Synthesis and Solution Properties of a Novel Hyperbranched Polymer Based on Chitosan for Enhanced Oil Recovery
Abstract
1. Introduction
2. Experimental Section
2.1. Experimental Materials
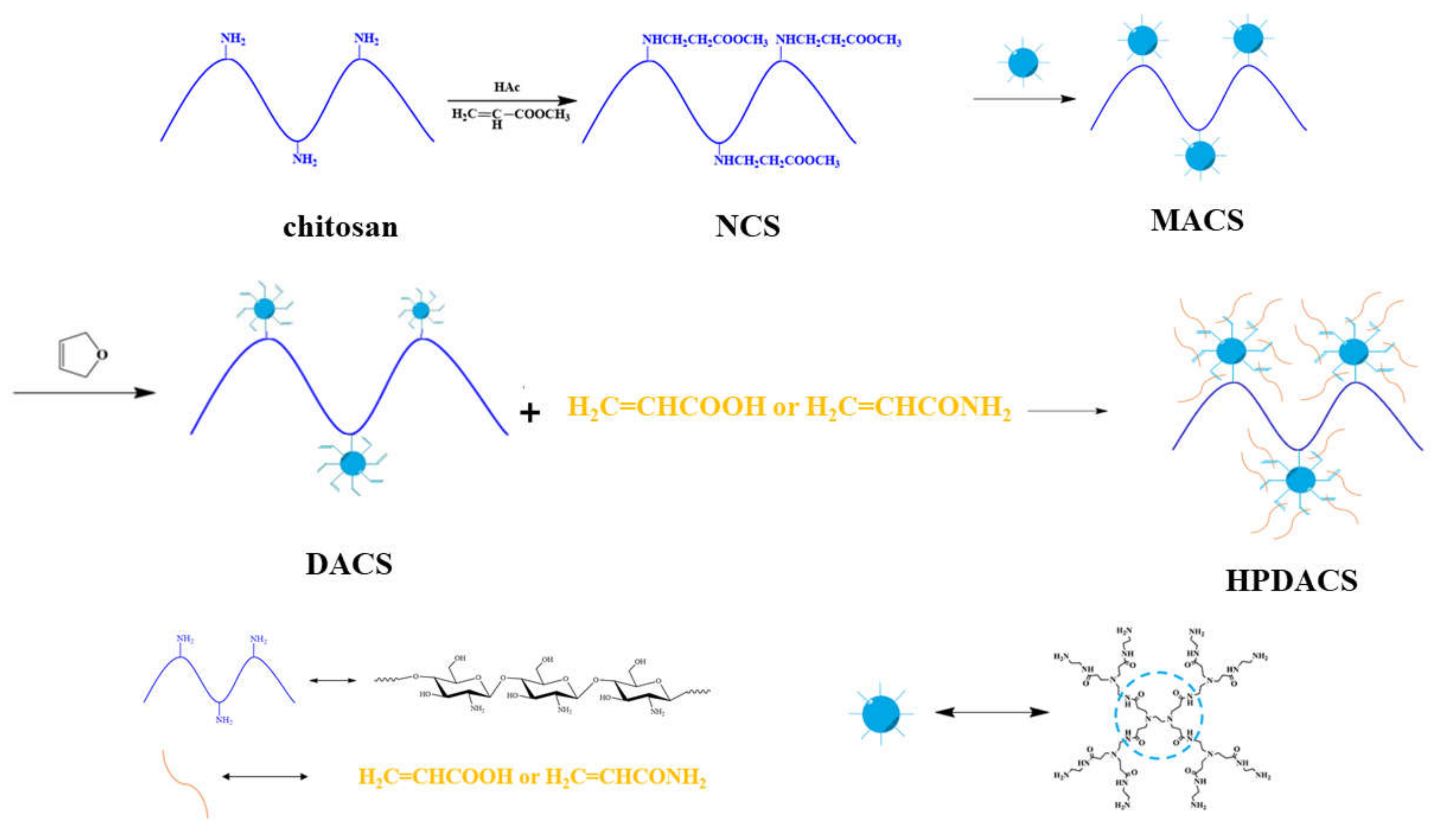
2.2. Synthesis of HPDACS
2.2.1. Synthesis of Branched Monomers
2.2.2. Synthesis of NCS
2.2.3. Synthesis of MACS
2.2.4. Synthesis of DACS
2.2.5. Synthesis of HPDACS
2.3. Characterization
2.4. Solution Properties
2.4.1. Solubility
2.4.2. Thickening Ability
2.4.3. Stability Experiments
2.4.4. Rheology and Viscoelasticity
2.5. Oil Displacement Capability of Polymers
3. Results and Discussion
3.1. Optimal Synthesis Conditions of HPDACS
3.2. Characterization
3.2.1. FT-IR Spectroscopy
3.2.2. X-ray Diffraction
3.2.3. C and N Elemental Analysis
3.2.4. 1HNMR
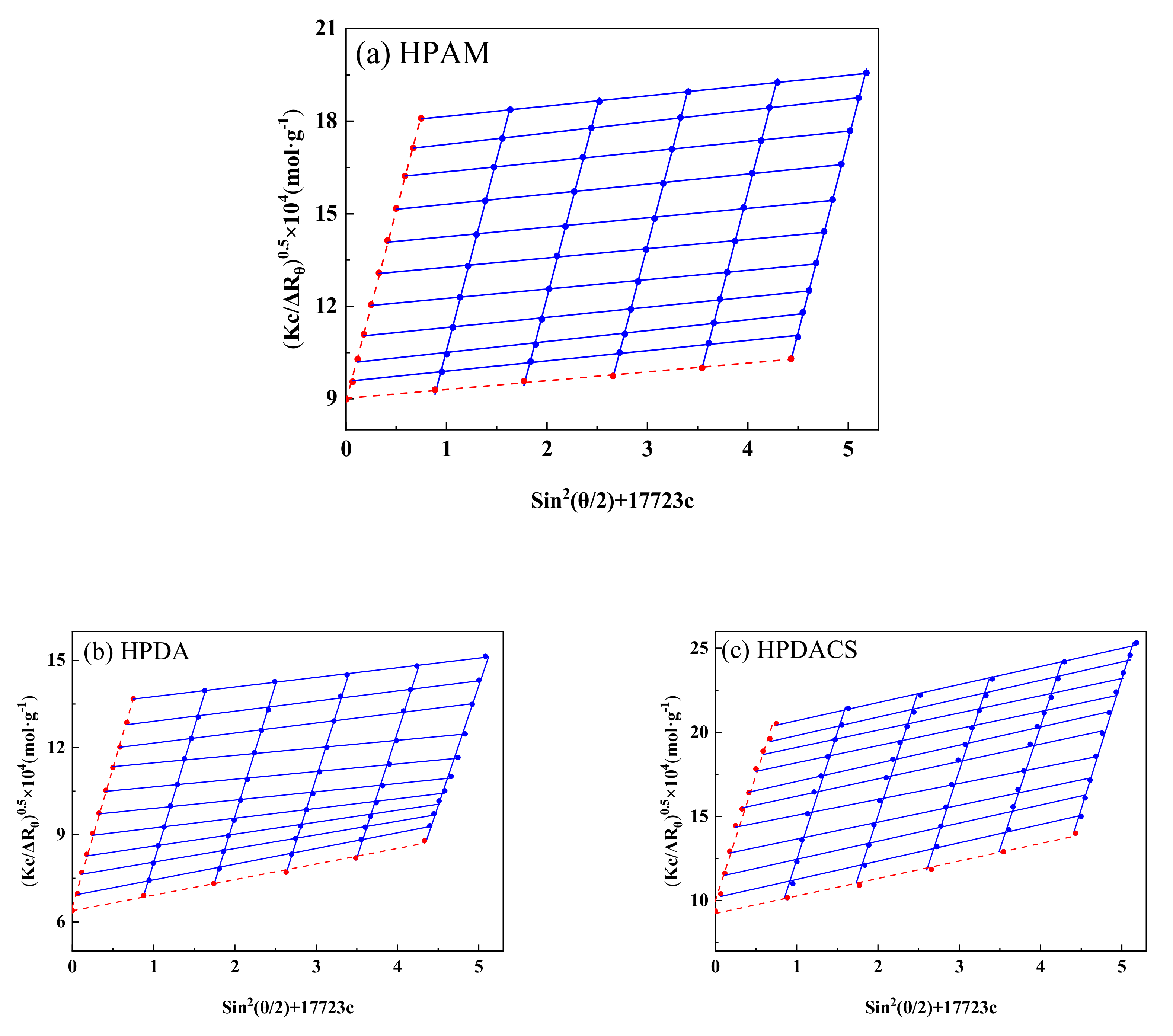
3.2.5. Molecular Weight
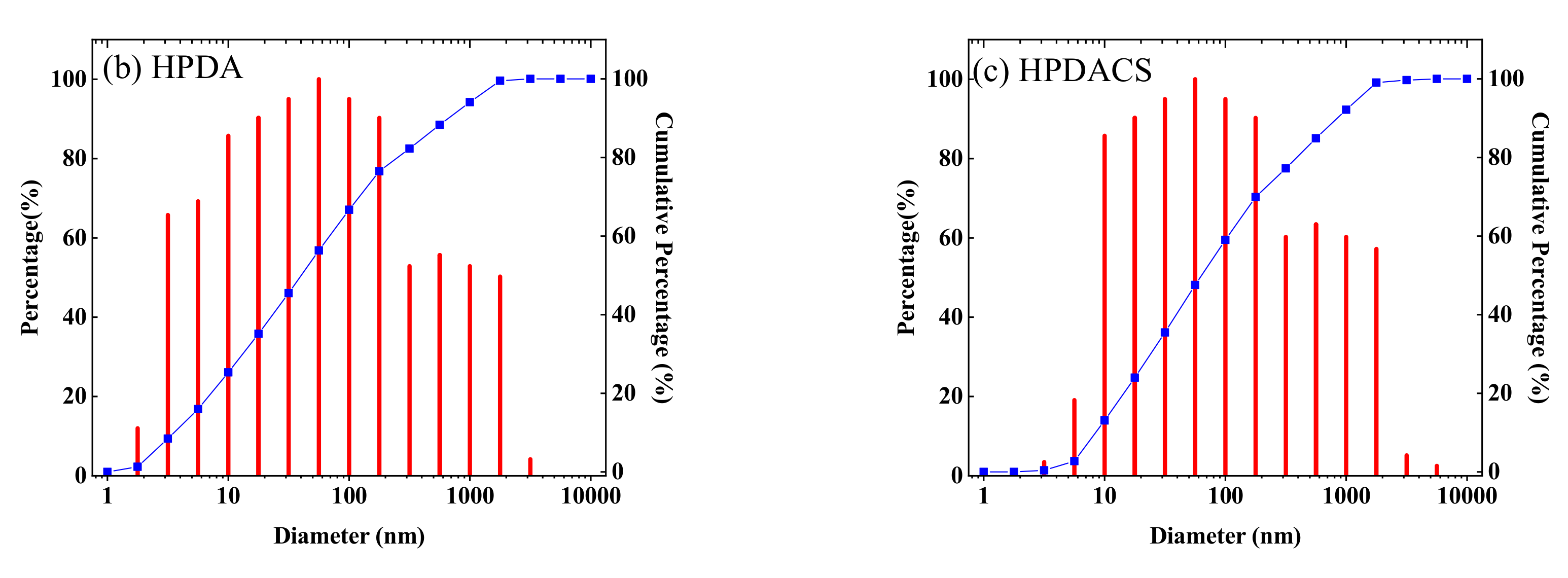
3.2.6. Hydrodynamic Radius
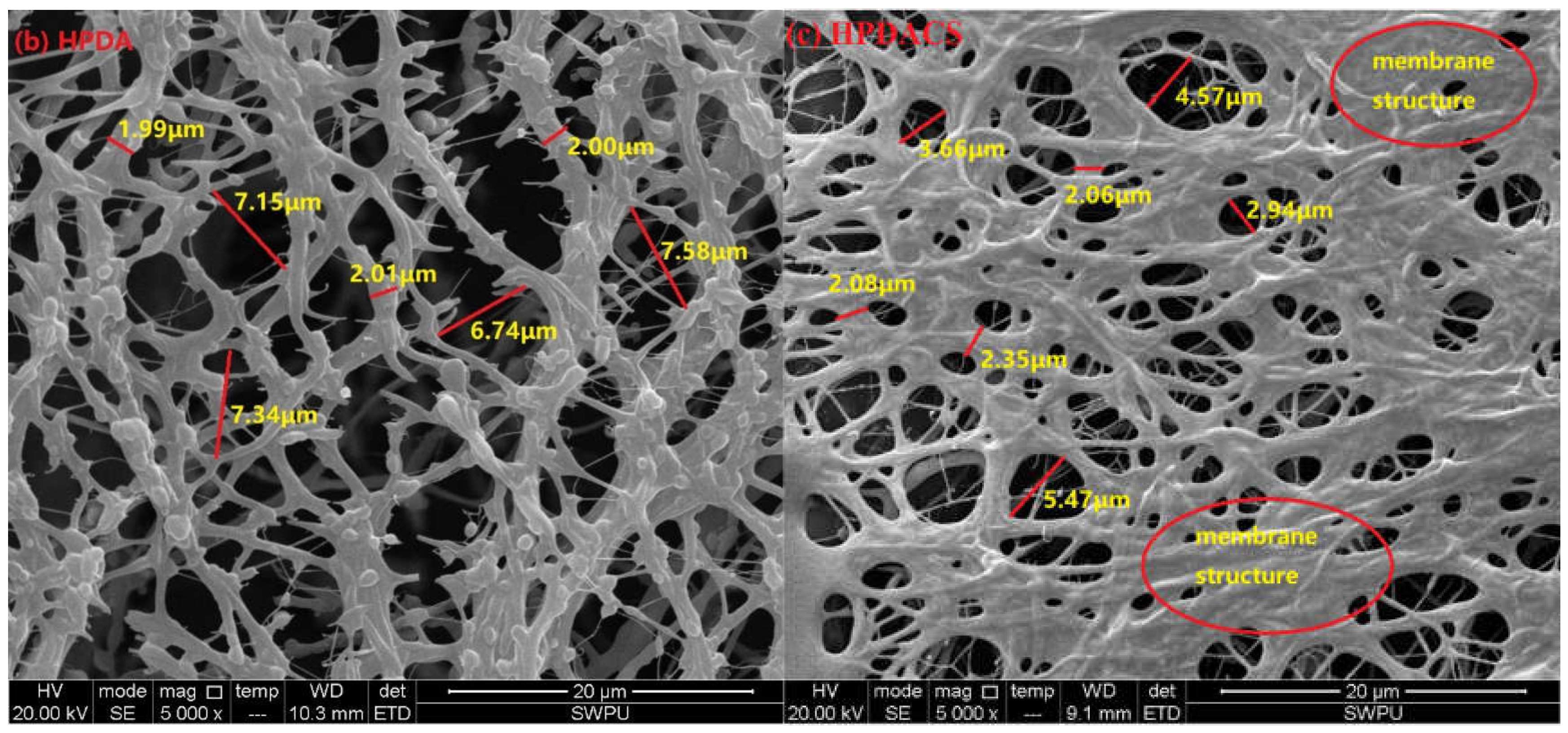
3.2.7. Morphology
3.3. Solution Properties
3.3.1. Solubility
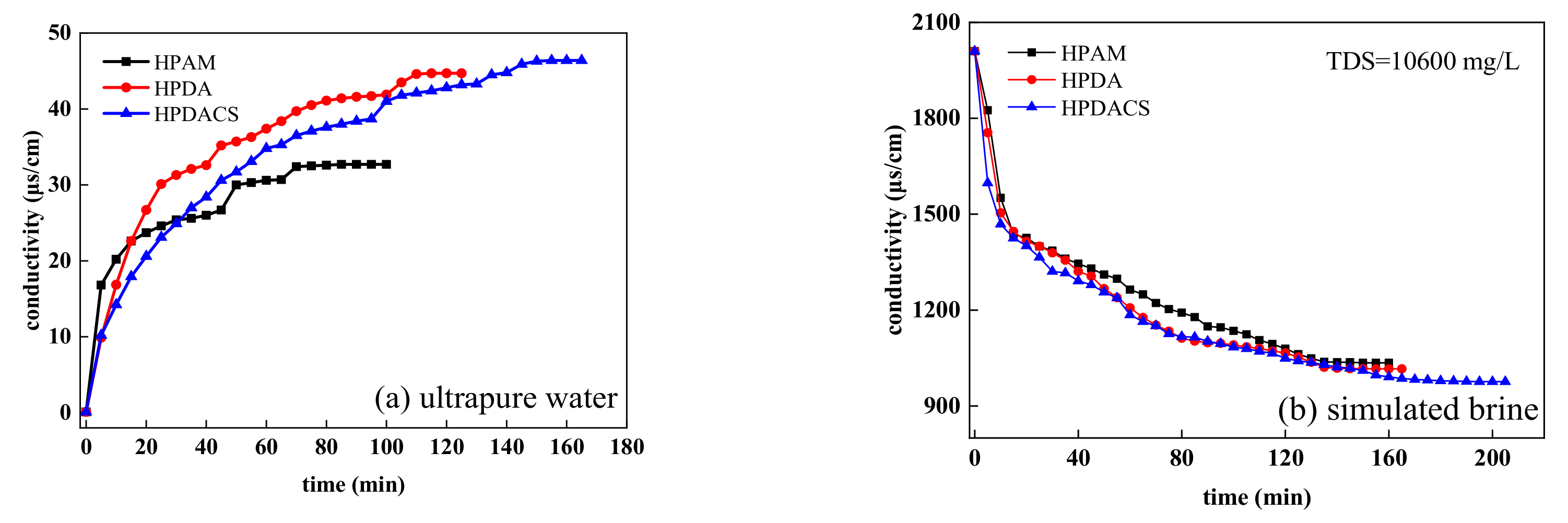
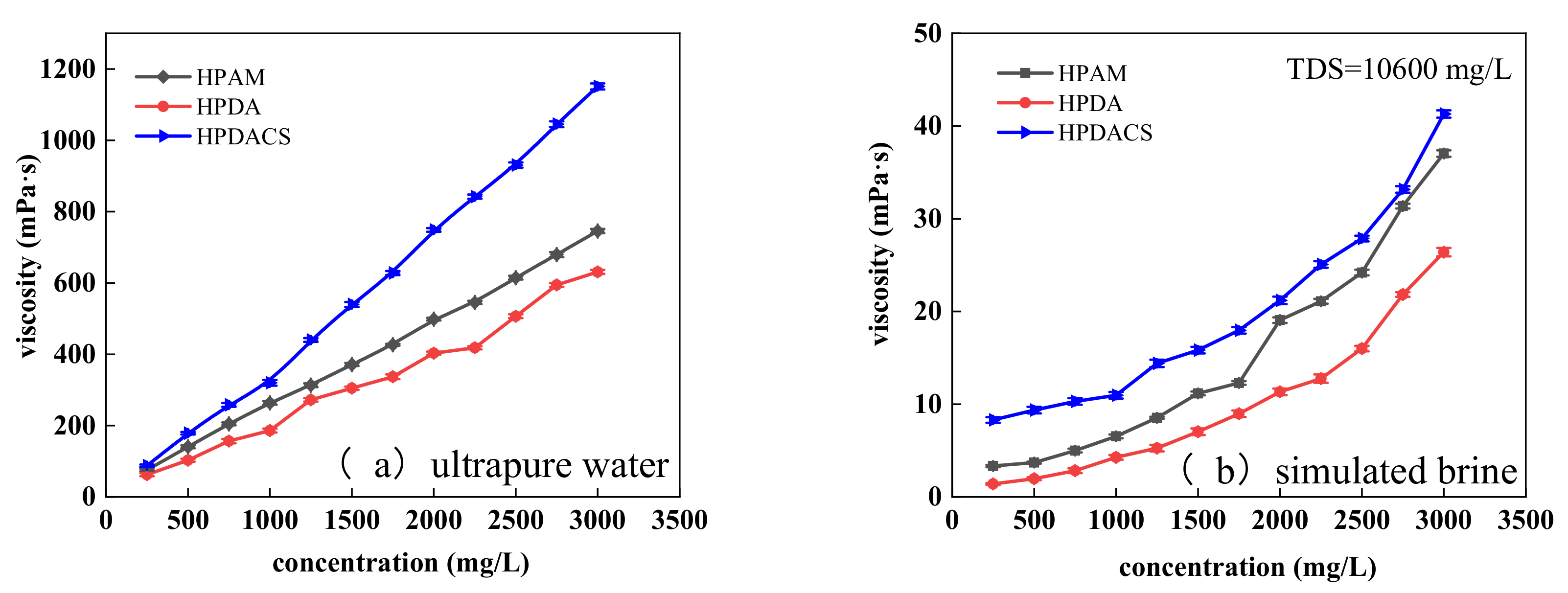
3.3.2. Thickening Ability
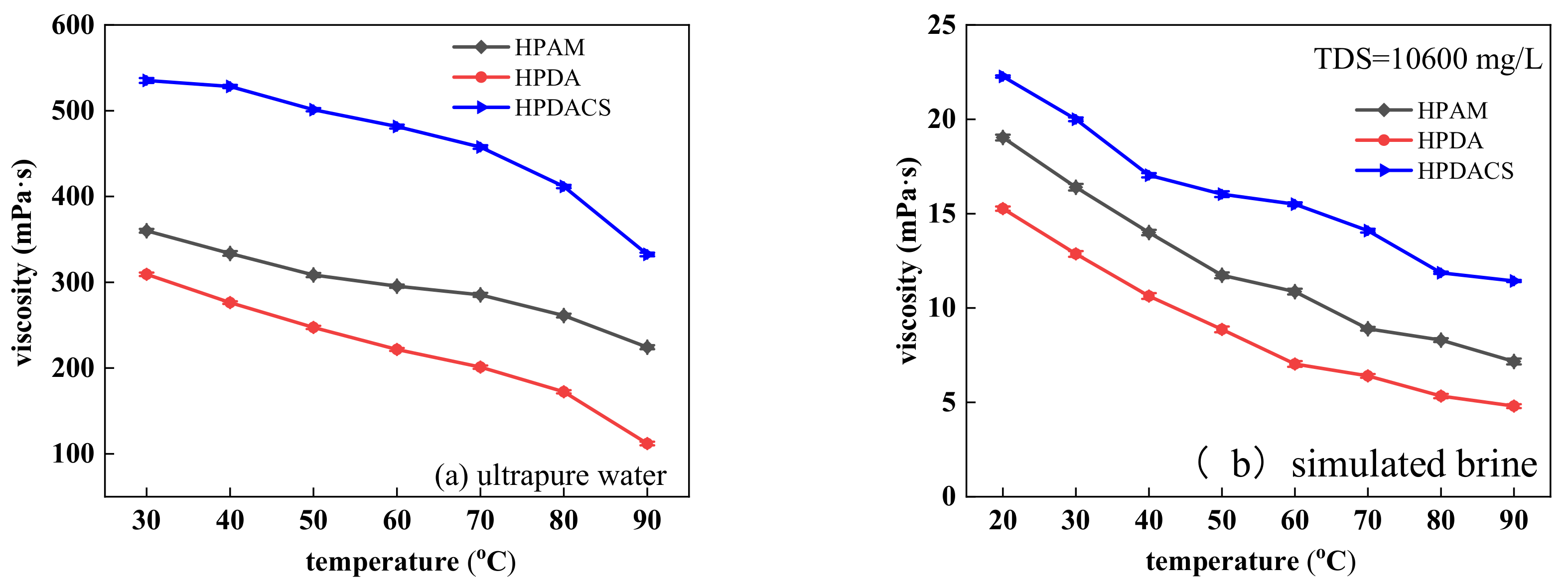
3.3.3. Temperature Resistance
3.3.4. Salt Resistance
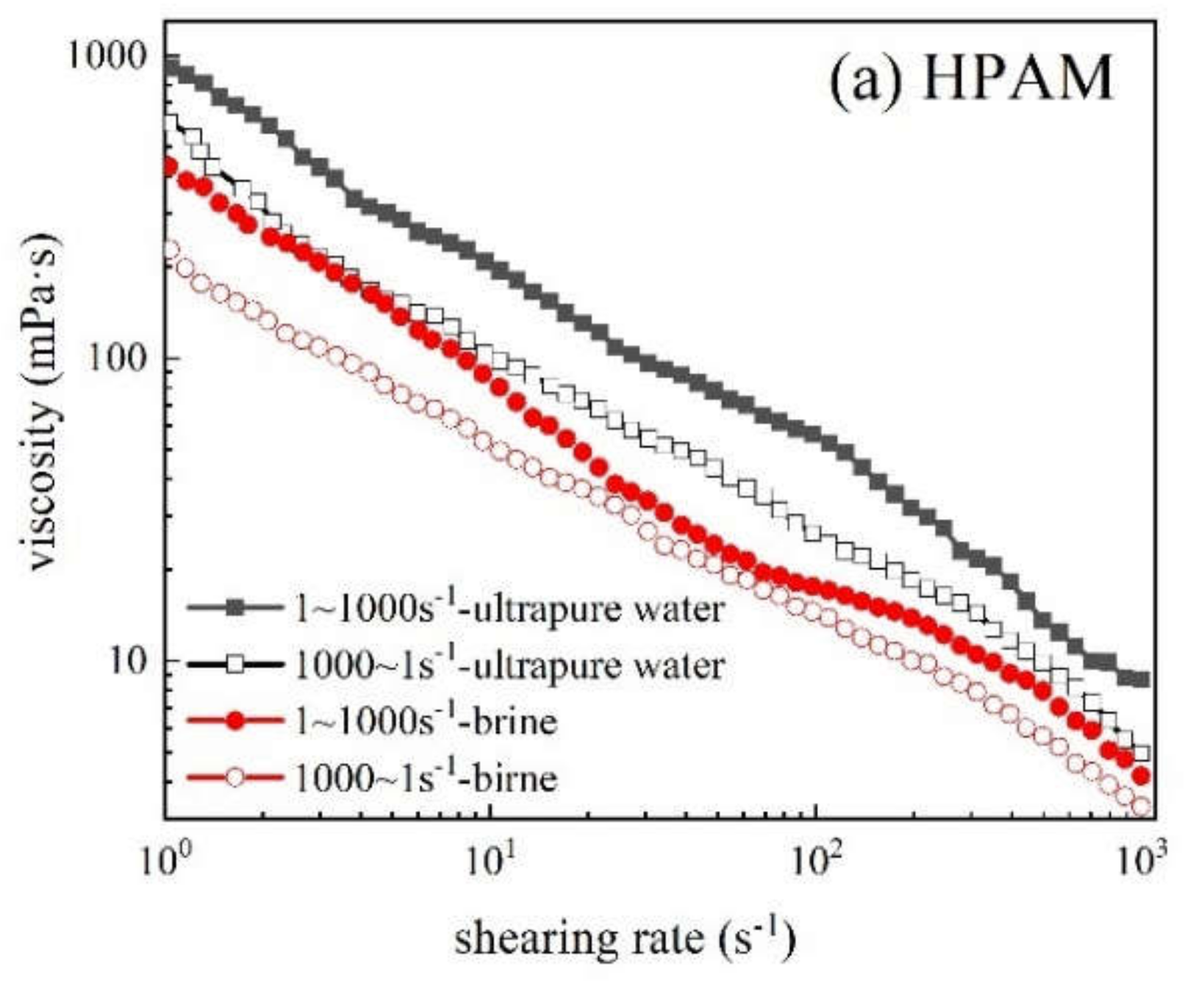
3.3.5. Anti-Shearing Ability
3.3.6. Anti-Aging Ability
3.3.7. Rheology
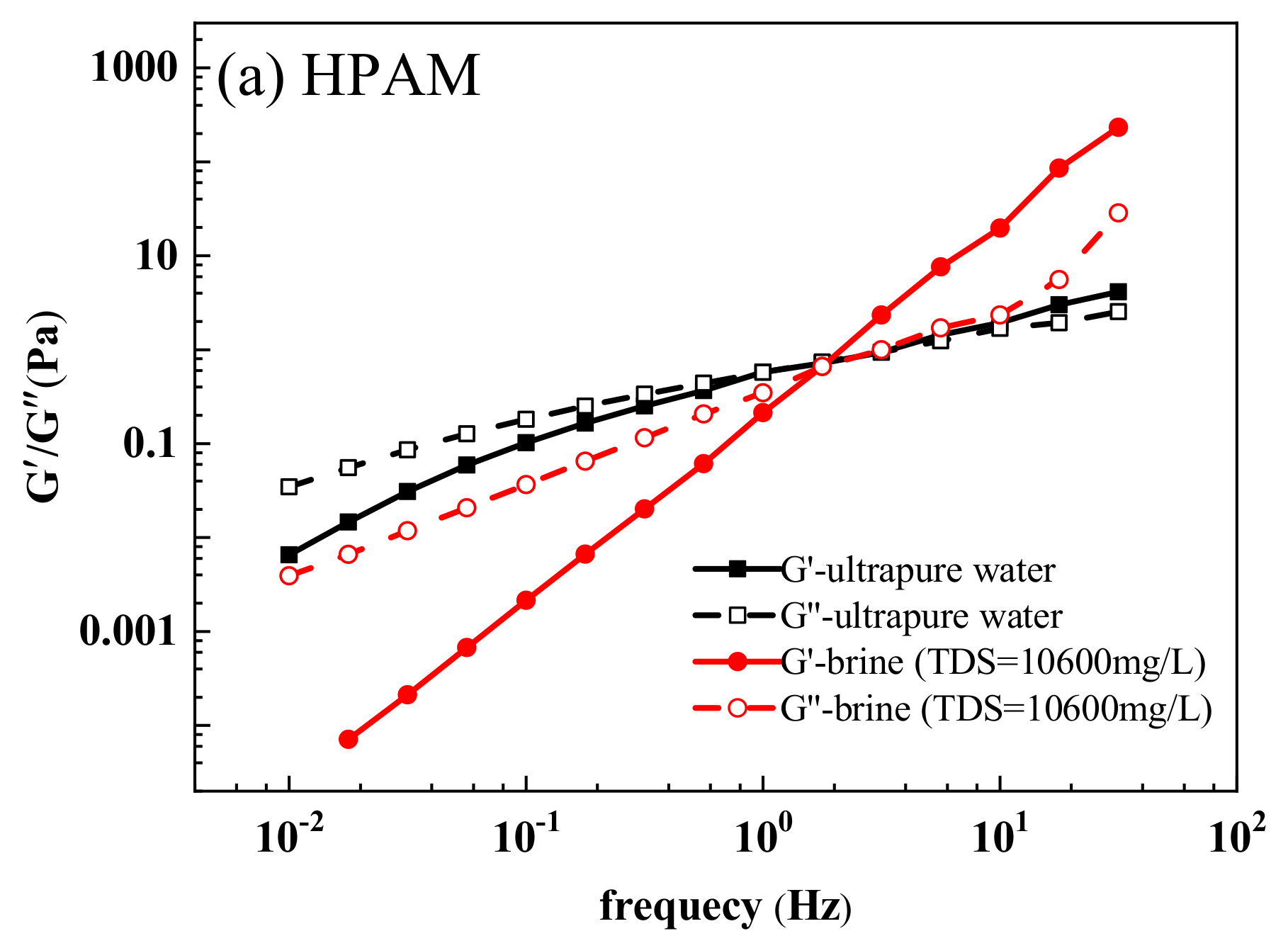
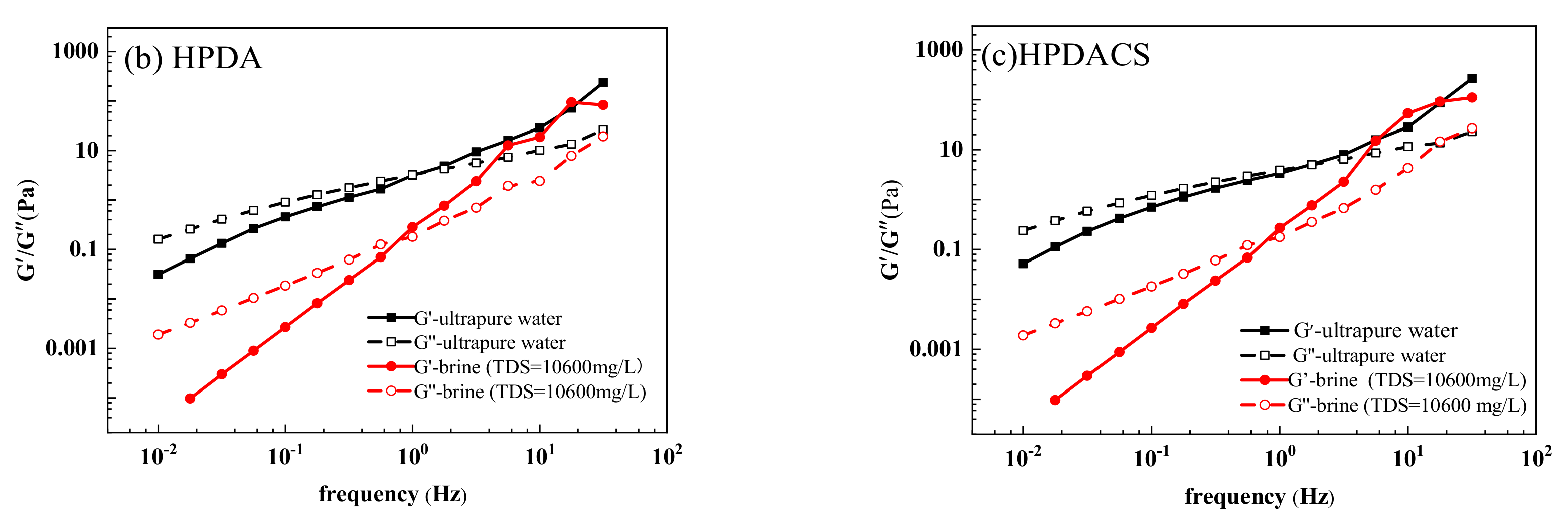
3.3.8. Viscoelasticity
3.4. EOR Ability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lai, N.; Wu, T.; Ye, Z.; Zhou, N.; Xu, Q.; Zeng, F. Preparation and properties of hyperbranched polymer containing functionalized Nano-SiO2 for low-moderate permeability reservoirs. Russ. J. Appl. Chem. 2016, 89, 1681–1693. [Google Scholar] [CrossRef]
- Sabhapondit, A.; Borthakur, A.; Haque, I. Characterization of acrylamide polymers for enhanced oil recovery. J. Appl. Polym. Sci. 2003, 87, 1869–1878. [Google Scholar] [CrossRef]
