Enhanced Dielectric and Hydrophobic Properties of Poly(vinylidene fluoride-trifluoroethylene)/TiO2 Nanowire Arrays Composite Film Surface Modified by Electrospinning
Abstract
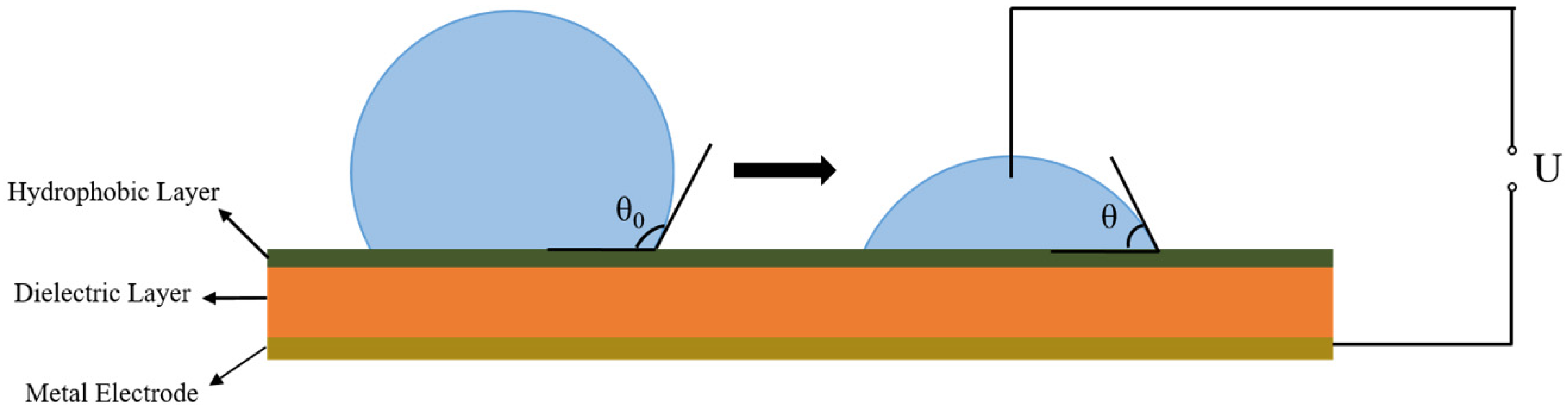
1. Introduction
2. Materials and Methods
2.1. Materials
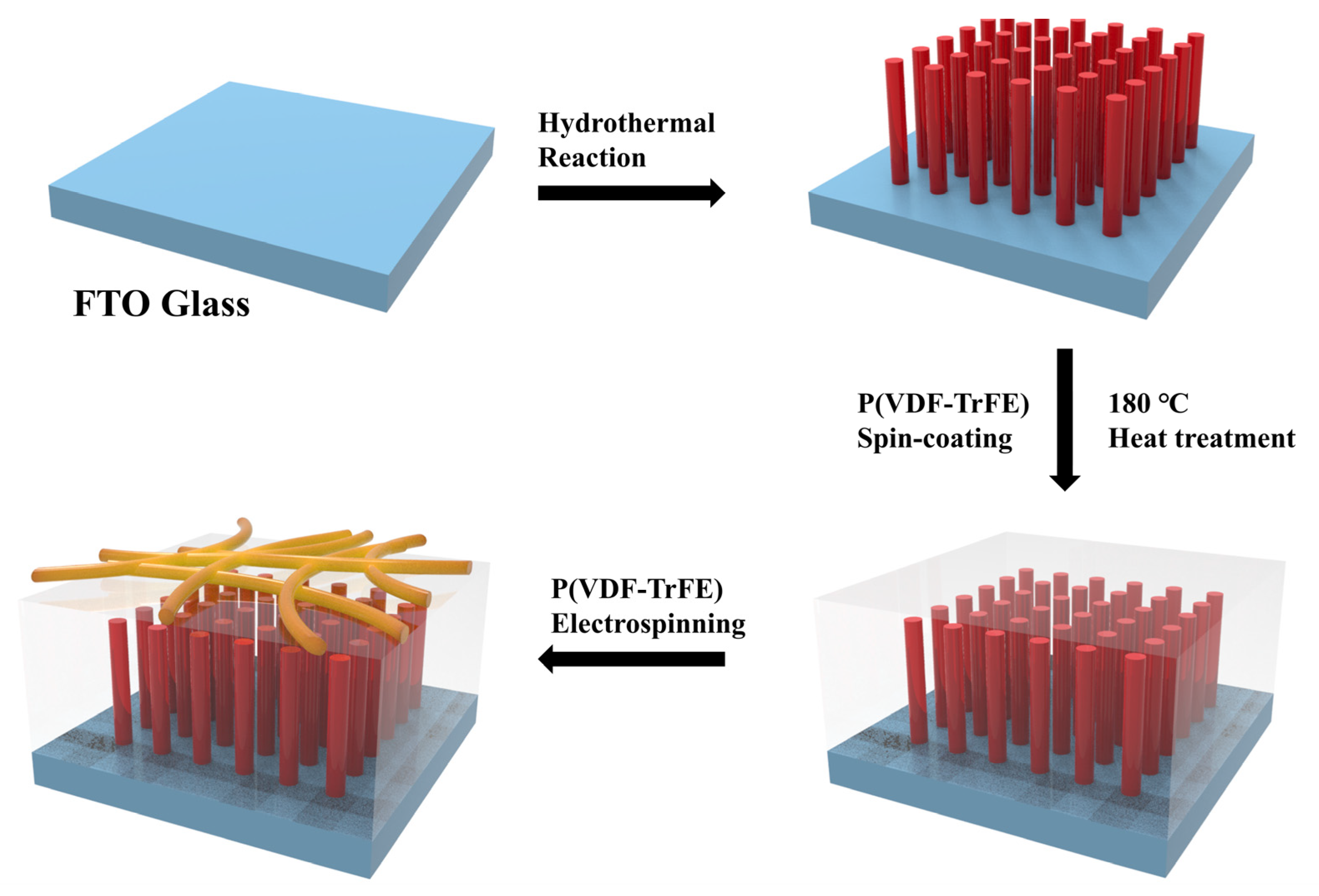
2.2. Preparation of TiO2 Nanowire Arrays (TNA)
2.3. Fabrication of P(VDF-TrFE) Nanofibers–P(VDF-TrFE)/TiO2 Nanowire Arrays (PVTNF-PVT/TNA)
2.4. Characterization
3. Results and Discussions
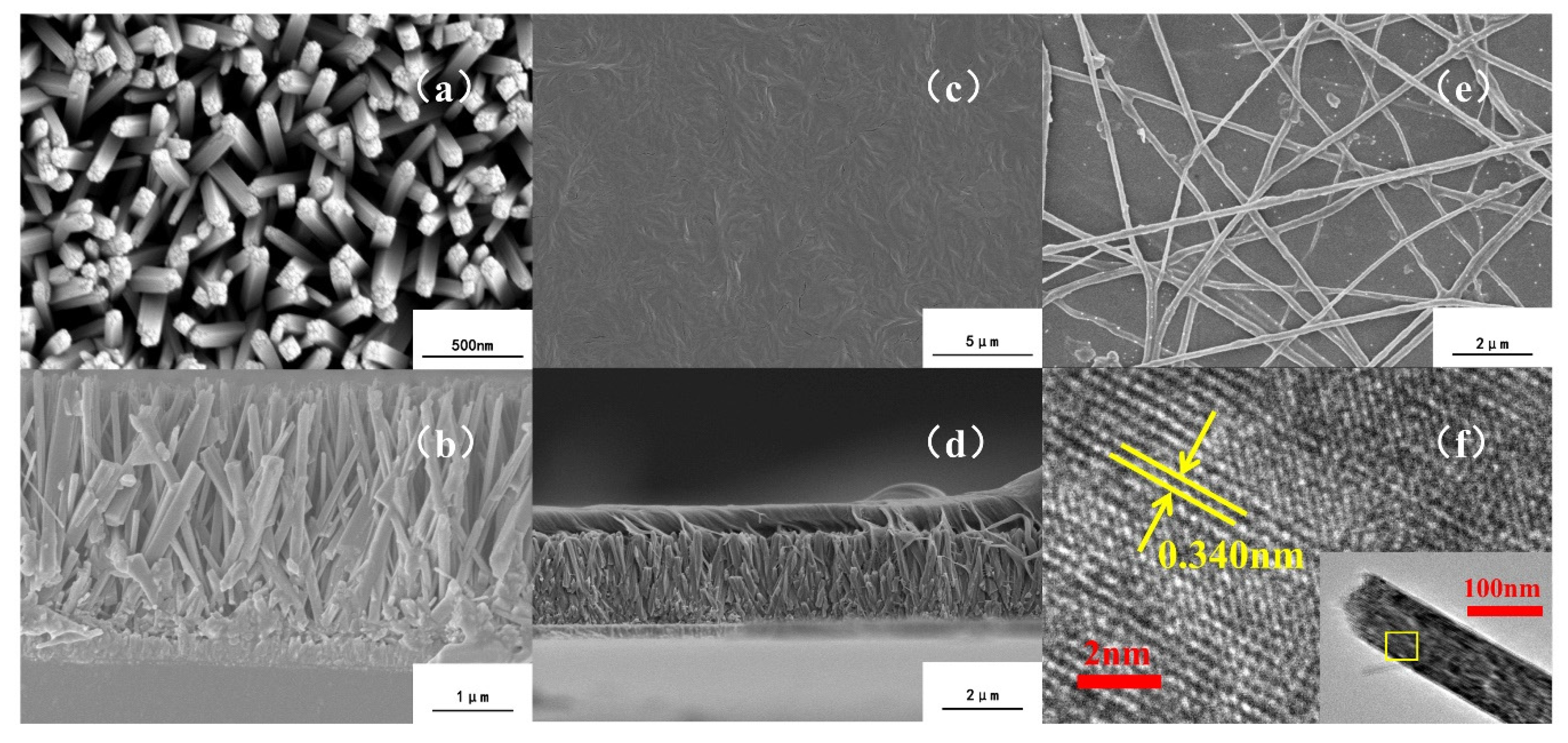
3.1. Surface and Cross-Section Morphology
3.2. FTIR and XRD Phase Analysis
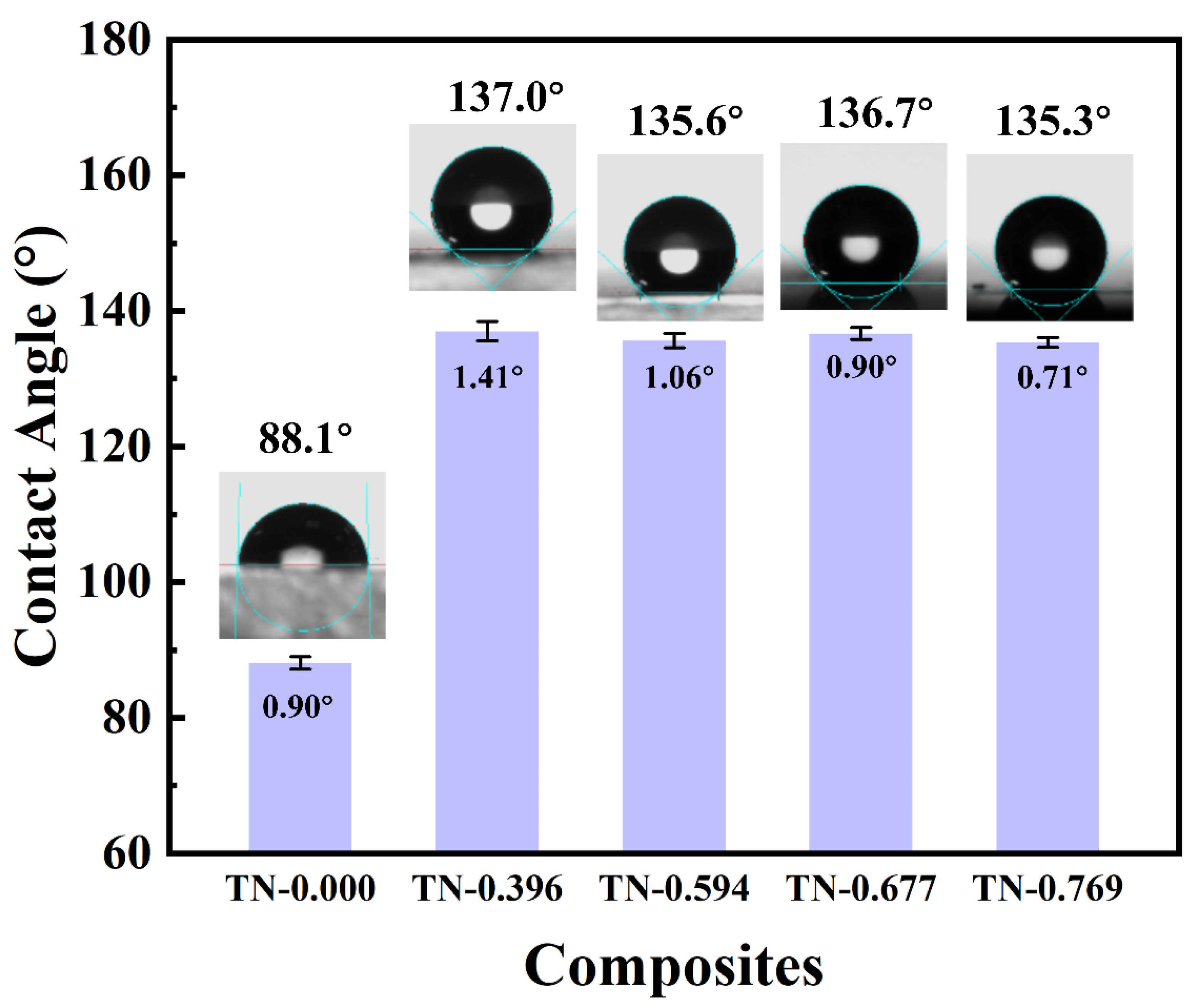
3.3. Surface Roughness and Contact Angle
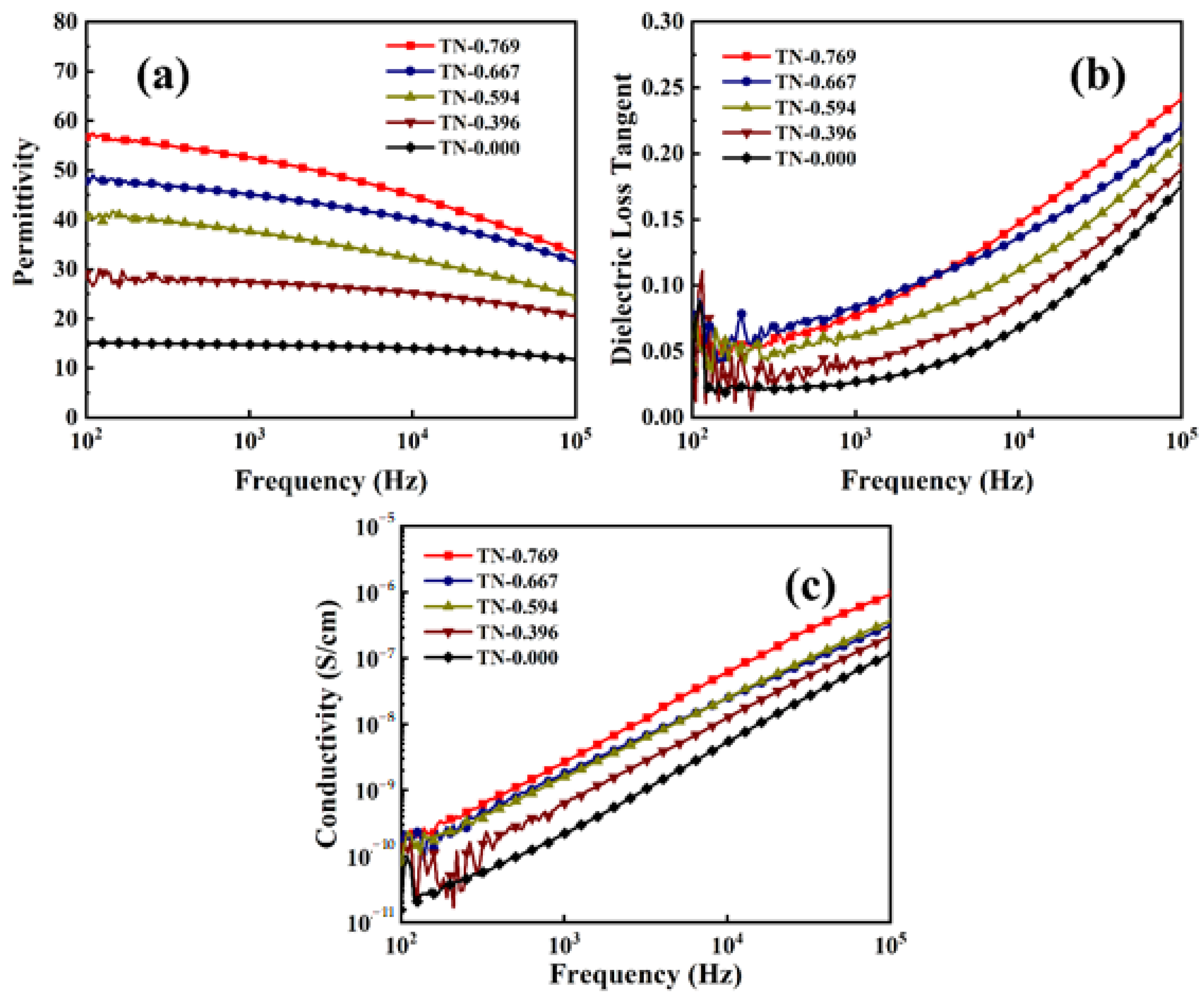
3.4. Dielectric Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shamai, R.; Andelman, D.; Berge, B.; Hayes, R. Water, electricity, and between... On electrowetting and its applications. Soft Matter 2008, 4, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.A.; Feenstra, B.J. Video-speed electronic paper based on electrowetting. Nature 2003, 425, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Mugele, F.; Duits, M.; van den Ende, D. Electrowetting: A versatile tool for drop manipulation, generation, and characterization. Adv. Colloid Interface Sci. 2010, 161, 115–123. [Google Scholar] [CrossRef]
