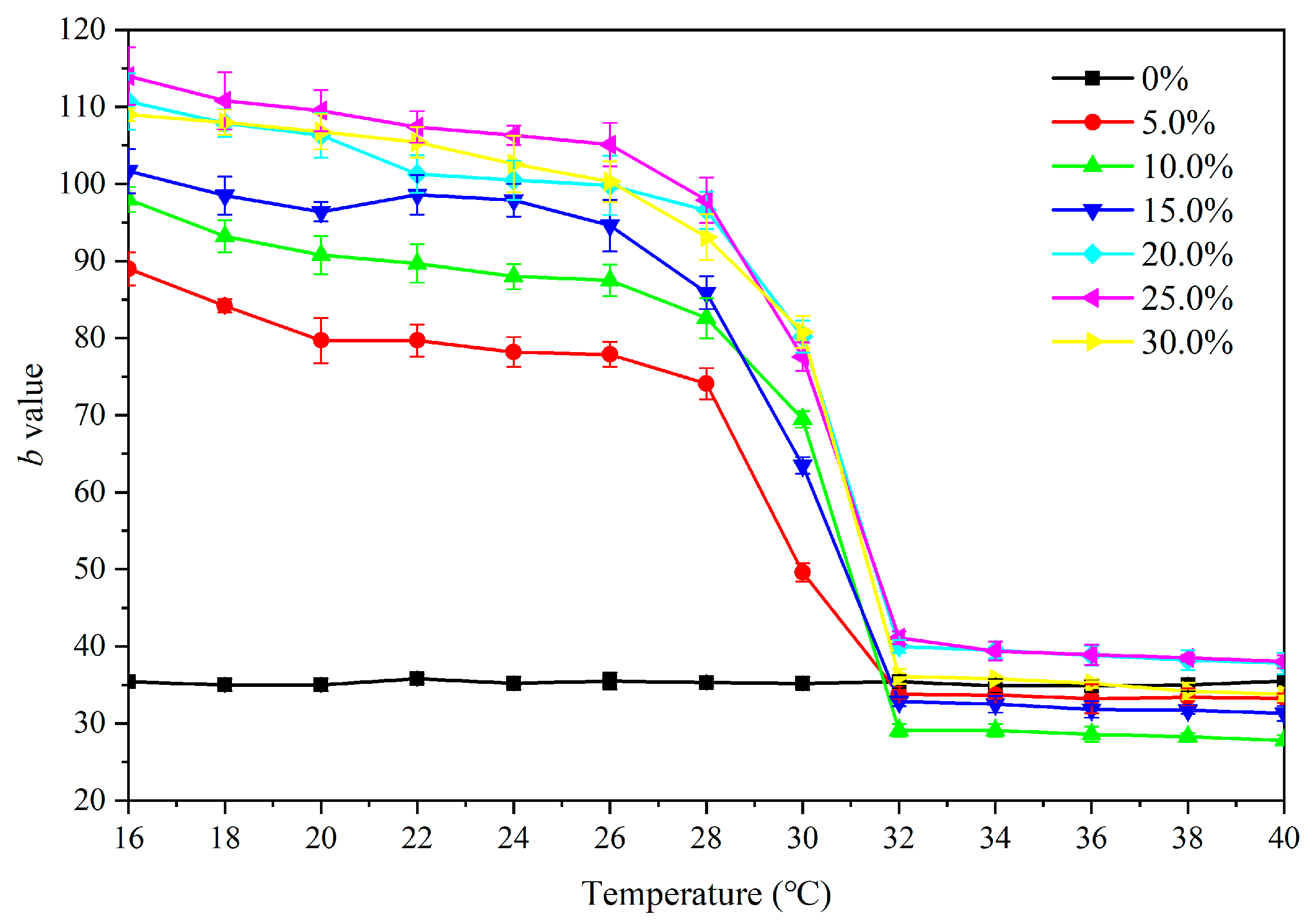
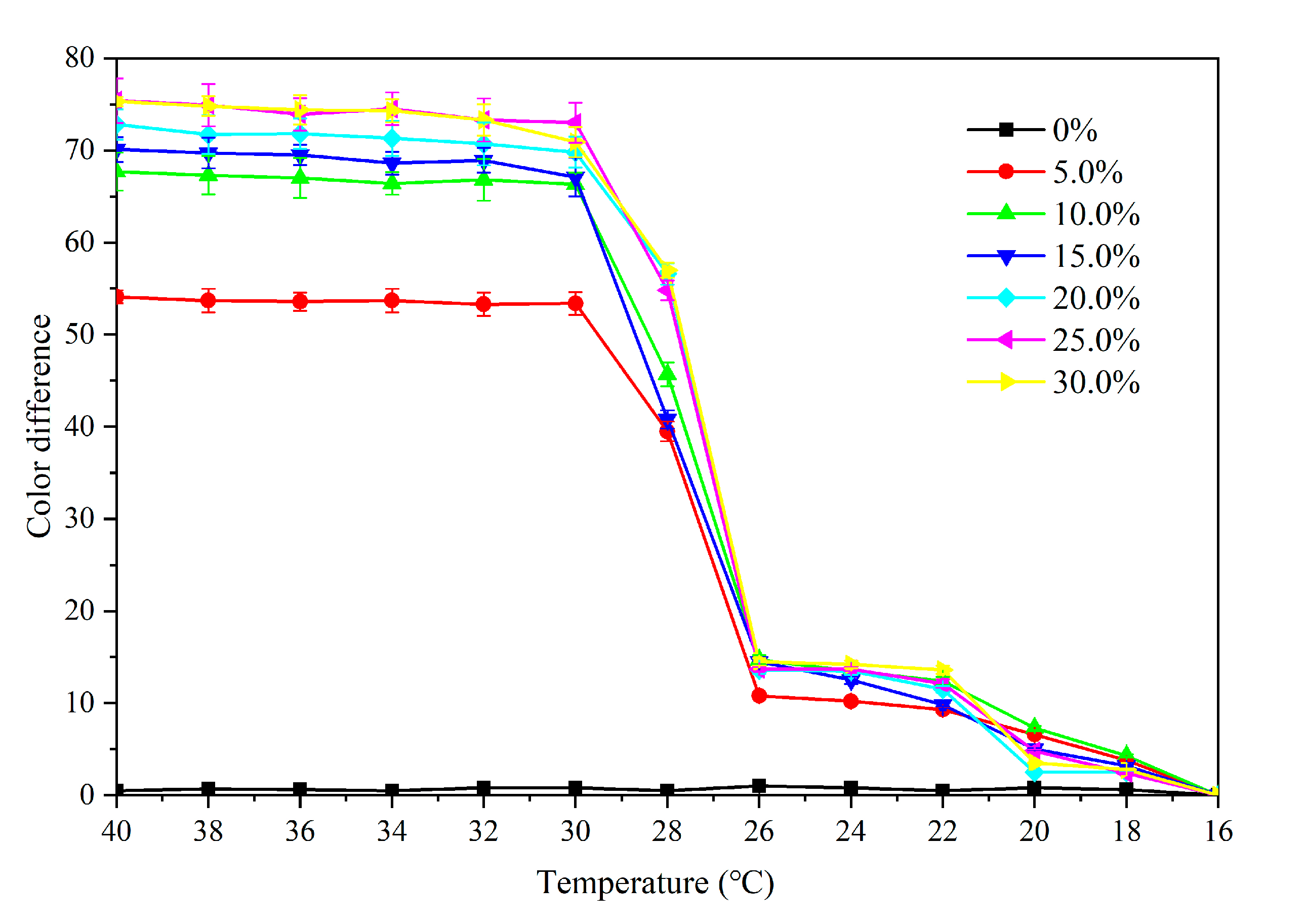
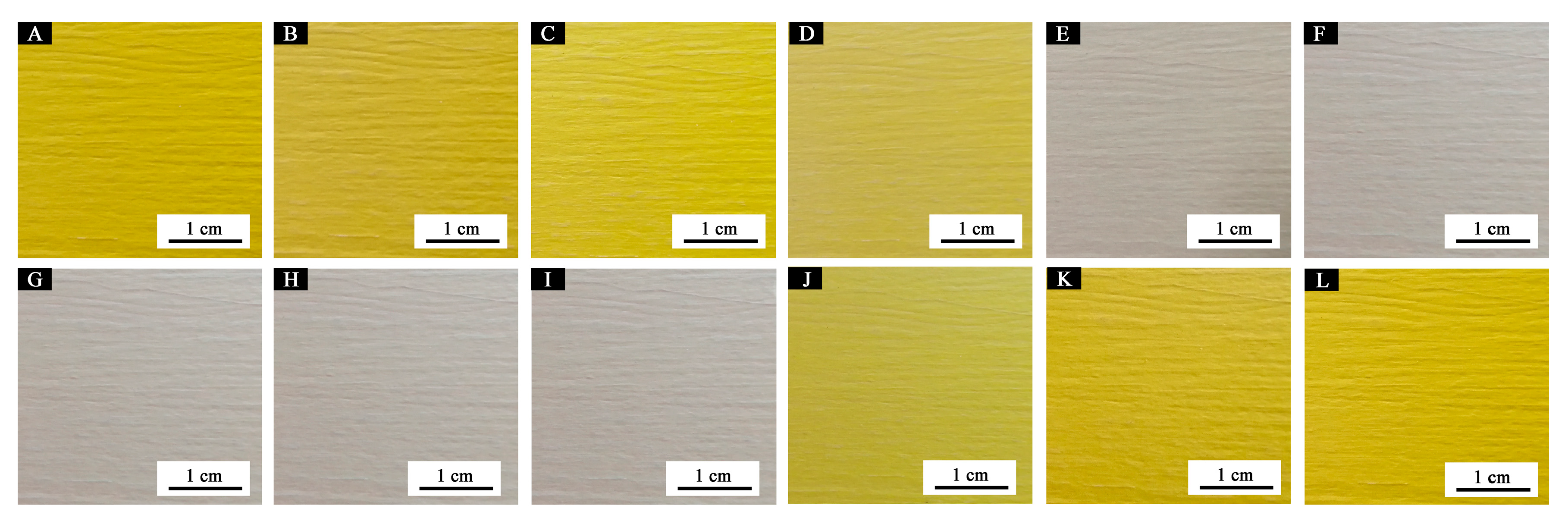
3.3.1. Effect of Microcapsule Concentration on Color Difference
3.3.2. Effect of Microcapsule Concentration on Gloss
3.3.3. Effect of Microcapsule Concentration on Mechanical Properties
3.3.4. Effect of Microcapsule Concentration on Liquid Resistance
3.3.5. Microstructure and Infrared Analysis of Films
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Teng, M.-J.; Wei, Y.-S.; Hu, T.-G.; Zhang, Y.; Feng, K.; Zong, M.-H.; Wu, H. Citric acid cross-linked zein microcapsule as an efficient intestine-specific oral delivery system for lipophilic bioactive compound. J. Food Eng. 2020, 281, 109993. [Google Scholar] [CrossRef]
- Cai, C.-W.; Ouyang, X.; Zhou, L.; Liu, G.-J.; Wang, Y.; Zhu, G.-C.; Yao, J.-M.; Militky, J.; Venkataraman, M.; Zhang, G.-Q. Co-solvent free interfacial polycondensation and properties of polyurea PCM microcapsules with dodecanol dodecanoate as core material. Sol. Energy 2020, 199, 721–730. [Google Scholar] [CrossRef]
- Du, F.-Y.; Wu, Y.-C.; Du, F.-T.; Zhang, L.-R.; Feng, W.-W.; Zhao, L.-L.; Cai, R.; Xu, L.-X.; Bian, G.-R.; Li, J.-G.; et al. Construction of catechol-grafted chitosan alginate/barium sulfate microcapsules for computed tomography real-time imaging and gastroretentive drug delivery. Int. J. Nanomed. 2019, 14, 6001–6018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.-M.; Hu, Q.; Cui, G.-Q.; Guo, X.-Y.; Wei, B.-Z.; Gan, C.-F.; Li, W.-G.; Mo, D.-M.; Lu, R.; Cui, J.