Facile One-Pot Synthesis of Hyperbranched Glycopolymers in Aqueous Solution via a Hydroxy/Cu(III) Redox Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
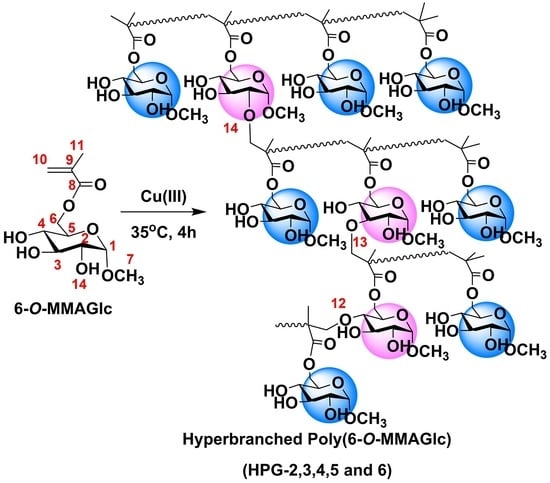
2.3. Synthesis
2.4. Cell Viability Assay
2.5. Amyloid Inhibition Test
2.5.1. Lysozyme Sample Preparation
2.5.2. Thioflavin T (ThT) Fluorescence Measurement
3. Results
3.1. Nuclear Magnetic Resonance (NMR) Spectra of Polymers
3.2. Gel Permeation Chromatography (GPC) Test for Polymers
3.3. Inhibitory Activity of Polymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Graham, B.; Fayter, A.E.R.; Houston, J.E.; Evans, R.C.; Gibson, M.I. Facially amphipathic glycopolymers inhibit ice recrystallization. J. Am. Chem. Soc. 2018, 140, 5682–5685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, D.A.; Zhang, Q.; Voorhaar, L.; Haddleton, D.M.; Herath, S.; Gleinich, A.S.; Randeva, H.S.; Crispin, M.; Lehnert, H.; Wallis, R.; et al. Manipulation of cytokine secretion in human dendritic cells using glycopolymers with picomolar affinity for DC-SIGN. Chem. Sci. 2017, 8, 6974–6980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jono, K.; Nagao, M.; Oh, T.; Sonoda, S.; Hoshino, Y.; Miura, Y. Controlling the lectin recognition of glycopolymers via distance arrangement of sugar blocks. Chem. Commun. 2018, 54, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Gerke, C.; Ebbesen, M.F.; Jansen, D.; Boden, S.; Freichel, T.; Hartmann, L. Sequence-controlled glycopolymers via step-growth polymerization of precision glycomacromolecules for lectin receptor clustering. Biomacromolecules 2017, 18, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Gorzkiewicz, M.; Jatczak-Pawlik, I.; Studzian, M.; Pułaski, Ł.; Appelhans, D.; Voit, B.; Klajnert-Maculewicz, B. Glycodendrimer nanocarriers for direct delivery of fludarabine triphosphate to leukemic cells: Improved pharmacokinetics and pharmacodynamics of fludarabine. Biomacromolecules 2018, 19, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Janaszewska, A.; Klajnert-Maculewicz, B.; Marcinkowska, M.; Duchnowicz, P.; Appelhans, D.; Grasso, G.; Deriu, M.A.; Danani, A.; Cangiotti, M.; Ottaviani, M.F. Multivalent interacting glycodendrimer to prevent amyloid-peptide fibril formation induced by Cu(II): A multidisciplinary approach. Nano Res. 2018, 11, 1204–1226. [Google Scholar] [CrossRef]
- Peng, Y.-Y.; Diaz-Dussan, D.; Kumar, P.; Narain, R. Tumor microenvironment-regulated redox responsive cationic galactose-based hyperbranched polymers for siRNA delivery. Bioconjugate Chem. 2019, 30, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Liu, C.-P.; Xie, F.-Y.; Ma, R.; Zhuo, L.-H.; Li, N.; Tian, W. Construction of β-cyclodextrin-based supramolecular hyperbranched polymers self-assemblies using AB2-type macromonomer and their application in the drug delivery field. Carbohydr. Polym. 2019, 213, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Haksar, D.; de Poel, E.; van Ufford, L.Q.; Bhatia, S.; Haag, R.; Beekman, J.; Pieters, R.J. Strong inhibition of cholera toxin B subunit by affordable, polymer-based multivalent inhibitors. Bioconjugate Chem. 2019, 30, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Grinstaff, M.W. Chemical synthesis of polysaccharides and polysaccharide mimetics. Prog. Polym. Sci. 2017, 74, 78–116. [Google Scholar] [CrossRef]
- Zhang, R.; Tao, Y.; Xu, W.; Xiao, S.; Du, S.; Zhou, Y.; Hasan, A. Rheological and controlled release properties of hydrogels based on mushroom hyperbranched polysaccharide and xanthan gum. Int. J. Biol. Macromol. 2018, 120, 2399–2409. [Google Scholar] [CrossRef] [PubMed]