- Nelson, W.C.; Kim, C.-J.C. Droplet Actuation by Electrowetting-on-Dielectric (EWOD): A Review. J. Adhes. Sci. Technol. 2012, 26, 1747–1771. [Google Scholar] [CrossRef]
- Lee, P.T.; Chiu, C.W.; Lee, T.M.; Chang, T.Y.; Wu, M.T.; Cheng, W.Y.; Kuo, S.W.; Lin, J.J. First fabrication of electrowetting display by using pigment-in-oil driving pixels. ACS Appl. Mater. Interfaces 2013, 5, 5914–5920. [Google Scholar] [CrossRef]
- He, T.; Jin, M.; Eijkel, J.C.; Zhou, G.; Shui, L. Two-phase microfluidics in electrowetting displays and its effect on optical performance. Biomicrofluidics 2016, 10, 011908. [Google Scholar] [CrossRef]
- Li, H.; Li, R.; Jiang, H.; Fang, X.; Yin, X.; Zhou, R. Fabrication and evaluation of flexible electrowetting display with support pillars. Nanoscale Adv. 2020, 2, 4077–4084. [Google Scholar] [CrossRef]
- Agarwal, A.; Salahuddin, A.; Wang, H.; Ahamed, M.J. Design and development of an efficient fluid mixing for 3D printed lab-on-a-chip. Microsyst. Technol. 2020, 26, 2465–2477. [Google Scholar] [CrossRef]
- Mark, D.; Haeberle, S.; Roth, G.; von Stetten, F.; Zengerle, R. Microfluidic lab-on-a-chip platforms: Requirements, characteristics and applications. Chem. Soc. Rev. 2010, 39, 1153–1182. [Google Scholar] [CrossRef]