-G. Release-controlled microcapsules of thiamethoxam encapsulated in beeswax and their application in field. J. Environ. Sci. Health B 2020, 55, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-F.; Huang, Y.-J.; Huang, Y.-X.; Zhang, J.-H.; Fang, C.; Yu, K.; Chen, Q.; Li, T.-R.; Han, R.; Yang, Z.-H.; et al. Laboratory and field study on the performance of microcapsule-based self-healing concrete in tunnel engineering. Constr. Build. Mater. 2019, 220, 90–101. [Google Scholar] [CrossRef]
- Liu, L.-C.; Xu, G.-Q. Preparation and stability of microcapsule wood preservative from neem extract. Bioresources 2019, 14, 3352–3363. [Google Scholar]
- Tozum, M.-S.; Alkan, C.; Aksoy, S.-A. Preparation of poly (methyl methacrylate-co-ethylene glycol dimethacrylate-co-glycidyl methacrylate) walled thermochromic microcapsules and their application to cotton fabrics. J. Appl. Polym. Sci. 2020, 137, 48815. [Google Scholar] [CrossRef]
- Pedaballi, S.; Li, C.-C.; Song, Y.-J. Dispersion of microcapsules for the improved thermochromic performance of smart coatings. RSC Adv. 2019, 9, 24175–24183. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, H.; Niu, J.-F.; Wang, X.-D.; Wu, D.-Z. Development of reversible and durable thermochromic phase-change microcapsules for real-time indication of thermal energy storage and management. Appl. Energy 2020, 264, 114729. [Google Scholar] [CrossRef]
- Dong, C.-H.; Liu, Y.; Long, Z.; Pang, Z.-Q.; Luo, Y.-H.; Li, X.-Z. Effect of papermaking conditions on the retention of reversible thermochromic microcapsule in paper. Bioresources 2012, 7, 66–77. [Google Scholar]