- You, Q.; Wen, Q.; Fang, J.; Guo, M.; Zhang, Q.; Dai, C. Experimental study on lateral flooding for enhanced oil recovery in bottom-water reservoir with high water cut. J. Pet. Sci. Eng. 2019, 174, 747–756. [Google Scholar] [CrossRef]
- Zhong, C.; Luo, P.; Ye, Z.; Chen, H. Characterization and solution properties of a novel water-soluble terpolymer for enhanced oil recovery. Polym. Bull. 2009, 62, 79–89. [Google Scholar] [CrossRef]
- Pu, W.; Liu, R.; Wang, K.; Li, K.; Yan, Z.; Li, B.; Zhao, L. Water-soluble core–shell hyperbranched polymers for enhanced oil recovery. Ind. Eng. Chem. Res. 2015, 54, 798–807. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, G. Aqueous dispersions of layered double hydroxide/polyacrylamide nanocomposites: Preparation and rheology. J. Mater. Chem. A 2014, 2, 13593–13601. [Google Scholar] [CrossRef]
- Li, K.; Duan, M.; Wang, H.; Zhang, J.; Jing, B. Investigation on the adsorption behavior of polyacrylamide on resin by dual polarization interferometry. RSC Adv. 2015, 5, 17389–17395. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, J.; Zhang, X.; Feng, R.; Li, H.; Zhang, J.; Lv, X.; Luo, P. Solution property investigation of combination flooding systems consisting of gemini–non-ionic mixed surfactant and hydrophobically associating polyacrylamide for enhanced oil recovery. Energy Fuels 2012, 26, 2116–2123. [Google Scholar] [CrossRef]
- Wever, D.; Picchioni, F.; Broekhuis, A. Polymers for enhanced oil recovery: A paradigm for structure–property relationship in aqueous solution. Prog. Polym. Sci. 2011, 36, 1558–1628. [Google Scholar] [CrossRef]
- Wu, G.; Yu, L.; Jiang, X. Synthesis and properties of an acrylamide-based polymer for enhanced oil recovery: A preliminary study. Adv. Polym. Technol. 2018, 37, 2763–2773. [Google Scholar] [CrossRef]