- Clement, C.E.; Thio, S.K.; Park, S.-Y. An optofluidic tunable Fresnel lens for spatial focal control based on electrowetting-on-dielectric (EWOD). Sens. Actuators B 2017, 240, 909–915. [Google Scholar] [CrossRef]
- Hu, G.-H.; Xu, A.-J.; Xu, Z.; Zhou, Z.-W. Dewetting of nanometer thin films under an electric field. Phys. Fluids 2008, 20. [Google Scholar] [CrossRef]
- Mugele, F.; Baret, J.-C. Electrowetting: From basics to applications. J. Phys. 2005, 17, R705–R774. [Google Scholar] [CrossRef]
- Chen, J.; Xiong, X.; Zhang, Q.; Shui, L.; Shen, S.; Yang, H.; Zhu, Z.; Zhang, F. P(VDF-TrFE)/PMMA Blended Films with Enhanced Electrowetting Responses and Superior Energy Storage Performance. Polymers 2019, 11, 526. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, H.; Zhu, Z.; Yang, H.; Zhang, Q. Enhanced dielectric, electromechanical and hydrophobic behaviors of core-shell AgNWs@SiO2/PDMS composites. Colloids Surf. A 2019, 563, 59–67. [Google Scholar] [CrossRef]
- Kavousanakis, M.E.; Chamakos, N.T.; Ellinas, K.; Tserepi, A.; Gogolides, E.; Papathanasiou, A.G. How to Achieve Reversible Electrowetting on Superhydrophobic Surfaces. Langmuir 2018, 34, 4173–4179. [Google Scholar] [CrossRef]
- Sohail, S.; Mistri, E.A.; Khan, A.; Banerjee, S.; Biswas, K. Fabrication and performance study of BST/Teflon nanocomposite thin film for low voltage electrowetting devices. Sens. Actuators A 2016, 238, 122–132. [Google Scholar] [CrossRef]