- Liu, B.-X.; Mazo, A.-R.; Gurr, P.-A.; Qiao, G.-G. Reversible nontoxic thermochromic microcapsules. Acs. Appl. Mater. Inter. 2020, 12, 9782–9789. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Gao, R.-N.; Li, J. Energy saving wood composite with temperature regulatory ability and thermoresponsive performance. Eur. Polym. J. 2019, 118, 163–169. [Google Scholar] [CrossRef]
- Mathis, D.; Blanchet, P.; Landry, V.; Lagiere, P. Impregnation of wood with microencapsulated bio-based phase change materials for high thermal mass engineered wood flooring. Appl. Sci. 2018, 8, 2696. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.-G.; Jeon, J.; Seo, J.; Lee, J.-H.; Kim, S. Performance evaluation of the microencapsulated PCM for wood-based flooring application. Energy Convers. Manag. 2012, 64, 516–521. [Google Scholar] [CrossRef]
- Zhu, X.-D.; Liu, Y.; Li, Z.; Wang, W.-C. Thermochromic microcapsules with highly transparent shells obtained through in-situ polymerization of urea formaldehyde around thermochromic cores for smart wood coatings. Sci. Rep. 2018, 8, 4015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.; Lyu, S.-Y.; Fu, F.; Huang, J.-D.; Wang, S.-Q. Preparation and properties of multifunctional thermochromic energy-storage wood materials. J. Mater. Sci. 2016, 51, 2716–2726. [Google Scholar] [CrossRef]
- Liu, Q.-Q.; Gao, D.; Xu, W. Effect of sanding processes on the surface properties of modified Poplar coated by primer compared with Mahogany. Coatings 2020, 10, 856. [Google Scholar] [CrossRef]
- Xu, F.; Qian, B.-R.; Hu, Z.; Chen, W.-D.; Zhuang, Z.-Y.; Zhu, B.-Y.; Zhang, H.-Q.; Zhu, K. A novel route to emulsifier-free, waterborne hydroxyl functional polyacrylate with low VOC level and its application in 2K-WPU coatings. J. Macromol. Sci. A 2013, 5, 555–561. [Google Scholar] [CrossRef]