- Howe, A.; Clarke, A.; Giernalczyk, D. Flow of concentrated viscoelastic polymer solutions in porous media: Effect of MW and concentration on elastic turbulence onset in various geometries. Soft Matter 2015, 11, 6419–6431. [Google Scholar] [CrossRef] [PubMed]
- Zaitoun, A.; Makakou, P.; Blin, N.; Al-Maamari, R.; Al-Hashmi, A.; Abdel-Goad, M. Shear stability of EOR polymers. SPE J. 2012, 17, 335–339. [Google Scholar] [CrossRef]
- Lu, X.; Yan, W.; Song, H.; Gao, Z. An experimental study on shear degradation of HPAM solutions flowing through porous media. Oilfield Chem. 1995, 4, 375–378. [Google Scholar]
- Shu, Z.; Ye, Z.; Zhang, J.; Peng, Y.; Xiang, W.; Zhao, W. Experiment design of velocity shearing simulation for polymer adjacent to borehole. Pet. Geol. Recovery Effic. 2010, 4, 55–58. [Google Scholar]
- Lyu, Y.; Gu, C.; Tao, J.; Yao, X.; Zhao, G.; Dai, C. Thermal-resistant, shear-stable and salt-tolerant polyacrylamide/surface-modified graphene oxide composite. J. Mater. Sci. 2019, 54, 14752–14762. [Google Scholar] [CrossRef]
- Li, X.; Xu, Z.; Yin, H.; Feng, Y.; Quan, H. Comparative studies on enhanced oil recovery: Thermoviscosifying polymer versus polyacrylamide. Energy Fuels 2017, 31, 2479–2487. [Google Scholar] [CrossRef]
- Giussi, J.; Azzaroni, O.; Hensel-Bielowka, S.; Wojnarowska, Z.; Knapik, J.; Paluch, M. Synthesis, characterization and dielectric relaxation study of hyperbranched polymers with different molecular architecture. Polymer 2016, 100, 227–237. [Google Scholar] [CrossRef]
- Konkolewicz, D.; Monteiro, M.; Perrier, S. Dendritic and hyperbranched polymers from macromolecular units: Elegant approaches to the synthesis of functional polymers. Macromolecules 2011, 44, 7067–7087. [Google Scholar] [CrossRef]
- Rosen, B.; Wilson, C.; Wilson, D.; Peterca, M.; Percec, V. Dendron-mediated self-assembly, disassembly, and self-organization of complex systems. Chem. Rev. 2009, 109, 6275–6540. [Google Scholar] [CrossRef]