- Bienia, M.; Quilliet, C.; Vallade, M. Modification of drop shape controlled by electrowetting. Langmuir 2003, 19, 9328–9333. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, Z.; Shen, D.; Yang, H. Enhanced dielectric and hydrophobic properties of PDMS/P(VDF-TrFE) blend films by embedding PS microspheres. Colloids Surf. A 2019, 569, 171–178. [Google Scholar] [CrossRef]
- Tamaddoni, N.; Taylor, G.; Hepburn, T.; Michael Kilbey, S.; Sarles, S.A. Reversible, voltage-activated formation of biomimetic membranes between triblock copolymer-coated aqueous droplets in good solvents. Soft Matter 2016, 12, 5096–5109. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.H.; Mirzaei, A.; Kim, H.W.; Tan, B.T.; Wu, P.; Kim, S.S. Electrowetting-on-dielectric characteristics of ZnO nanorods. Sci. Rep. 2020, 10, 14194. [Google Scholar] [CrossRef]
- Papadopoulou, E.L.; Pagkozidis, A.; Barberoglou, M.; Fotakis, C.; Stratakis, E. Electrowetting Properties of ZnO and TiO2 Nanostructured Thin Films. J. Phys. Chem. C 2010, 114, 10249–10253. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Zhang, J.; Pei, X.; Li, Y.; Wang, C. The electrowetting-on-dielectric enhancement of the reduced and bamboo-like layered TiO2 nanotube array and the visual sensor application. J. Alloys Compd. 2020, 846, 156375. [Google Scholar] [CrossRef]