- Lai, W.-D.; Chen, Y.-J.; Li, X.-Z.; An, W.; Li, X.-W. Photochromic characteristic of cationic photoinitiator and fluorane dye system microencapsulated in sub-micrometre scale. J. Photopolym. Sci. Technol. 2013, 26, 453–458. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.-X.; Chang, Y.-J.; Qian, X.-Y. Effect of concentration of thermochromic ink on performance of waterborne finish films for the surface of Cunninghamia Lanceolata. Polymers 2020, 12, 552. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.-L.; Zhang, L.; Yu, R.-L.; Yuan, L.-Y.; Yang, Y.-H.; He, X.-D.; Wang, J.-M.; Li, Z.-P. Microencapsulation of ethylenediamine and its application in binary self-healing system using dual-microcapsule. Mater. Des. 2020, 189, 108535. [Google Scholar] [CrossRef]
- Barapatre, A.; Aadil, K.-R.; Jha, H. Biodegradation of malachite green by the ligninolytic fungus aspergillus flavus. Clean Soil Air Water 2017, 45, 1600045. [Google Scholar] [CrossRef]
- Ma, X.-G.; Wang, L.; Li, L.; Bian, L.-R.; Yang, W.-F.; Meng, Q.-T. The novel thermochromic and energy-storage microcapsules with significant extension of color change range to different tones. J. Macromol. Sci. A 2019, 56, 588–596. [Google Scholar] [CrossRef]
- Vieira, A.-P.; Santana, S.-A.-A.; Bezerra, C.-W.-B.; Silva, H.-A.-S.; Chaves, J.-A.-P.; Melo, J.-C.-P.; Silva, E.-C.; Airoldi, C. Removal of textile dyes from aqueous solution by babassu coconut epicarp (Orbignya speciosa). Chem. Eng. J. 2011, 173, 334–340. [Google Scholar] [CrossRef]
