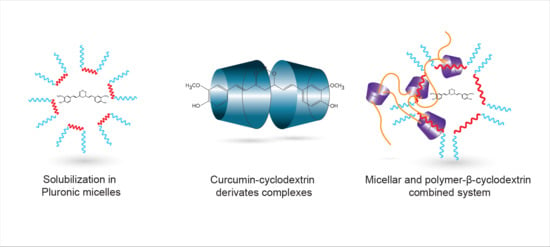
Highly Enhanced Curcumin Delivery Applying Association Type Nanostructures of Block Copolymers, Cyclodextrins and Polycyclodextrins
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Preparation of the Curcumin Loaded Pluronic Micelles
2.2.2. Dissolution of Curcuminin Cyclodextrin Solutions
2.3. Characterization of Curcumin Loaded Nanostructures
2.3.1. Drug Content
2.3.2. FTIR Spectroscopy
2.3.3. NMR Spectroscopy
2.3.4. In vitro Drug Release and Release Kinetics
3. Results and Discussion
3.1. Solubilisation in Pluronic Micelles
3.2. Curcumin—Cyclodextrin Derivates Complexes
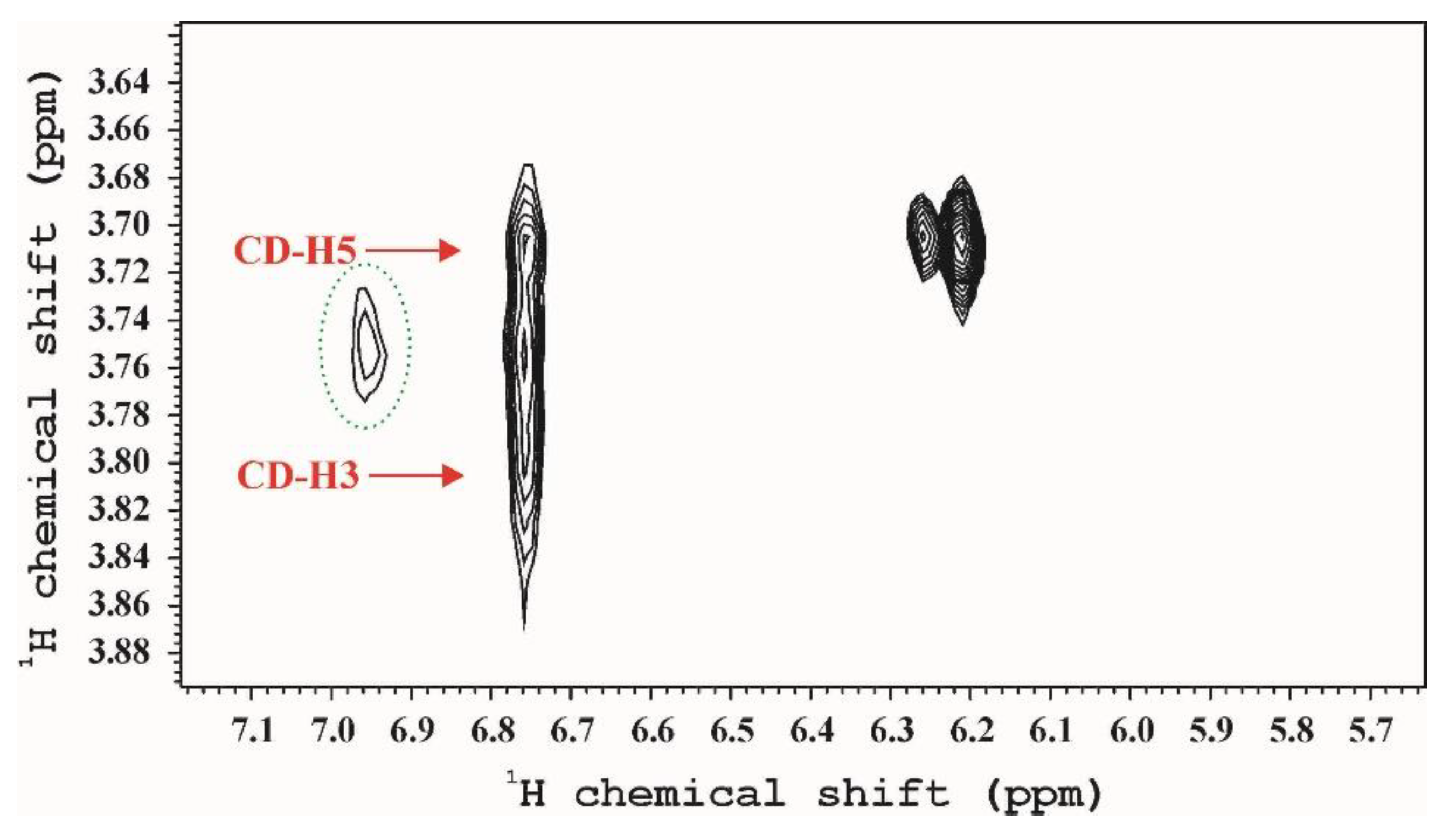
3.2.1. NMR
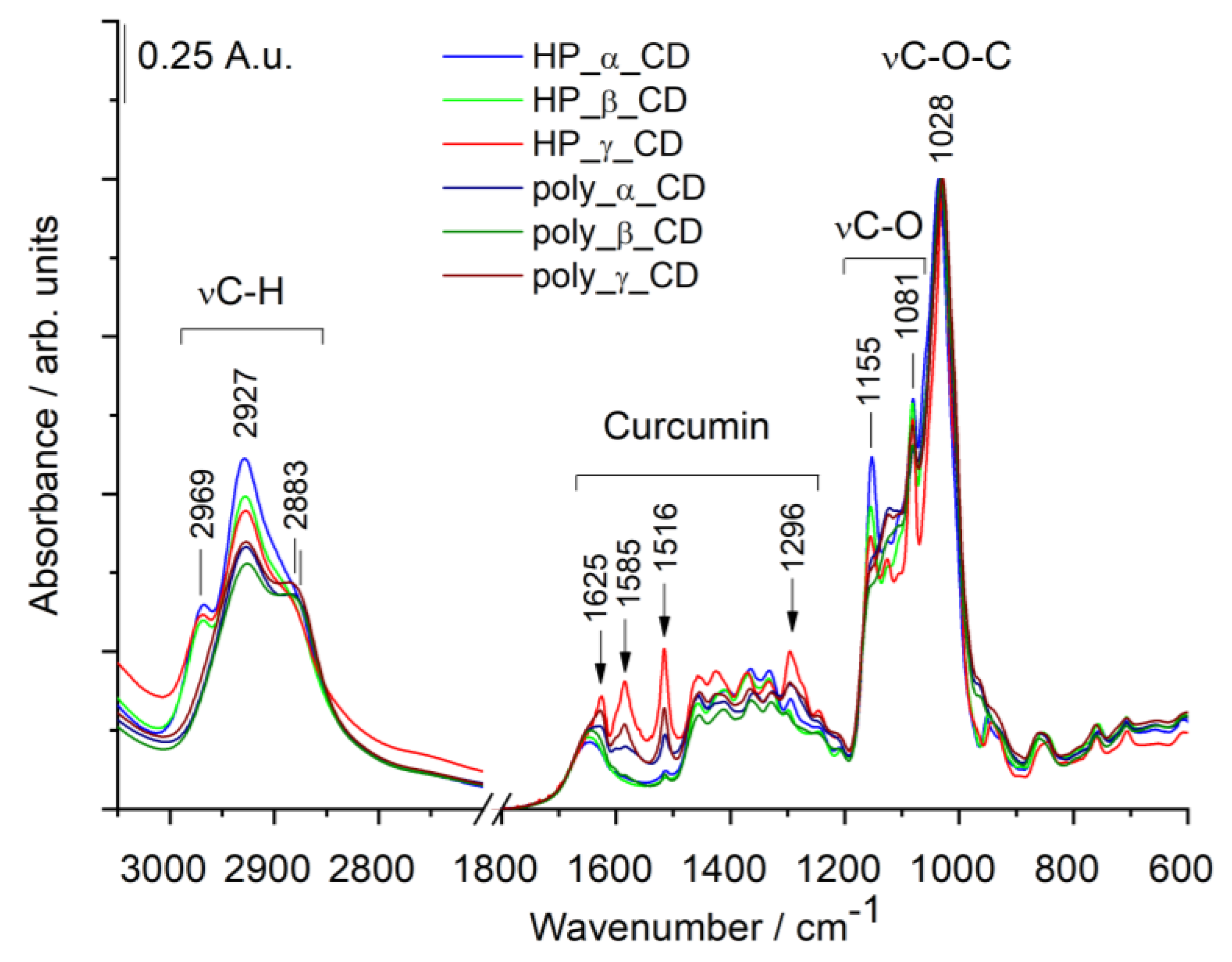
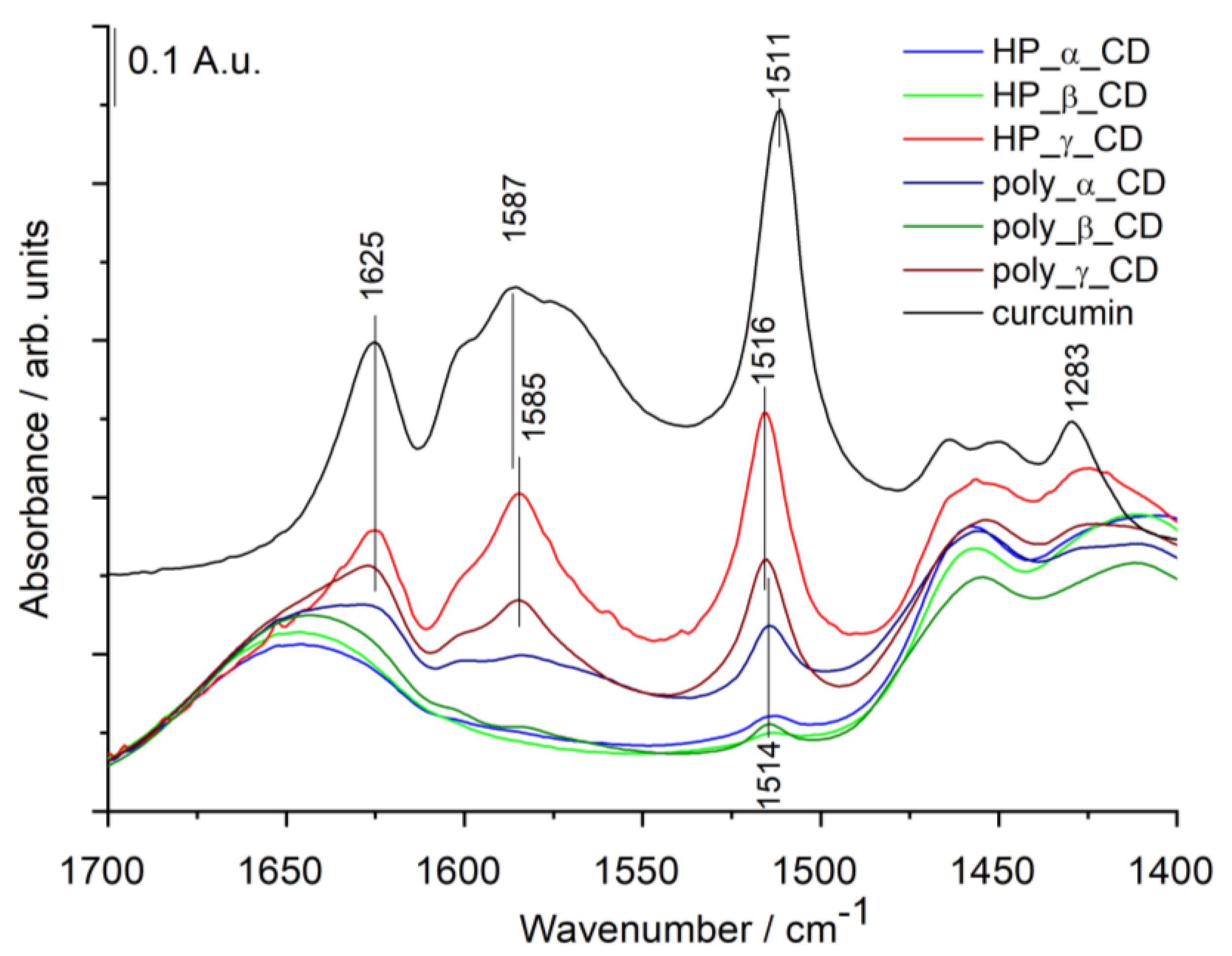
3.2.2. Infrared (IR) Spectra of Cyclodextrin—Curcumin Complexes
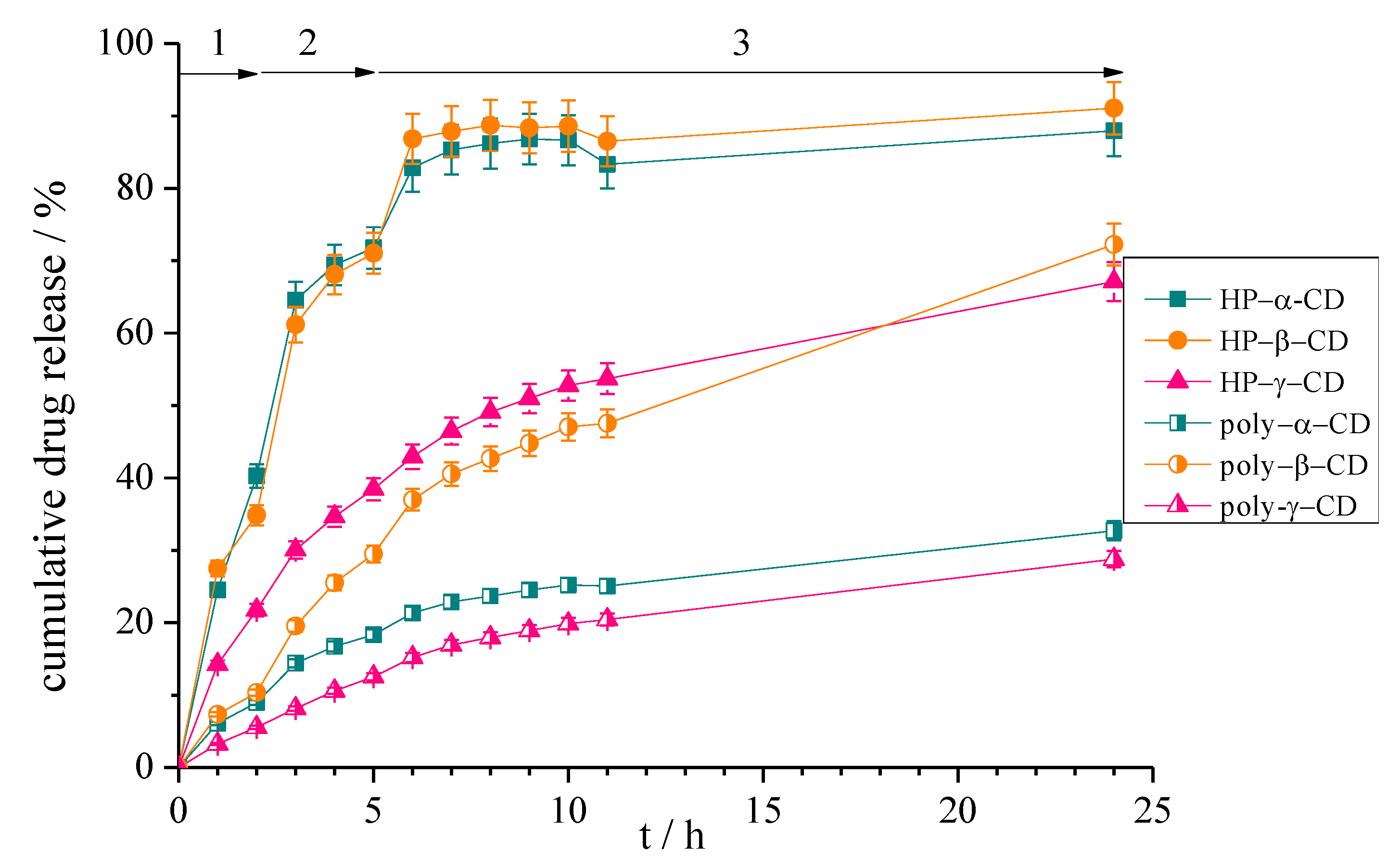
3.2.3. Drug Release
3.3. Curcumin Loaded Micellar System Stabilized by Poly-β-CD
Release from the Micellarand Poly-β-CD Combined Systems
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [PubMed]
- Naksuriya, O.; Okonogi, S.; Schiffelers, R.M.; Hennink, W.E. Curcuminnanoformulations: A review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials 2014, 35, 3365–3383. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Y.; Hu, C.H.; Huang, W.C.; Ho, C.Y.; Yao, C.H.; Huang, C.H. Effects of Bilayer Nanofibrous Scaffolds ContainingCurcumin/Lithospermi Radix Extract on WoundHealing in Streptozotocin-Induced Diabetic Rats. Polymers 2019, 11, 1745. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.U.; Zia, K.M.; Nazir, A.; Iqbal, J.; Ejaz, S.A.; Akash, M.S.H. Pluronic-Based Mixed Polymeric Micelles Enhance the Therapeutic Potential of Curcumin. AAPS Pharm. Sci. Tech. 2018, 19, 2719–2739. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Zhong, Q.; Baek, S.J. Enhanced dispersibilty and bioactivity of curcumin by encapsulation in casein nanocapsules. J. Agric. Food Chem. 2013, 61, 6036–6043. [Google Scholar] [CrossRef]