- Lee, J.K.; Park, K.-W.; Kim, H.-R.; Kong, S.H. Dielectrically stabilized electrowetting on AF1600/Si3N4/ TiO2 dielectric composite film. Sens. Actuators B 2011, 160, 1593–1598. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, J.; Zhu, Z.; Zhang, Q.; Yang, H.; Wang, Q. Significantly enhanced dielectric and hydrophobic properties of SiO2@MgO/PMMA composite films. RSC Adv. 2018, 8, 4032–4038. [Google Scholar] [CrossRef]
- Chen, J.; Xiong, X.; Shui, L.; Zhang, Q.; Yang, H.; Zhang, F. Enhanced dielectric constant and hydrophobicity of P(VDF–TrFE)-based composites. J. Mater. Sci. 2018, 29, 17612–17621. [Google Scholar] [CrossRef]
- Hou, J.; Feng, Y.; Liao, J.; Ding, W.; Shui, L.; Li, H.; Wang, Y.; Tang, B.; Umar, A.; Zhou, G. Multiscale Interface Effect on Homogeneous Dielectric Structure of ZrO(2)/Teflon Nanocomposite for Electrowetting Application. Polymers 2018, 10, 1119. [Google Scholar] [CrossRef]
- Magisetty, R.; Shukla, A.; Kandasubramanian, B. Dielectric, Hydrophobic Investigation of ABS/NiFe2O4 Nanocomposites Fabricated by Atomized Spray Assisted and Solution Casted Techniques for Miniaturized Electronic Applications. J. Electron. Mater. 2018, 47, 5640–5656. [Google Scholar] [CrossRef]
- Sedach, P.A.; Gordon, T.J.; Sayed, S.Y.; Fürstenhaupt, T.; Sui, R.; Baumgartner, T.; Berlinguette, C.P. Solution growth of anatase TiO2 nanowires from transparent conducting glass substrates. J. Mater. Chem. 2010, 20, 5063–5069. [Google Scholar] [CrossRef]