- Raditoiu, V.; Raditoiu, A.; Wagner, L.; Amariutei, V.; Nicolae, C.-A. Thermochromic systems based on complexes of some triarylmethane dyes. Rev. Chim. Bucharest 2013, 64, 147–151. [Google Scholar]
- Santana, S.-A.-A.; Vieira, A.-P.; da Silva, E.-C.; Melo, J.-C.-P.; Airoldi, C. Immobilization of ethylenesulfide on babassu coconut epicarp and mesocarp for divalent cation sorption. J. Hazard. Mater. 2010, 174, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, D.; Lakshmi, S.-N.-S.; Raja, K.-S.; Vasanthi, N.-S. Biopolishing of cotton fabric with fungal cellulase and its effect on the morphology of cotton fibres. Indian J. Fibre Text. Res. 2013, 38, 156–160. [Google Scholar]
- Kulcar, R.; Friskovec, M.; Hauptman, N.; Vesel, A.; Gunde, M.-K. Colorimetric properties of reversible thermochromic printing inks. Dyes Pigments 2010, 86, 271–277. [Google Scholar] [CrossRef]
- Nesterova, T.; Dam-Johansen, K.; Pedersen, L.-T.; Kiil, S. Microcapsule-based self-healing anticorrosive coatings: Capsule size, coating formulation, and exposure testing. Prog. Org. Coat. 2012, 75, 309–318. [Google Scholar] [CrossRef]
- Lin, C.-M.; Gong, F.-Y.; Yang, Z.-J.; Pan, L.-P.; Liu, S.-J.; Li, J.; Guo, S.-Y. Bio-inspired fabrication of core@shell structured TATB/polydopamine microparticles via in situ polymerization with tunable mechanical properties. Polym. Test. 2018, 68, 126–134. [Google Scholar] [CrossRef]
- Comlekci, G.-K.; Ulutan, S. Acquired self-healing ability of an epoxy coating through microcapsules having linseed oil and its alkyd. Prog. Org. Coat. 2019, 129, 292–299. [Google Scholar] [CrossRef]


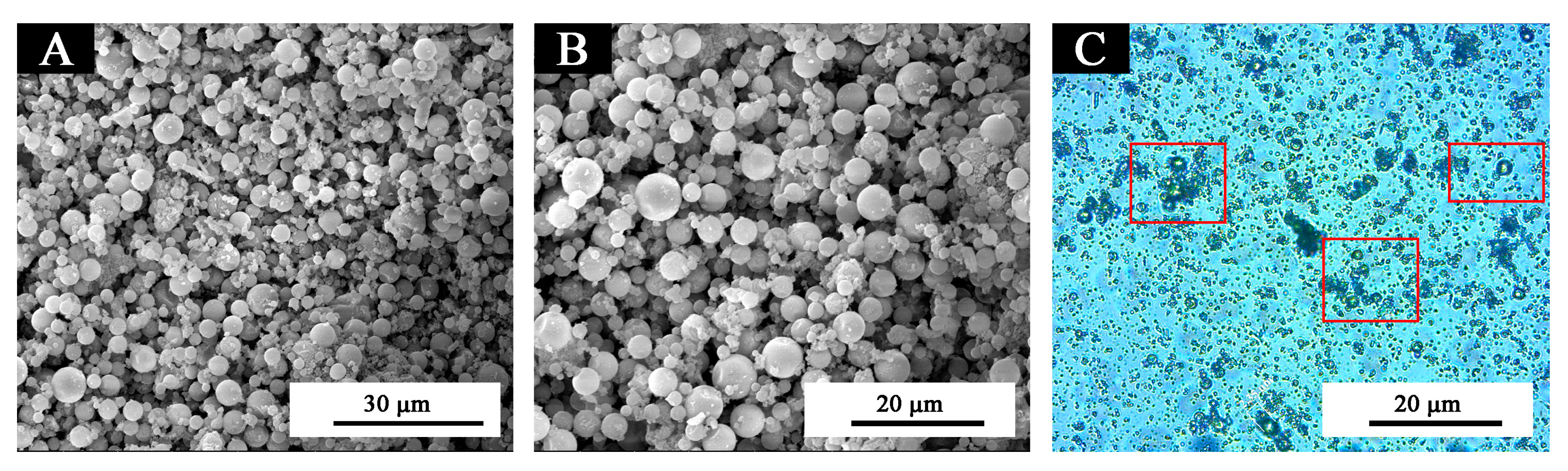
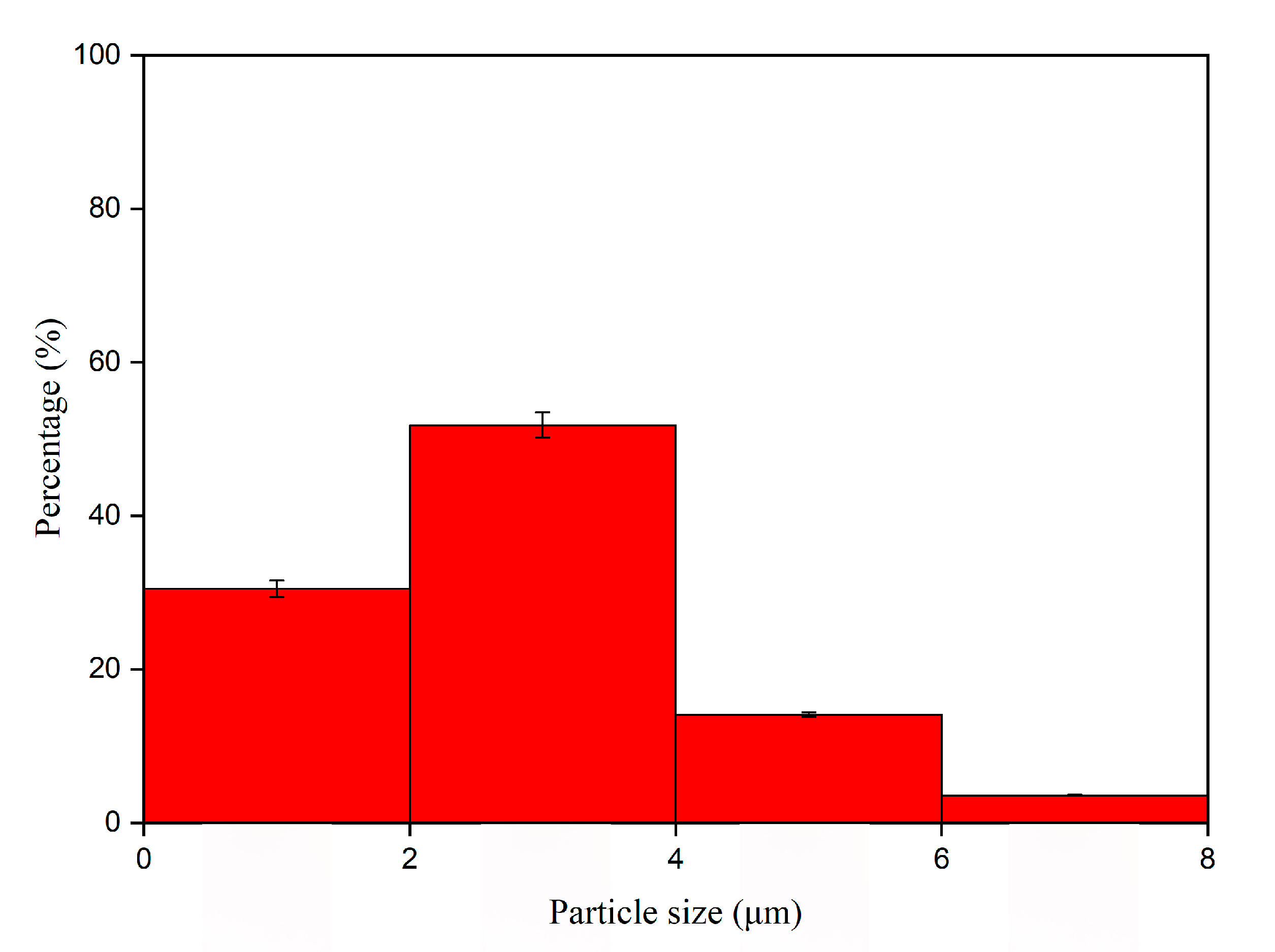
