Adsorption Properties for La(III), Ce(III), and Y(III) with Poly(6-acryloylamino-hexyl hydroxamic acid) Resin
Abstract
:1. Introduction
2. Experimental and Methods
2.1. Quantum Chemical Calculation of Poly(6-acryloylamino-hexyl hydroxamic acid) Resin (PAMHA)
2.2. Materials and Apparatus
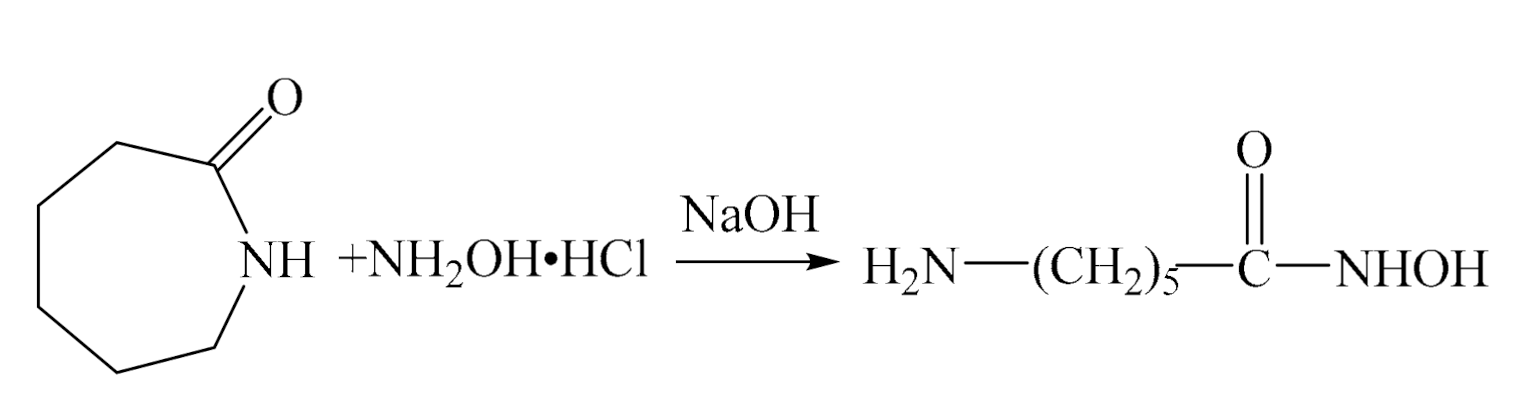
2.3. Preparation of 6-aminohexyl hydroxamic acid
2.4. Preparation of PAMHA
2.5. The Adsorption and Desorption Experiments
2.6. Determination of Metal Ion Concentration