- Kaur, K.; Kumar, R.; Mehta, S.K. Nanoemulsion: A new medium to study the interactions and stability of curcumin with bovine serum albumin. J. Molec. Liquids 2015, 209, 62–70. [Google Scholar] [CrossRef]
- Cui, J.; Zhou, J.; Huang, L.; Jing, J.; Wang, N.; Wang, L. Curcumin encapsulation and protection based on lysozyme nanoparticles. Food Sci. Nutr. 2019, 7, 2702–2707. [Google Scholar] [CrossRef]
- Saxena, V.; Hussain, M.D. Polymeric Mixed Micelles for Delivery of Curcumin to Multidrug Resistant Ovarian Cancer. J. Biomed. Nanotechnol. 2013, 9, 7. [Google Scholar] [CrossRef]
- Davis, B.M.; Pahlitzsch, M.; Guo, L.; Balendra, S.; Shah, P.; Ravindran, N.; Malaguarnera, G.; Sisa, C.; Shamsher, E.; Hamze, H.; et al. Topical CurcuminNanocarriers are Neuroprotective in Eye Disease. Sci. Rep. 2018, 8, 1106. [Google Scholar] [CrossRef]
- Iurciuc-Tincu, C.E.; Cretan, M.S.; Purcar, V.; Popa, M.; Daraba, O.M.; Atanase, L.I.; Ochiuz, L. Drug Delivery System Based on pH-Sensitive Biocompatible Poly(2-vinyl pyridine)-b-poly(ethyleneoxide) Nanomicelles Loaded with Curcuminand 5-Fluorouracil. Polymers 2020, 12, 1450. [Google Scholar] [CrossRef]
- Beloqui, A.; Memvanga, P.B.; Coco, R.; Reimondez-Troitino, S.; Alhouayek, M.; Mucciolie, G.G.; Alonsoc, M.J.; Csaba, N.; de la Fuente, M.; Préat, V. A comparative study of curcumin-loaded lipid-based nanocarriersinthe treatment of inflammatory bowel disease. Colloids Surf. B Biointerfaces 2016, 143, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Araujo, V.H.S.; Bento da Silva, P.; Szlachetka, I.O.; William da Silvac, S.; Santos, B.F.; Chorilli, M.; Ganassin, R.; Tavares de Oliveira, G.R.; Oliveira da Rocha, M.C.; Fernandese, R.P.; et al. The influence of NLC composition on curcumin loading under a physicochemical perspective and in vitro evaluation. Colloids Surf. A 2020, 602, 125070. [Google Scholar] [CrossRef]