- Wei, Z.; Li, R.; Huang, T.; Yu, A. Fabrication of morphology controllable rutile TiO2 nanowire arrays by solvothermal route for dye-sensitized solar cells. Electrochim. Acta 2011, 56, 7696–7702. [Google Scholar] [CrossRef]
- Neese, B.; Chu, B.J.; Lu, S.G.; Wang, Y.; Furman, E.; Zhang, Q.M. Large electrocaloric effect in ferroelectric polymers near room temperature. Science 2008, 321, 821–823. [Google Scholar] [CrossRef]
- Wang, J.W.; Shen, Q.D.; Yang, C.Z.; Zhang, Q.M. High dielectric constant composite of P(VDF-TrFE) with grafted copper phthalocyanine oligomer. Macromolecules 2004, 37, 2294–2298. [Google Scholar] [CrossRef]
- Park, C.-H.; Van Duong, Q.; Moon, Y.-J.; Ha, K.; Choi, S.T. Enhanced thermo-electro-mechanical characteristics of purified P(VDF-TrFE) films for ultrasonic transducers. Sens. Actuators A 2018, 279, 586–592. [Google Scholar] [CrossRef]
- Li, K.; Hou, D.; Fu, C.; Wang, K.; Wang, J. Fabrication of PVDF nanofibrous hydrophobic composite membranes reinforced with fabric substrates via electrospinning for membrane distillation desalination. J. Environ. Sci. 2019, 75, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Lin, Y.; Sodano, H.A. Enhanced Energy Storage in Nanocomposite Capacitors through Aligned PZT Nanowires by Uniaxial Strain Assembly. Adv. Energy Mater. 2012, 2, 469–476. [Google Scholar] [CrossRef]