3. Results and Discussions
3.1. Quantum Chemical Calculation
3.2. Characterization
3.2.1. Scanning Electron Microscopy (SEM)
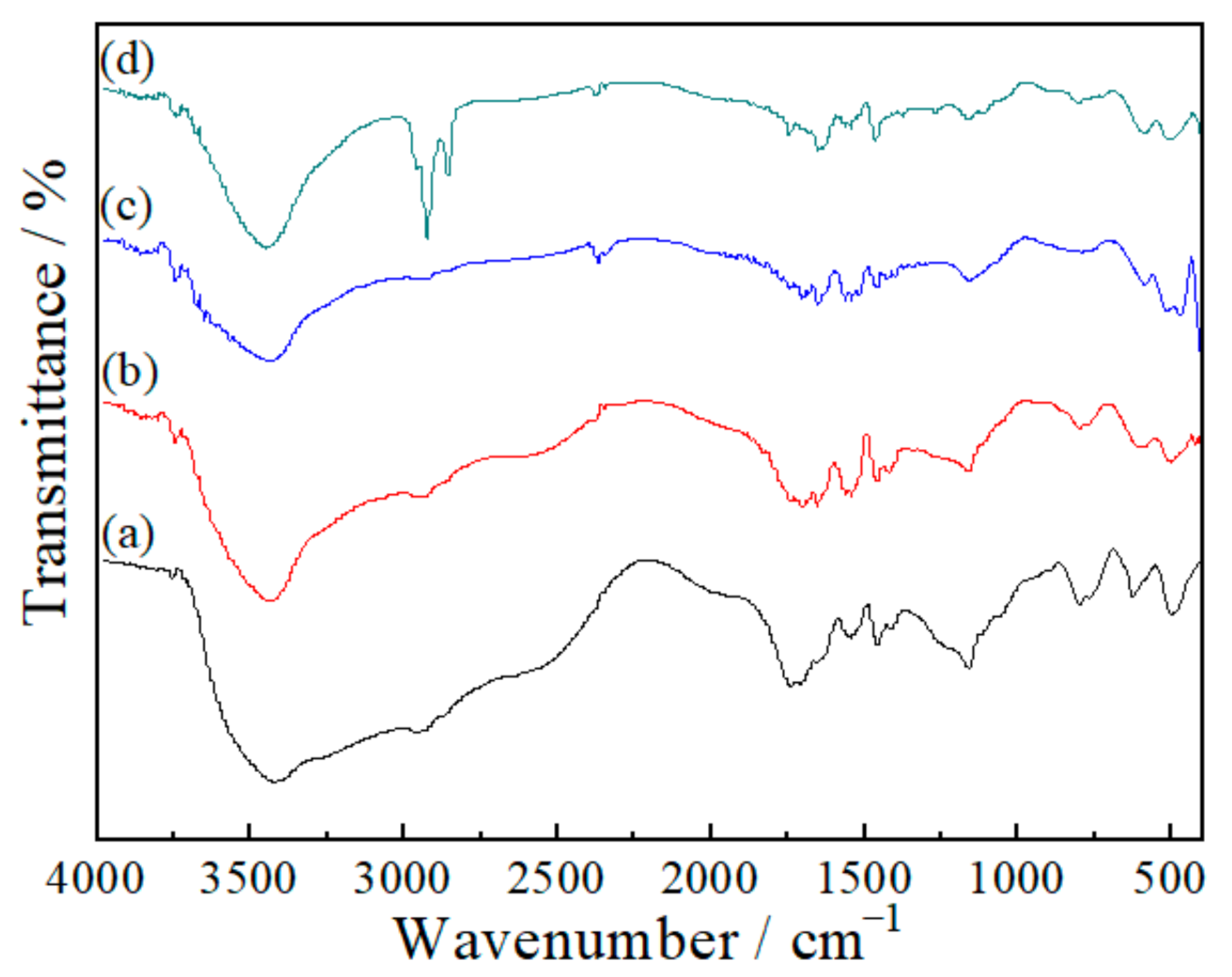
3.2.2. Fourier Transform Infrared Spectroscopy (FTIR)
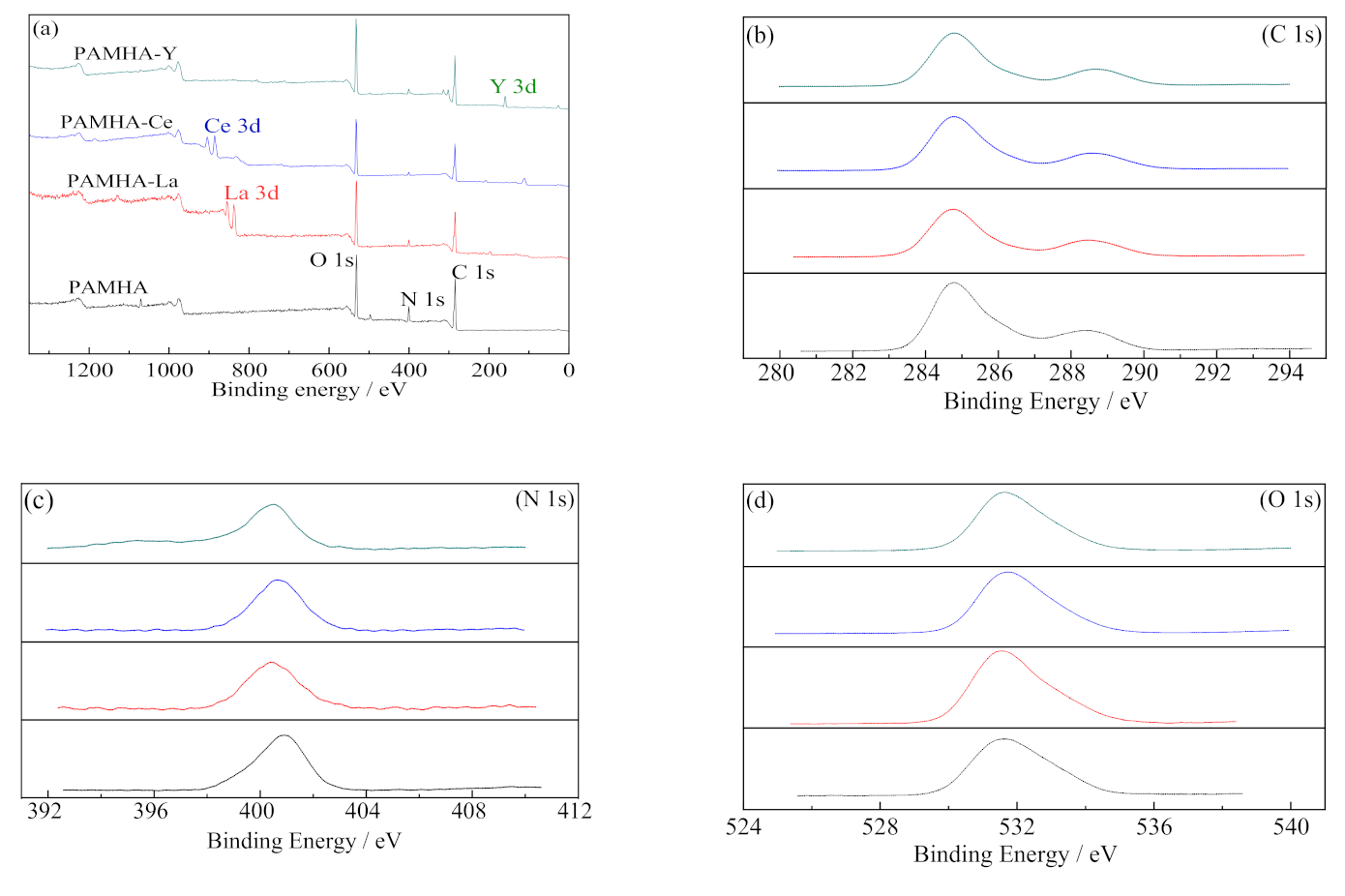