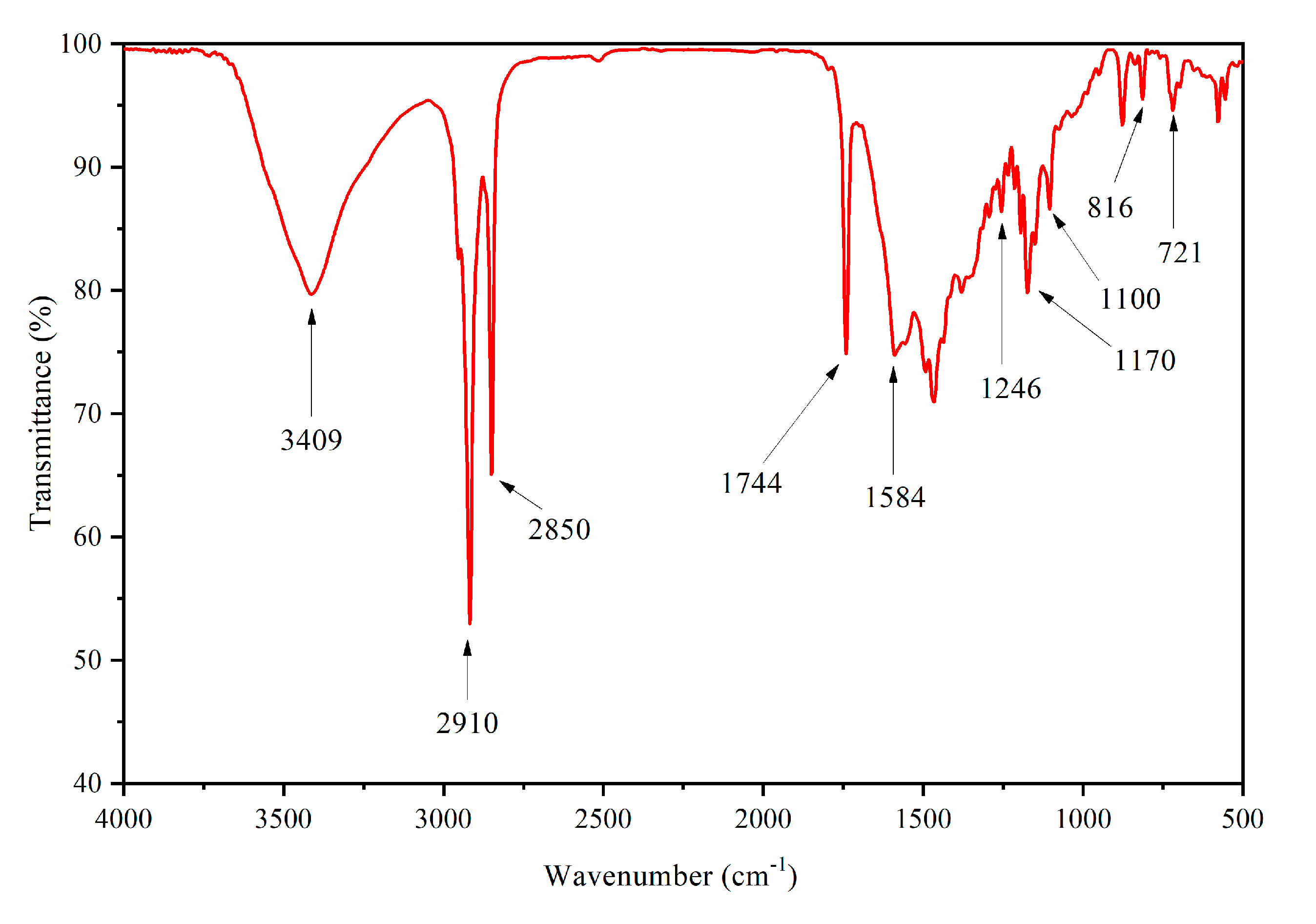
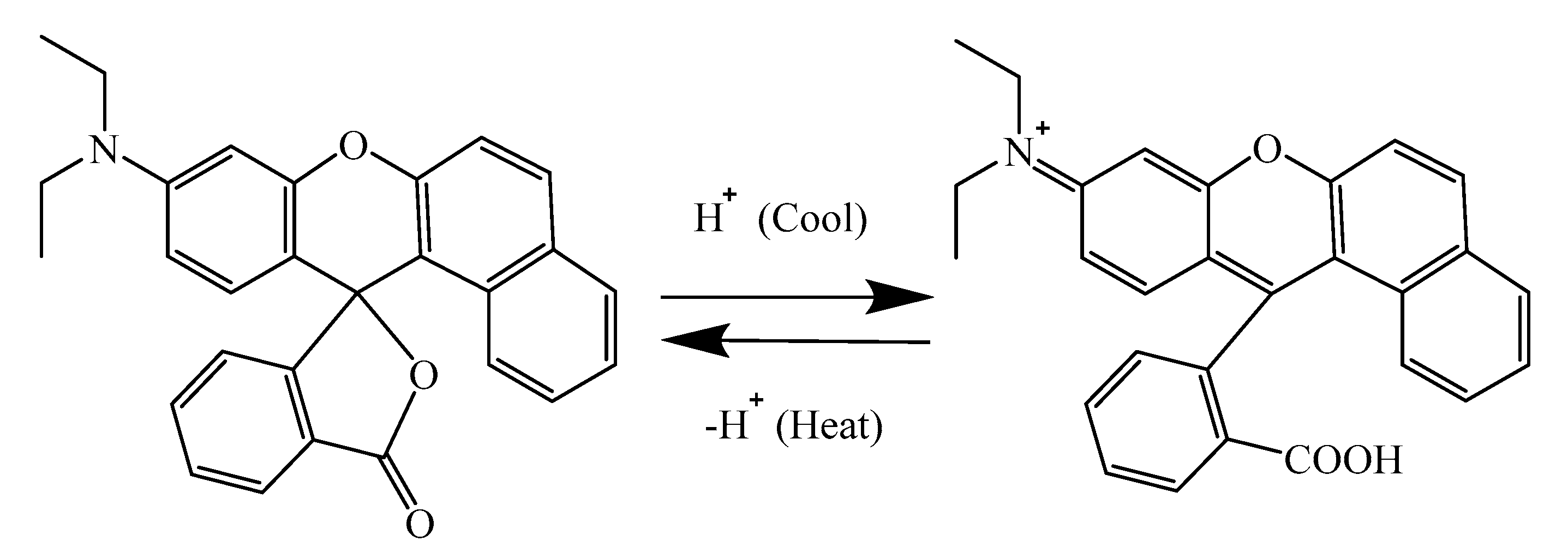


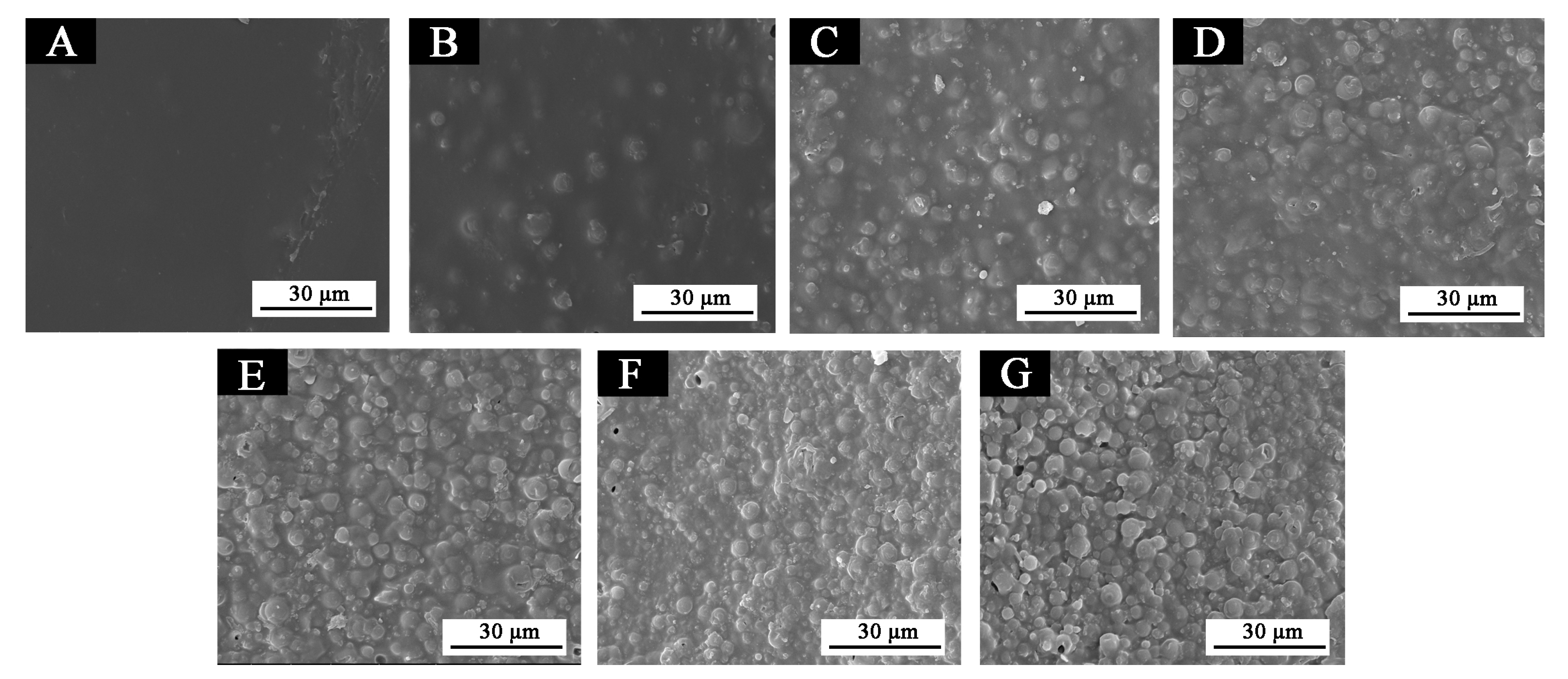
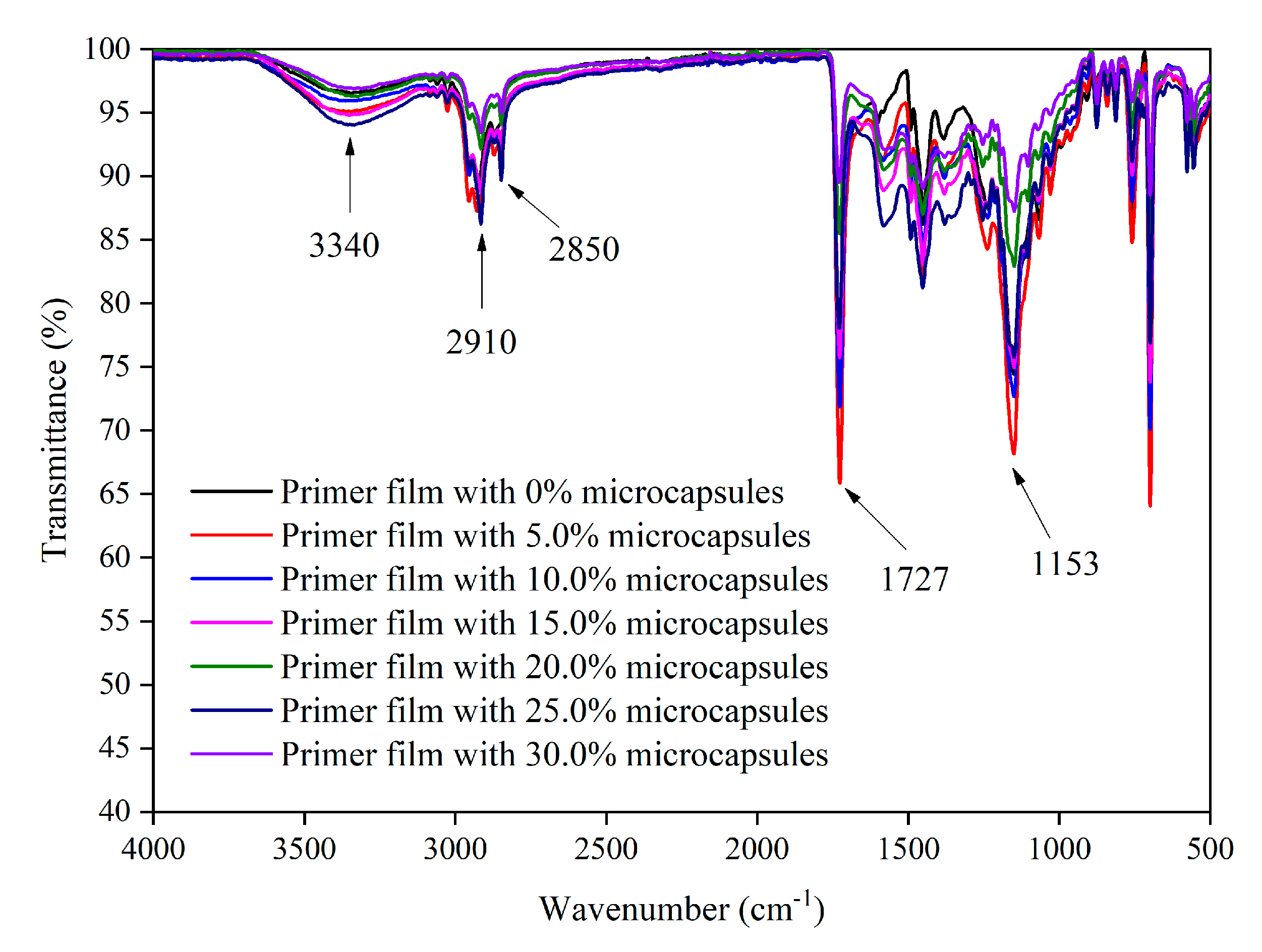
| Sample (#) | Microcapsules Concentration (%) | Drying Temperature (°C) | Drying Time (h) |
|---|---|---|---|
| 1 | 15.0 | 35.0 | 2.0 |
| 2 | 15.0 | 60.0 | 4.0 |
| 3 | 30.0 | 35.0 | 4.0 |
| 4 | 30.0 | 60.0 | 2.0 |
| Sample (#) | Microcapsules Concentration (%) | Weight of Thermochromic Microcapsule (g) | Weight of Waterborne Primer (g) |
|---|---|---|---|
| 1 | 15.0 | 15.0 | 85.0 |
| 2 | 15.0 | 15.0 | 85.0 |
| 3 | 30.0 | 30.0 | 70.0 |
| 4 | 30.0 | 30.0 | 70.0 |
| 5 | 0.0 | 0.0 | 100.0 |
| 6 | 5.0 | 5.0 | 95.0 |
| 7 | 10.0 | 10.0 | 90.0 |
| 8 | 15.0 | 15.0 | 85.0 |
| 9 | 20.0 | 20.0 | 80.0 |
| 10 | 25.0 | 25.0 | 75.0 |
| 11 | 30.0 | 30.0 | 70.0 |
| Sample (#) | Microcapsules Concentration (%) | Drying Temperature (°C) | Drying Time (h) | The Color Difference of 16 °C and 32 °C in the Heating Process |
|---|---|---|---|---|
| 1 | 15.0 | 35.0 | 2.0 | 68.5 ± 1.47 |
| 2 | 15.0 | 60.0 | 4.0 | 65.4 ± 1.18 |
| 3 | 30.0 | 35.0 | 4.0 | 73.9 ± 1.49 |
| 4 | 30.0 | 60.0 | 2.0 | 74.0 ± 2.45 |
| Mean 1 | 66.950 | 71.200 | - | - |
| Mean 2 | 73.950 | 69.700 | - | - |
| Range | 7.000 | 1.500 | - | - |
| Microcapsules Concentration (%) | 20° Gloss (%) | 60° Gloss (%) | 85° Gloss (%) |
|---|---|---|---|
| 0 | 8.60 ± 0.29 | 39.00 ± 1.02 | 51.20 ± 0.86 |
| 5.0 | 2.90 ± 0.08 | 10.60 ± 0.29 | 22.20 ± 0.67 |
| 10.0 | 1.80 ± 0.04 | 4.60 ± 0.16 | 10.20 ± 0.28 |
| 15.0 | 1.60 ± 0.04 | 3.10 ± 0.08 | 12.20 ± 0.22 |
| 20.0 | 1.70 ± 0.04 | 2.60 ± 0.08 | 10.90 ± 0.22 |