- Segawa, Y.; Higashihara, T.; Ueda, M. Hyperbranched polymers with controlled degree of branching from 0 to 100%. J. Am. Chem. Soc. 2010, 132, 11000–11001. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Weng, Z.; Gao, C. Hyperbranched polymers: Advances from synthesis to applications. Chem. Soc. Rev. 2015, 44, 4091–4130. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D. Birth of a new macromolecular architecture: Dendrimers as quantized building blocks for nanoscale synthetic polymer chemistry. Prog. Polym. Sci. 2005, 30, 294–324. [Google Scholar] [CrossRef]
- Deshmukh, S.; Sudhakar, K.; Singh, R. Drag-reduction efficiency, shear stability, and biodegradation resistance of carboxymethyl cellulose-based and starch-based graft copolymers. J. Appl. Polym. Sci. 1991, 43, 1091–1101. [Google Scholar] [CrossRef]
- Kim, O.; Little, R.; Patterson, R.; Ting, R. Polymer structures and turbulent shear stability of drag reducing solutions. Nature 1974, 250, 408–410. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, H.; Chen, H.; Zhang, J.; Xiang, W.; Gao, J. Experiment Study on the Impact of Injection Water Quality Index by Residual Polymer from Polymer Flooding Oilfield. Chem. Eng. Oil Gas 2011, 40, 63–65. [Google Scholar]
- Lai, N.; Guo, X.; Zhou, N.; Xu, Q. Shear resistance properties of modified nano-SiO2/AA/AM copolymer oil displacement agent. Energies 2016, 9, 1037. [Google Scholar] [CrossRef]
- Lai, N.; Tang, L.; Jia, N.; Qiao, D.; Chen, J.; Wang, Y.; Zhao, X. Feasibility Study of Applying Modified Nano-SiO2 Hyperbranched Copolymers for Enhanced Oil Recovery in Low-Mid Permeability Reservoirs. Polymers 2019, 11, 1483. [Google Scholar] [CrossRef]
- Lai, N.; Zhang, Y.; Xu, Q.; Zhou, N.; Wang, H.; Ye, Z. A water-soluble hyperbranched copolymer based on a dendritic structure for low-to-moderate permeability reservoirs. RSC Adv. 2016, 6, 32586–32597. [Google Scholar] [CrossRef]