- Rafiee, Z.; Nejatian, M.; Daeihamed, M.; Jafari, S.M. Application of different nanocarriers for encapsulation of curcumin. Crit. Rev. Food Sci. Nutr. 2018, 3468–3497. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sahoo, S.K.; Padhee, K.; Kochar, P.P.S.; Satapathy, A.; Pathak, N. Review onSolubility Enhancement Techniques for Hydrophobic Drugs. Pharm. Glob. 2011, 2, 1–7. [Google Scholar]
- Araiza-Calahorra, A.; Akhtar, M.; Sarkar, A. Recent advances in emulsionbased delivery approaches for curcumin: From encapsulation to bioaccessibility. Trends Food Sci. Technol. 2018, 71, 155–169. [Google Scholar] [CrossRef]
- Szejtli, J. Cyclodextrins and molecular encapsulation. In Encyclopedia of Nanoscience and Nanotechnology; Nalwa, H.S., Ed.; American Scientific Publishers: Stevenson Ranch, CA, USA, 2004; Volume 2, pp. 283–304. ISBN 1-58883-001-2. [Google Scholar]
- Raval, A.; Pillai, S.A.; Bahadur, A.; Bahadur, P. Systematic characterization of Pluronic®micelles and their application for solubilization and in vitro release of some hydrophobic anticancer drugs. J. Mol. Liq. 2017, 230, 473–481. [Google Scholar] [CrossRef]
- Liu, R.; Forrest, M.L.; Kwon, G.S.; Xiong, X.; Zhang, Z. Polymeric Micelles in Water-Insoluble Drug Delivery. In Water-Insoluble Drug Formulation; Liu, R., Ed.; CRC Press Taylor and Frances Group: Boca Raton, FL, USA, 2018; pp. 336–404. [Google Scholar]
- Malmsten, M. Block copolymers in pharmaceutics. In Amphiphilic Block Copolymers, Self-Assembly and Applications; Alexandridis, P., Lindman, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 319–347. [Google Scholar]
- Alakhova, D.Y.; Kabanov, A.V. Pluronics and MDR Reversal: An Update. Mol. Pharmac. 2014, 11, 2566–2578. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Batrakova, E.V.; Alakhov, V.Y. Pluronic® block copolymers for overcoming drug resistance in cancer. Adv. Drug Deliv. Rev. 2002, 54, 759–779. [Google Scholar] [CrossRef]