- Sarac, A.S. Redox polymerization. Prog. Polym. Sci. 1999, 24, 1149–1204. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, C.; Song, H.; Bai, L.; Cheng, Y.; Ba, X.; Wu, Y. A facile one-step grafting of polyphosphonium onto halloysite nanotubes initiated by Ce(IV). Chem. Commun. 2019, 55, 1040–1043. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wu, Y.; Bai, L.; Peng, X.; Zhang, H.; Zhang, Y.; An, P.; Wang, S.; Ma, G.; Ba, X. Facile preparation of hyperbranched glycopolymers via an AB3* inimer promoted by a hydroxy/cerium(IV) redox process. Polym. Chem. 2018, 9, 5024–5031. [Google Scholar] [CrossRef]
- Jose, T.P.; Tuwar, S.M. Oxidation of threonine by the analytical reagent diperiodatocuprate(III)—An autocatalysed reaction. J. Mol. Struct. 2007, 827, 137–144. [Google Scholar] [CrossRef]
- Albertin, L.; Stenzel, M.; Barner-Kowollik, C.; Foster, L.J.R.; Davis, T.P. Well-defined glycopolymers from RAFT polymerization: poly(methyl 6-O-methacryloyl-α-d-glucoside) and its block copolymer with 2-hydroxyethyl methacrylate. Macromolecules 2004, 37, 7530–7537. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, Y.; Roberts, E.; Duxbury, C.J.; Ding, L.; Irvine, D.J.; Howdle, S.M. Controlling chain growth: A new strategy to hyperbranched materials. Macromolecules 2007, 40, 7184–7194. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Y.; Liu, Q.; Li, S.; Perrier, S.; Zhao, Y. Facile synthesis of hyperbranched and star-shaped polymers by RAFT polymerization based on a polymerizable trithiocarbonate. Macromolecules 2011, 44, 2034–2049. [Google Scholar] [CrossRef]
- Lin, K.; Kasko, A.M. Effect of branching density on avidity of hyperbranched glycomimetics for mannose binding lectin. Biomacromolecules 2013, 14, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Narain, R. The effect of molecular weight, compositions and lectin type on the properties of hyperbranched glycopolymers as non-viral gene delivery systems. Biomaterials 2012, 33, 3990–4001. [Google Scholar] [CrossRef] [PubMed]






| Sample | γ a | Yield (%) | Mnb (104 g/mol) | Mwb (104 g/mol) | PDI (Mw/Mn) | α c | DBd |
|---|---|---|---|---|---|---|---|
| LPG-1 | - | 67 | 3.3 | 4.5 | 1.36 | 0.6 | 0 |
| HPG-2 | 0.1 | 35 | 3.4 | 5.7 | 1.67 | 0.52 | 0.21 |
| HPG-3 | 0.2 | 33 | 3.1 | 5.5 | 1.77 | 0.50 | 0.25 |
| HPG-4 | 0.3 | 35 | 2.8 | 4.8 | 1.71 | 0.49 | 0.27 |
| HPG-5 | 0.4 | 30 | 1.8 | 3.6 | 1.97 | 0.48 | 0.30 |
| HPG-6 | 0.5 | 29 | 1.0 | 2.8 | 2.87 | 0.45 | 0.32 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Zhang, Y.; Hao, X.; Zhou, Q.; Zheng, Y.; Bai, L.; Zhang, H. Facile One-Pot Synthesis of Hyperbranched Glycopolymers in Aqueous Solution via a Hydroxy/Cu(III) Redox Process. Polymers 2020, 12, 2065. https://doi.org/10.3390/polym12092065
Liu F, Zhang Y, Hao X, Zhou Q, Zheng Y, Bai L, Zhang H. Facile One-Pot Synthesis of Hyperbranched Glycopolymers in Aqueous Solution via a Hydroxy/Cu(III) Redox Process. Polymers. 2020; 12(9):2065. https://doi.org/10.3390/polym12092065
Chicago/Turabian StyleLiu, Feng, Yuangong Zhang, Xiaohui Hao, Qian Zhou, Ying Zheng, Libin Bai, and Hailei Zhang. 2020. "Facile One-Pot Synthesis of Hyperbranched Glycopolymers in Aqueous Solution via a Hydroxy/Cu(III) Redox Process" Polymers 12, no. 9: 2065. https://doi.org/10.3390/polym12092065
APA StyleLiu, F., Zhang, Y., Hao, X., Zhou, Q., Zheng, Y., Bai, L., & Zhang, H. (2020). Facile One-Pot Synthesis of Hyperbranched Glycopolymers in Aqueous Solution via a Hydroxy/Cu(III) Redox Process. Polymers, 12(9), 2065. https://doi.org/10.3390/polym12092065