- Lai, N.; Zhang, Y.; Zeng, F.; Wu, T.; Zhou, N.; Xu, Q.; Ye, Z. Effect of degree of branching on the mechanism of hyperbranched polymer to establish the residual resistance factor in high-permeability porous media. Energy Fuels 2016, 30, 5576–5584. [Google Scholar] [CrossRef]
- Li, H.; Hou, W.; Zhang, Y. Rheological properties of aqueous solution of new exopolysaccharide secreted by a deep-sea mesophilic bacterium. Carbohydr. Polym. 2011, 84, 1117–1125. [Google Scholar] [CrossRef]
- Zong, Z.; Kimura, Y.; Takahashi, M.; Yamane, H. Characterization of chemical and solid state structures of acylated chitosans. Polymer 2000, 41, 899–906. [Google Scholar] [CrossRef]
- Tømmeraas, K.; Köping-Höggård, M.; Vårum, K.; Christensen, B.; Artursson, P.; Smidsrød, O. Preparation and characterisation of chitosans with oligosaccharide branches. Carbohydr. Res. 2002, 337, 2455–2462. [Google Scholar] [CrossRef]
- Baumann, H.; Faust, V. Concepts for improved regioselective placement of O-sulfo, N-sulfo, N-acetyl, and N-carboxymethyl groups in chitosan derivatives. Carbohydr. Res. 2001, 331, 43–57. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Zhao, K. Biomedical applications of chitosan and its derivative nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef]
- Yuan, Y.; Jia, D. Study on the Preparation of Chitosan/Sodium Alginate Copolymer. Adv. Mater. Res. 2012, 531, 39–42. [Google Scholar] [CrossRef]
- Moura, M.; Faneca, H.; Lima, M.; Gil, M.; Figueiredo, M. In situ forming chitosan hydrogels prepared via ionic/covalent co-cross-linking. Biomacromolecules 2011, 12, 3275–3284. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.; Felt, O.; Peppas, N.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Masuda, Y.; Tang, K.; Miyazawa, M.; Tanaka, S. 1D simulation of polymer flooding including the viscoelastic effect of polymer solution. SPE Reserv. Eng. 1992, 7, 247–252. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Zhou, J. Microscopic roles of “viscoelasticity” in HPMA polymer flooding for EOR. Transp. Porous Media 2011, 86, 229–244. [Google Scholar] [CrossRef]