- Sahu, A.; Kasoju, N.; Goswami, P.; Bora, U. Encapsulation of Curcumin in Pluronic Block Copolymer Micelles for Drug Delivery Applications. J. Biomat. Appl. 2011, 25, 619–639. [Google Scholar] [CrossRef]
- Zhao, L.; Du, J.; Duan, Y.; Zang, Y.; Zhang, H.; Yang, C.; Cao, F.; Zhai, G. Curcumin loaded mixed micelles composed of Pluronic P123 and F68: Preparation, optimization and in vitro characterization. Colloids Surf. B Biointerfaces 2012, 97, 101–108. [Google Scholar] [CrossRef]
- Chiappetta, D.A.; Sosnik, A. Poly(ethylene oxide)–poly(propylene oxide) block copolymer micelles as drug delivery agents: Improved hydrosolubility, stability and bioavailability of drugs. Eur. J. Pharm. Biopharm. 2007, 66, 303–317. [Google Scholar] [CrossRef]
- Oh, K.T.; Bronich, T.K.; Kabanov, A.V. Micellar formulations for drug delivery based on mixtures of hydrophobic and hydrophilic Pluronic block copolymers. J. Control. Release 2004, 94, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Ramdani, L.; Bourboulou, R.L.; Belkouch, M.; Jebors, S.; Tauran, Y.; Parizot, C.; Suwinska, K.; Coleman, A.W.; Duyckaerts, C.; Lazar, A.N. MulifunctionalCurcumin-Nanocarriers Based on Host-Guest Interactions for Alzheimer Disease Diagnostic. J. Nanomed. Nanotechnol. 2015, 6, 2. [Google Scholar] [CrossRef]
- Tonnesen, H.H.; Masson, M.; Loftsson, T. Studies of curcumin and curcuminoids XXVII. Cyclodextrincomplexation: Solubility, chemical and photochemical stability. Int. J. Pham. 2002, 244, 127–135. [Google Scholar] [CrossRef]
- Baglole, K.N.; Boland, P.G.; Wagner, B.D. Fluorescence enhancement of curcumin upon inclusion intoparent and modified cyclodextrins. J. Photochem. Photobiol. A Chem. 2005, 173, 230–237. [Google Scholar] [CrossRef]
- Kozlowska, J.; Kolodziejska, M.; Morin, S.; Sliwa, W. Selected applications of cyclodextrin polymers. Ars Sep. Acta 2012, 9–10, 37–57. [Google Scholar]