- Liao, S.; Shen, Z.; Pan, H.; Zhang, X.; Shen, Y.; Lin, Y.-H.; Nan, C.-W. A surface-modified TiO2 nanorod array/P(VDF–HFP) dielectric capacitor with ultra high energy density and efficiency. J. Mater. Chem. C 2017, 5, 12777–12784. [Google Scholar] [CrossRef]
- Xie, B.; Zhang, H.; Zhang, Q.; Zang, J.; Yang, C.; Wang, Q.; Li, M.-Y.; Jiang, S. Enhanced energy density of polymer nanocomposites at a low electric field through aligned BaTiO3 nanowires. J. Mater. Chem. A 2017, 5, 6070–6078. [Google Scholar] [CrossRef]
- Yao, L.; Pan, Z.; Liu, S.; Zhai, J.; Chen, H.H. Significantly Enhanced Energy Density in Nanocomposite Capacitors Combining the TiO2 Nanorod Array with Poly(vinylidene fluoride). ACS Appl. Mater. Interfaces 2016, 8, 26343–26351. [Google Scholar] [CrossRef]
- Greenhoe, B.M.; Hassan, M.K.; Wiggins, J.S.; Mauritz, K.A. Universal power law behavior of the AC conductivity versus frequency of agglomerate morphologies in conductive carbon nanotube-reinforced epoxy networks. J. Polym. Sci. Part B 2016, 54, 1918–1923. [Google Scholar] [CrossRef]



| Composites | Length of TiO2 Nanowires (μm) | Film Thickness (μm) | TN |
|---|---|---|---|
| TN-0.769 | 2.0 | 2.6 | 0.769 |
| TN-0.667 | 2.0 | 3.0 | 0.667 |