- Clarke, A.; Howe, A.; Mitchell, J.; Staniland, J.; Hawkes, L. How viscoelastic-polymer flooding enhances displacement efficiency. SPE J. 2016, 21, 675–687. [Google Scholar] [CrossRef]
- Luo, C.; Zhang, L.; Hou, J.; Guo, M.; Qiao, J. Molecular weight characterization of polyacrylamide for oil recovery by multiangle light scattering. Acta Polym. Sin. 2012, 3, 313–317. [Google Scholar] [CrossRef]
- Hatzignatiou, D.; Giske, N.; Stavland, A. Polymers and polymer-based gelants for improved oil recovery and water control in naturally fractured chalk formations. Chem. Eng. Sci. 2018, 187, 302–317. [Google Scholar] [CrossRef]
- Lai, N.; Wen, Y.; Yang, Z.; Chen, J.; Zhao, X.; Wang, D.; He, W.; Chen, Y. Polymer flooding in high-temperature and high-salinity heterogeneous reservoir by using diutan gum. J. Pet. Sci. Eng. 2020, 108, 106902. [Google Scholar] [CrossRef]
- Liu, R.; Pu, W.; Du, D. Synthesis and characterization of core-shell associative polymer that prepared with oilfield formation water for polymer flooding. J. Ind. Eng. Chem. 2016, 46, 89–90. [Google Scholar] [CrossRef]











| Ion | Na+ | Ca2+ | Mg2+ | Cl– | TDS |
|---|---|---|---|---|---|
| Content (mg/L) | 5000 | 100 | 100 | 5400 | 10,600 |
| Polymer | Concentration (mg/L) | Permeability (×10–3 μm2) | Porosity (%) | Oil saturation (%) |
|---|---|---|---|---|
| HPAM | 1500 | 317 | 30.59 | 79.35 |
| HPDA | 346 | 30.77 | 85.19 | |
| HPDACS | 327 | 30.63 | 82.49 |
| Polymer | Total Monomer Concentration (wt.%) | AM:AA | Initiator (wt.%) | Functional Monomer (wt.%) | Temperature (°C) | pH | Reaction Time (h) | ||
|---|---|---|---|---|---|---|---|---|---|
| HPAM | 17.6 | 6.9:3.1 | 0.46 | - | 39 | 7 | 6 | ||
| HPDA | 0.26 (MA2.0) | ||||||||
| HPDACS | 0.26 (DACS) | ||||||||
| Monomer | Element | Experimental Value (%) | Theoretical Value (%) |
|---|---|---|---|
| NCS | C | 48.55 | 48.58 |
| N | 0.056 | 0.057 | |