- Bahadur, P.; Pandya, K. Aggregation Behavior of Pluronic P-94 in Water. Langmuir 1992, 8, 2666–2670. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Kabanov, A.V. Pluronic block copolymers: Evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J. Control. Release 2008, 130, 98–106. [Google Scholar] [CrossRef]
- Fromming, K.-H.; Szejtli, J. Cyclodextrins in Pharmacy; Springer: Dordrecht, The Netherlands, 1994. [Google Scholar] [CrossRef]
- Malanga, M.; Bálint, M.; Puskás, I.; Tuza, K.; Sohajda, S.; Jicsinszky, L.; Szente, L.; Fenyvesi, É. Synthetic strategies for the fluorescent labeling of epichlorohydrin-branched cyclodextrin polymers. Beilstein J. Org. Chem. 2014, 10, 3007–3018. [Google Scholar] [CrossRef]
- Akl, M.A.; Kartal-Hodzic, A.; Oksanen, T.; Ismael, H.R.; Afouna, M.M.; Yliperttula, M.; Samy, A.M.; Viitala, T. Factorial design formulation optimization and in vitro characterizationofcurcumin-loaded PLGA nanoparticles for colon delivery. J. Drug Deliv. Sci. Technol. 2016, 32, 10–20. [Google Scholar] [CrossRef]
- Nagy, N.Z.; Varga, Z.; Mihaly, J.; Kasza, G.; Ivan, B.; Kiss, E. Highly efficient encapsulation of curcumin into and pH-controlled drug release from poly(ε-caprolactone)nanoparticles stabilized with a novel amphiphilichyperbranchedpolyglycerol. Expr. Polym. Lett. 2020, 14, 90–101. [Google Scholar] [CrossRef]
- Alexandridis, P.; Nivaggioli, T.; Hatton, T.A. Temperature Effects on Structural Properties of Pluronic P104 and F108 PEO-PPO-PEO Block Copolymer Solutions. Langmuir 1995, 11, 1468–1476. [Google Scholar] [CrossRef]
- Alexandridis, P.; Zhou, D.; Khan, A. Lyotropic Liquid Crystallinity in Amphiphilic Block Copolymers: Temperature Effects on Phase Behavior and Structure for Poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) Copolymers of Different Composition. Langmuir 1996, 12, 2690–2700. [Google Scholar] [CrossRef]