| TN-0.594 | 1.9 | 3.2 | 0.594 |
| TN-0.396 | 2.1 | 5.3 | 0.396 |
| TN-0.000 | 0 | 3.2 | 0.000 |
| TN-1.000 | 2.1 | 2.1 | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, D.; Zhang, Q.; Zhang, Z.; Yang, H.; Sheng, J. Enhanced Dielectric and Hydrophobic Properties of Poly(vinylidene fluoride-trifluoroethylene)/TiO2 Nanowire Arrays Composite Film Surface Modified by Electrospinning. Polymers 2021, 13, 105. https://doi.org/10.3390/polym13010105
Shen D, Zhang Q, Zhang Z, Yang H, Sheng J. Enhanced Dielectric and Hydrophobic Properties of Poly(vinylidene fluoride-trifluoroethylene)/TiO2 Nanowire Arrays Composite Film Surface Modified by Electrospinning. Polymers. 2021; 13(1):105. https://doi.org/10.3390/polym13010105
Chicago/Turabian StyleShen, Da, Qilong Zhang, Zhao Zhang, Hui Yang, and Jiansong Sheng. 2021. "Enhanced Dielectric and Hydrophobic Properties of Poly(vinylidene fluoride-trifluoroethylene)/TiO2 Nanowire Arrays Composite Film Surface Modified by Electrospinning" Polymers 13, no. 1: 105. https://doi.org/10.3390/polym13010105
APA StyleShen, D., Zhang, Q., Zhang, Z., Yang, H., & Sheng, J. (2021). Enhanced Dielectric and Hydrophobic Properties of Poly(vinylidene fluoride-trifluoroethylene)/TiO2 Nanowire Arrays Composite Film Surface Modified by Electrospinning. Polymers, 13(1), 105. https://doi.org/10.3390/polym13010105