| MACS | C | 51.85 | 51.86 |
| N | 0.22 | 0.23 | |
| DACS | C | 57.62 | 57.66 |
| N | 0.16 | 0.16 |
| Polymer | ηb (mPa·s) | Shear Rate (r/min) | ηa at Different Times (mPa·s) | Viscosity Retention (%) | ||
|---|---|---|---|---|---|---|
| 0 h | 12 h | 24 h | ||||
| HPAM | 373.3 | 3500 | 307.1 | 308.7 | 308.7 | 82.69 |
| 7000 | 278.8 | 280.4 | 282.0 | 75.54 | ||
| 11,500 | 231.7 | 233.6 | 233.6 | 62.58 | ||
| HPDA | 304.7 | 3500 | 269.3 | 270.8 | 270.8 | 88.87 |
| 7000 | 233.8 | 240.2 | 241.8 | 79.36 | ||
| 11,500 | 214.2 | 220.6 | 220.6 | 72.40 | ||
| HPDACS | 539.2 | 3500 | 494.6 | 496.0 | 497.6 | 92.28 |
| 7000 | 453.9 | 458.7 | 460.3 | 85.36 | ||
| 11,500 | 429.2 | 435.5 | 437.1 | 81.06 | ||
| Polymer | ηb (mPa·s) | Shear Rate (r/min) | ηa at Different Times (mPa·s) | Viscosity Retention (%) | ||
|---|---|---|---|---|---|---|
| 0 h | 12 h | 24 h | ||||
| HPAM | 11.0 | 3500 | 5.2 | 5.3 | 5.3 | 48.18 |
| 7000 | 4.1 | 4.2 | 4.3 | 39.09 | ||
| 11,500 | 3.3 | 3.3 | 3.4 | 30.91 | ||
| HPDA | 7.0 | 3500 | 4.1 | 4.1 | 4.1 | 58.57 |
| 7000 | 3.5 | 3.5 | 3.6 | 51.43 | ||
| 11,500 | 2.8 | 2.9 | 3.0 | 42.86 | ||
| HPDACS | 15.6 | 3500 | 11.8 | 11.8 | 11.9 | 76.28 |
| 7000 | 10.6 | 10.7 | 10.8 | 69.23 | ||
| 11,500 | 9.4 | 9.5 | 9.5 | 60.90 | ||
| Aging Time (Days) | Viscosity (mPa·s) | |||||
|---|---|---|---|---|---|---|
| Ultrapure Water | Simulated Brine | |||||
| HPAM | HPDA | HPDACS | HPAM | HPDA | HPDACS | |
| 0 | 373.3 | 304.7 | 539.2 | 11.0 | 7.0 | 15.6 |
| 5 | 366.9 | 289.5 | 528.4 | 10.7 | 6.7 | 15.3 |
| 10 | 355.7 | 280.3 | 512.2 | 10.5 | 6.4 | 14.7 |
| 20 | 342.6 | 262.0 | 496.1 | 9.6 | 6.0 | 14.0 |
| 30 | 330.1 | 246.8 | 479.9 | 9.0 | 5.7 | 13.4 |
| 60 | 312.9 | 240.7 | 458.3 | 8.6 | 4.9 | 12.8 |
| 90 | 294.9 | 228.5 | 442.1 | 7.8 | 4.3 | 12.4 |
| Polymers | Shearing Rate (s−1) | Ultrapure Water | Simulated Brine | ||||
|---|---|---|---|---|---|---|---|
| K1 (Pa·sn−1) | n | R2 | K2 (Pa·sn−1) | n | R2 | ||