- Meznarich, N.A.K. Effect of Ternary Solutes on the Evolution of Structure and Gel Formation in Amphiphilic Copolymer Solutions. Ph.D. Thesis, The University of Michigan, Ann Arbor, MI, USA, 2012. [Google Scholar]
- Ivanova, R.; Lindman, B.; Alexandridis, P. Effect of Glycols on the Self-Assembly of AmphiphilicBlock Copolymers in Water. 1. Phase Diagrams and Structure Identification. Langmuir 2000, 16, 3660–3675. [Google Scholar] [CrossRef]
- Jang, J.D.; Kim, E.; Lee, M.J.; Han, Y.S.; Bang, J.; Kim, T.H. Unexpected Phase Behavior of Pluronic Polymer-Organic Derivative Mixtures Depending on Temperature in Aqueous Solution. Micromachines 2018, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, N.; Caldwell, K. Structural transitions in micellar solutions of Pluronic P-105 and their effect on the conformation of dissolved Cytochrome C: An electron paramagnetic resonance investigation. Colloids Surf. B Biointerfaces 1994, 3, 217–228. [Google Scholar] [CrossRef]
- Tang, B.; Ma, L.; Wang, H.; Zhang, G. Study on the Supramolecular Interaction of Curcumin and cyclodextrin by Spectrophotometry and Its Analytical Application. J. Agricult. Food Chem. 2002, 50, 1355–1361. [Google Scholar] [CrossRef]
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Fundamentals and Applications of Cyclodextrins. In Cyclodextrin Fundamentals, Reactivity and Analysis; Fourmentin, S., Crini, G., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2018; pp. 3–55. [Google Scholar]
- Payton, F.; Sandusky, P.; Alwort, W.L. NMR Study of the Solution Structure of Curcumin. J. Nat. Prod. 2007, 70, 2143–2146. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. β-Cyclodextrin-Curcumin Self-Assembly Enhances Curcumin Delivery in Prostate Cancer Cells. Colloids Surf. BBiointerfaces 2010, 79, 113–125. [Google Scholar] [CrossRef]