| 25.0 | 1.70 ± 0.03 | 2.50 ± 0.08 | 10.50 ± 0.41 |
| 30.0 | 1.70 ± 0.02 | 2.20 ± 0.08 | 11.20 ± 0.25 |
| Microcapsules Concentration (%) | Hardness | Adhesion (Grade) | Impact Resistance (kg cm) |
|---|---|---|---|
| 0 | H ± 0 | 0 ± 0 | 5.00 ± 0.08 |
| 5.0 | 2H ± 0 | 0 ± 0 | 7.00 ± 0.14 |
| 10.0 | 3H ± 0 | 0 ± 0 | 7.00 ± 0.22 |
| 15.0 | 3H ± 0 | 0 ± 0 | 10.00 ± 0.29 |
| 20.0 | 3H ± 0 | 1 ± 0 | 11.00 ± 0.14 |
| 25.0 | 4H ± 0 | 1 ± 0 | 11.00 ± 0.41 |
| 30.0 | 4H ± 0 | 1 ± 0 | 12.00 ± 0.16 |
| Microcapsules Concentration (%) | After the Test (Red Ink) | After the Test (NaCl Solution) | After the Test (Ethanol) | After the Test (Detergent) |
|---|---|---|---|---|
| 0 | 6.40 ± 0.22 | 1.80 ± 0.04 | 0.80 ± 0 | 1.80 ± 0.05 |
| 5.0 | 21.70 ± 0.57 | 1.20 ± 0.03 | 3.00 ± 0.08 | 3.00 ± 0.08 |
| 10.0 | 26.90 ± 0.94 | 3.00 ± 0.08 | 2.50 ± 0.08 | 2.80 ± 0.08 |
| 15.0 | 35.70 ± 0.42 | 3.00 ± 0.08 | 2.70 ± 0.08 | 3.00 ± 0.08 |
| 20.0 | 42.50 ± 1.16 | 2.60 ± 0.08 | 2.30 ± 0.08 | 5.70 ± 0.16 |
| 25.0 | 60.40 ± 1.18 | 2.00 ± 0.08 | 2.50 ± 0.08 | 10.10 ± 0.29 |
| 30.0 | 63.20 ± 1.88 | 2.50 ± 0.08 | 3.00 ± 0.08 | 19.90 ± 0.70 |
| Grade | Situation |
|---|---|
| 1 | No visual change (no damage). |
| 2 | Only when the light reaches the test surface or is very close to the mark and reflects to the eye of the observer, there is slight visible discoloration or discontinuous marks. |
| 3 | Slight impression, visible in several directions, such as a nearly complete ring or circle mark. |
| 4 | The surface structure has not changed significantly. |
| 5 | Severe effect, change of surface structure, tearing of surface material in whole or in part, or adhesion of paper to test surface. |