| HPAM | 1~1000 | 946.89916 | 0.31522 | 0.9969 | 436.8173 | 0.2905 | 0.9984 |
| 1000~1 | 577.3057 | 0.2094 | 0.9853 | 213.9437 | 0.3909 | 0.9963 | |
| HPDA | 1~1000 | 1276.2056 | 0.2744 | 0.9968 | 627.7289 | 0.2340 | 0.9919 |
| 1000~1 | 948.7144 | 0.3462 | 0.9985 | 414.0435 | 0.1500 | 0.9970 | |
| HPDACS | 1~1000 | 1945.5635 | 0.1772 | 0.9900 | 797.2804 | 0.1414 | 0.9961 |
| 1000~1 | 1820.0343 | 0.0936 | 0.9976 | 571.9893 | 0.0377 | 0.9934 | |
| Polymers | Ultrapure Water | Simulated Brine | ||
|---|---|---|---|---|
| Gc (Pa) | λc (s) | Gc (Pa) | λc (s) | |
| HPAM | 0.5903 | 1.0156 | 0.6478 | 0.5583 |
| HPDA | 3.1931 | 0.9916 | 0.1490 | 1.2192 |
| HPDACS | 4.8897 | 0.5583 | 0.1531 | 1.3071 |
| Polymer | Permeability (×10−3μm2) | E2 (%) | E1 (%) | EOR (%) |
|---|---|---|---|---|
| HPAM | 317 | 55.14 | 44.49 | 10.65 |
| HPDA | 346 | 70.57 | 56.85 | 13.72 |
| HPDACS | 327 | 70.65 | 51.45 | 19.20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Ye, Z.; Tang, L.; Wu, T.; Jiang, Q.; Lai, N. Synthesis and Solution Properties of a Novel Hyperbranched Polymer Based on Chitosan for Enhanced Oil Recovery. Polymers 2020, 12, 2130. https://doi.org/10.3390/polym12092130
Chen Q, Ye Z, Tang L, Wu T, Jiang Q, Lai N. Synthesis and Solution Properties of a Novel Hyperbranched Polymer Based on Chitosan for Enhanced Oil Recovery. Polymers. 2020; 12(9):2130. https://doi.org/10.3390/polym12092130
Chicago/Turabian StyleChen, Qingyuan, Zhongbin Ye, Lei Tang, Tao Wu, Qian Jiang, and Nanjun Lai. 2020. "Synthesis and Solution Properties of a Novel Hyperbranched Polymer Based on Chitosan for Enhanced Oil Recovery" Polymers 12, no. 9: 2130. https://doi.org/10.3390/polym12092130
APA StyleChen, Q., Ye, Z., Tang, L., Wu, T., Jiang, Q., & Lai, N. (2020). Synthesis and Solution Properties of a Novel Hyperbranched Polymer Based on Chitosan for Enhanced Oil Recovery. Polymers, 12(9), 2130. https://doi.org/10.3390/polym12092130