- Chen, J.; Qin, X.; Zhong, S.; Chen, S.; Su, W.; Liu, Y. Characterization of Curcumin/Cyclodextrin Polymer Inclusion Complex and Investigation on Its Antioxidant and Antiproliferative Activities. Molecules 2018, 23, 1179. [Google Scholar] [CrossRef]
- Szegedi, Á.; Shestakova, P.; Trendafilova, I.; Mihaly, J.; Tsacheva, I.; Mitova, V.; Kyulavska, M.; Koseva, N.; Momekova, D.; Konstantinov, S. Modified Mesoporous Silica Nanoparticles Coated by Polymer Complex as Novel Curcumin Delivery Carriers. J. Drug Deliv. Sci. Technol. 2019, 49, 700–712. [Google Scholar] [CrossRef]
- Mangolim, C.S.; Moriwaki, C.; Nogueira, A.C.; Sato, F.; Baesso, M.L.; Neto, A.M.; Matioli, G. Curcumin–β-Cyclodextrin Inclusion Complex: Stability, Solubility, Characterisation by FT-IR, FT-Raman, X-Ray Diffraction and Photoacoustic Spectroscopy, and Food Application. Food Chem. 2014, 153, 361–370. [Google Scholar] [CrossRef] [PubMed]
- López-Tobar, E.; Blanch, G.P.; Ruiz del CastilloSanchez-Cortes, M.L.S. Encapsulation and isomerization of curcumin with cyclodextrins characterized by electronic and vibrational spectroscopy. Vib. Spectrosc. 2012, 62, 292–298. [Google Scholar] [CrossRef]
- Manolova, Y.; Deneva, V.; Drakalska, E.; Momekova, D.; Lambov, N. The effect of the water on the curcumintautomerism: A quantitative approach. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wyman, I.W. Supramolecular Nanostructures Based on Cyclodextrin and Poly(ethylene oxide): Syntheses, Structural Characterizations and Applications for Drug Delivery. Polymers 2016, 8, 198. [Google Scholar] [CrossRef] [PubMed]






| Pluronic | M/g·mol−1 | nPEO-mPPO-nPEO | cmc/mol·L−1 (37 °C) | cmc/g·L−1 (37 °C) | HLB |
|---|---|---|---|---|---|
| 94 | 4600 | 24-47-24 | 4.3 × 10−6 a | 0.020 | 13.5 |
| 105 | 6500 | 37-56-37 | 1.5 × 10−5 b | 0.097 | 15 |
| 108 | 14,600 | 132-56-132 | 2.2 × 10−5 c | 0.32 | 27 |
| 127 | 12,600 | 97-63-97 | 2.8 × 10−6 c | 0.034 | 22 |