| Microcapsules Concentration (%) | Red Ink | NaCl Solution | Ethanol | Detergent |
|---|---|---|---|---|
| 0 | 2 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 |
| 5.0 | 3 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 |
| 10.0 | 3 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 |
| 15.0 | 3 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 |
| 20.0 | 4 ± 0 | 1 ± 0 | 1 ± 0 | 2 ± 0 |
| 25.0 | 4 ± 0 | 1 ± 0 | 1 ± 0 | 2 ± 0 |
| 30.0 | 4 ± 0 | 1 ± 0 | 1 ± 0 | 2 ± 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Yan, X. Mechanical and Optical Properties of Thermochromic Reversible Waterborne Primer Film on Tilia europaea with 1,2-Benzo-6-diethylaminofluorane Based Microcapsules. Polymers 2020, 12, 2062. https://doi.org/10.3390/polym12092062
Wang L, Yan X. Mechanical and Optical Properties of Thermochromic Reversible Waterborne Primer Film on Tilia europaea with 1,2-Benzo-6-diethylaminofluorane Based Microcapsules. Polymers. 2020; 12(9):2062. https://doi.org/10.3390/polym12092062
Chicago/Turabian StyleWang, Lin, and Xiaoxing Yan. 2020. "Mechanical and Optical Properties of Thermochromic Reversible Waterborne Primer Film on Tilia europaea with 1,2-Benzo-6-diethylaminofluorane Based Microcapsules" Polymers 12, no. 9: 2062. https://doi.org/10.3390/polym12092062
APA StyleWang, L., & Yan, X. (2020). Mechanical and Optical Properties of Thermochromic Reversible Waterborne Primer Film on Tilia europaea with 1,2-Benzo-6-diethylaminofluorane Based Microcapsules. Polymers, 12(9), 2062. https://doi.org/10.3390/polym12092062