| Cyclodextrin | M/g·mol−1 | Inner Diameter of Cavity a/nm | Degree of Substitution |
|---|---|---|---|
| HP-α-CD | 1180 | 0.53 | 3.6 |
| HP-β-CD | 1396 | 0.65 | 4.6 |
| HP-γ-CD | 1580 | 0.83 | 4.8 |
| poly-α-CD | ~150,000 | 5–6 b | |
| poly-β-CD | ~165,000 | 5–6 b | |
| poly-γ-CD | ~227,000 | 5–6 b |
| Pluronic | S/molcurcumin/molPluronic | S*/molcurcumin/molPluronic |
|---|---|---|
| 108 | 0.287 | 0.39 |
| 127 | 0.362 | 0.530 |
| 105 | 0.473 | 0.875 |
| 94 | 0.526 | 0.526 |
| Sample | IR: Relative Amount of curc/C-O-C bonds (A (1516 cm−1)/A (1027 cm−1)) | NMR: |
|---|---|---|
| HP-α-CD | 0.12 | 0.027 |
| HP-β-CD | 0.06 | 0.007 |
| HP-γ-CD | 2.44 | 0.402 |
| poly-α-CD | 0.50 | 0.093 |
| poly-β-CD | 0.14 | 0.088 |
| poly-γ-CD | 1.39 | 0.179 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagy, N.Z.; Varga, Z.; Mihály, J.; Domján, A.; Fenyvesi, É.; Kiss, É. Highly Enhanced Curcumin Delivery Applying Association Type Nanostructures of Block Copolymers, Cyclodextrins and Polycyclodextrins. Polymers 2020, 12, 2167. https://doi.org/10.3390/polym12092167
Nagy NZ, Varga Z, Mihály J, Domján A, Fenyvesi É, Kiss É. Highly Enhanced Curcumin Delivery Applying Association Type Nanostructures of Block Copolymers, Cyclodextrins and Polycyclodextrins. Polymers. 2020; 12(9):2167. https://doi.org/10.3390/polym12092167
Chicago/Turabian StyleNagy, Nóra Zsuzsanna, Zoltán Varga, Judith Mihály, Attila Domján, Éva Fenyvesi, and Éva Kiss. 2020. "Highly Enhanced Curcumin Delivery Applying Association Type Nanostructures of Block Copolymers, Cyclodextrins and Polycyclodextrins" Polymers 12, no. 9: 2167. https://doi.org/10.3390/polym12092167
APA StyleNagy, N. Z., Varga, Z., Mihály, J., Domján, A., Fenyvesi, É., & Kiss, É. (2020). Highly Enhanced Curcumin Delivery Applying Association Type Nanostructures of Block Copolymers, Cyclodextrins and Polycyclodextrins. Polymers, 12(9), 2167. https://doi.org/10.3390/polym12092167