3.2.3. X-ray Photoelectron Spectroscopy (XPS)
3.3. Adsorption Capacity of PAMHA on Rare Earth Ions
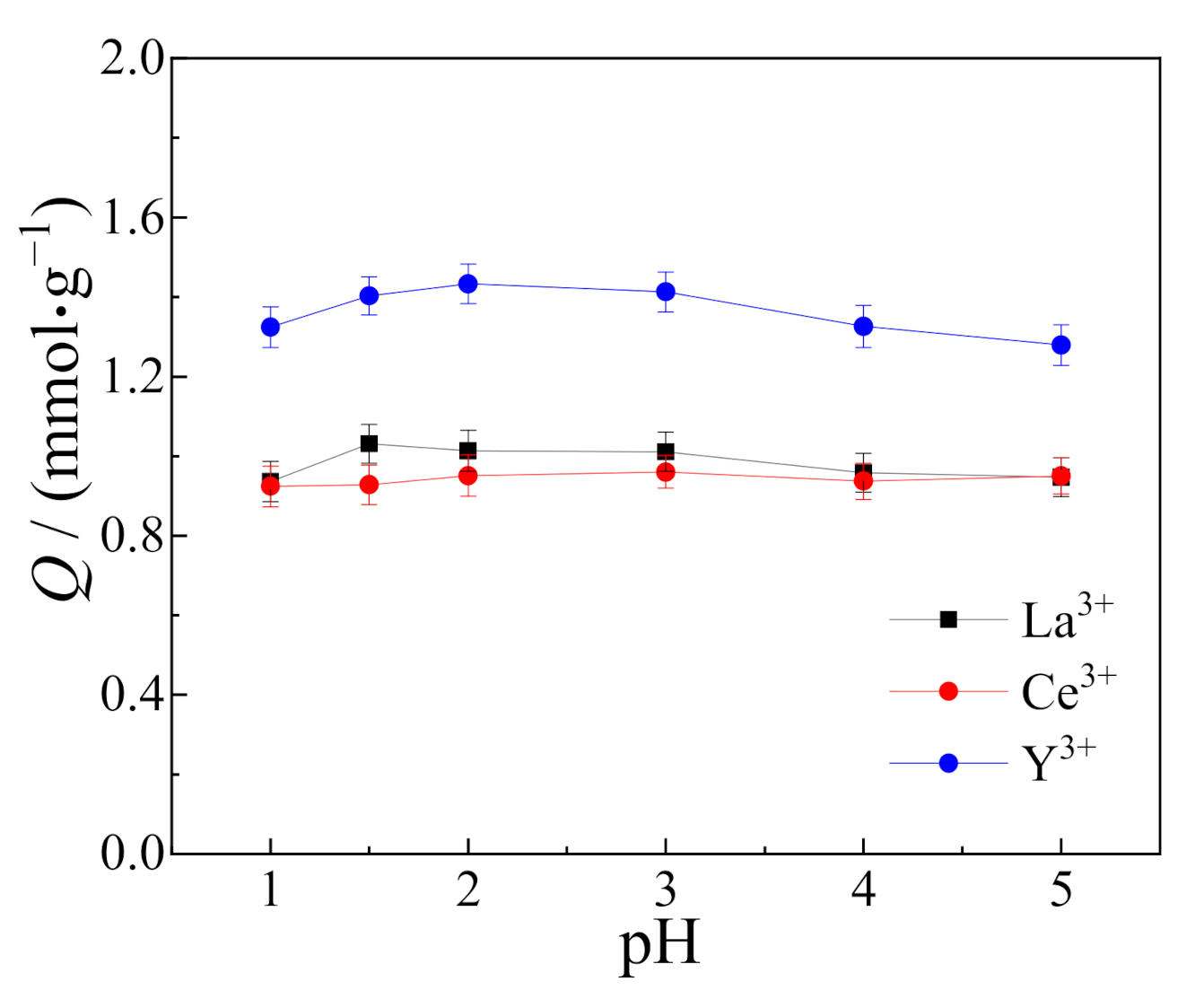
3.4. Effect of Solution pH on Adsorption
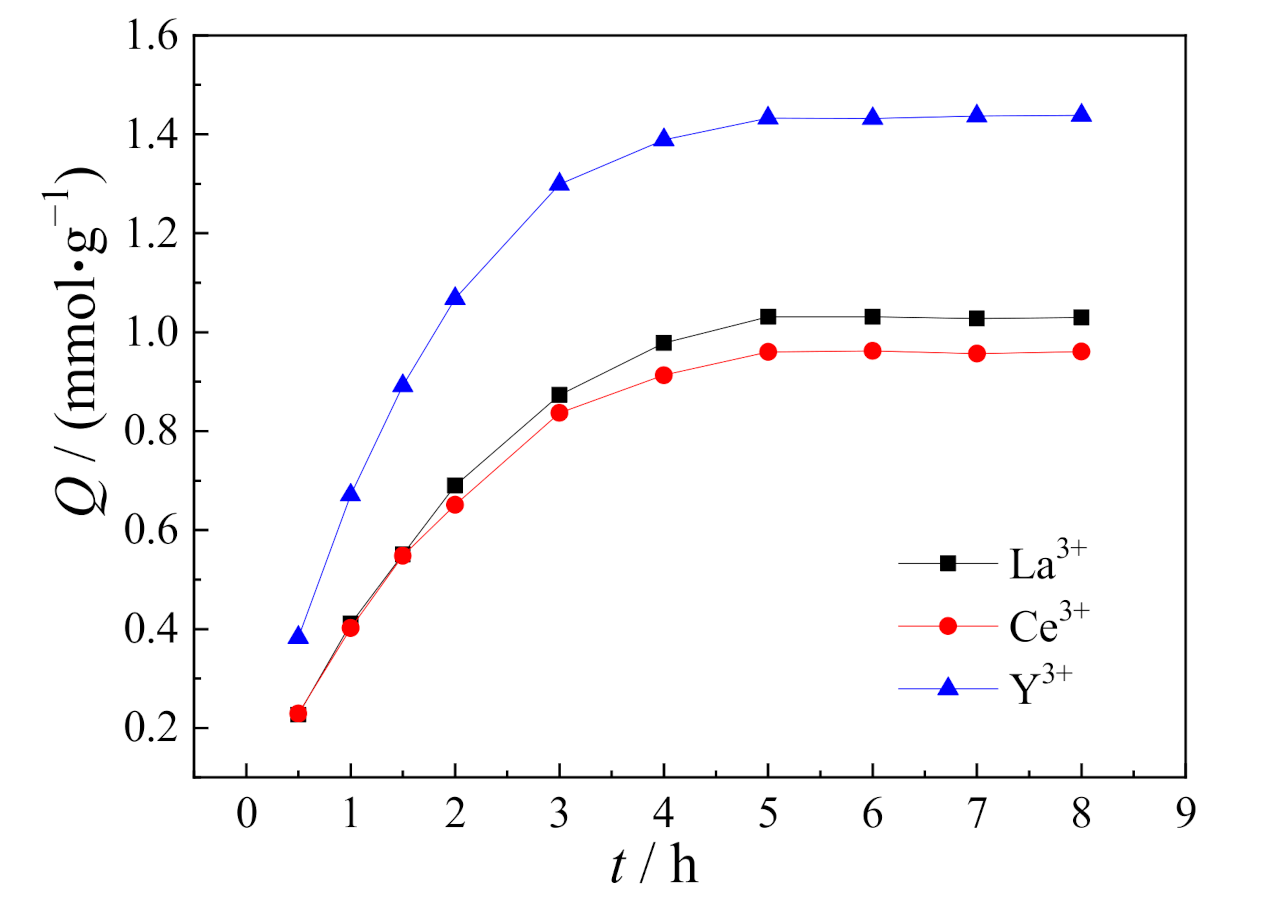
3.5. Kinetic Adsorption
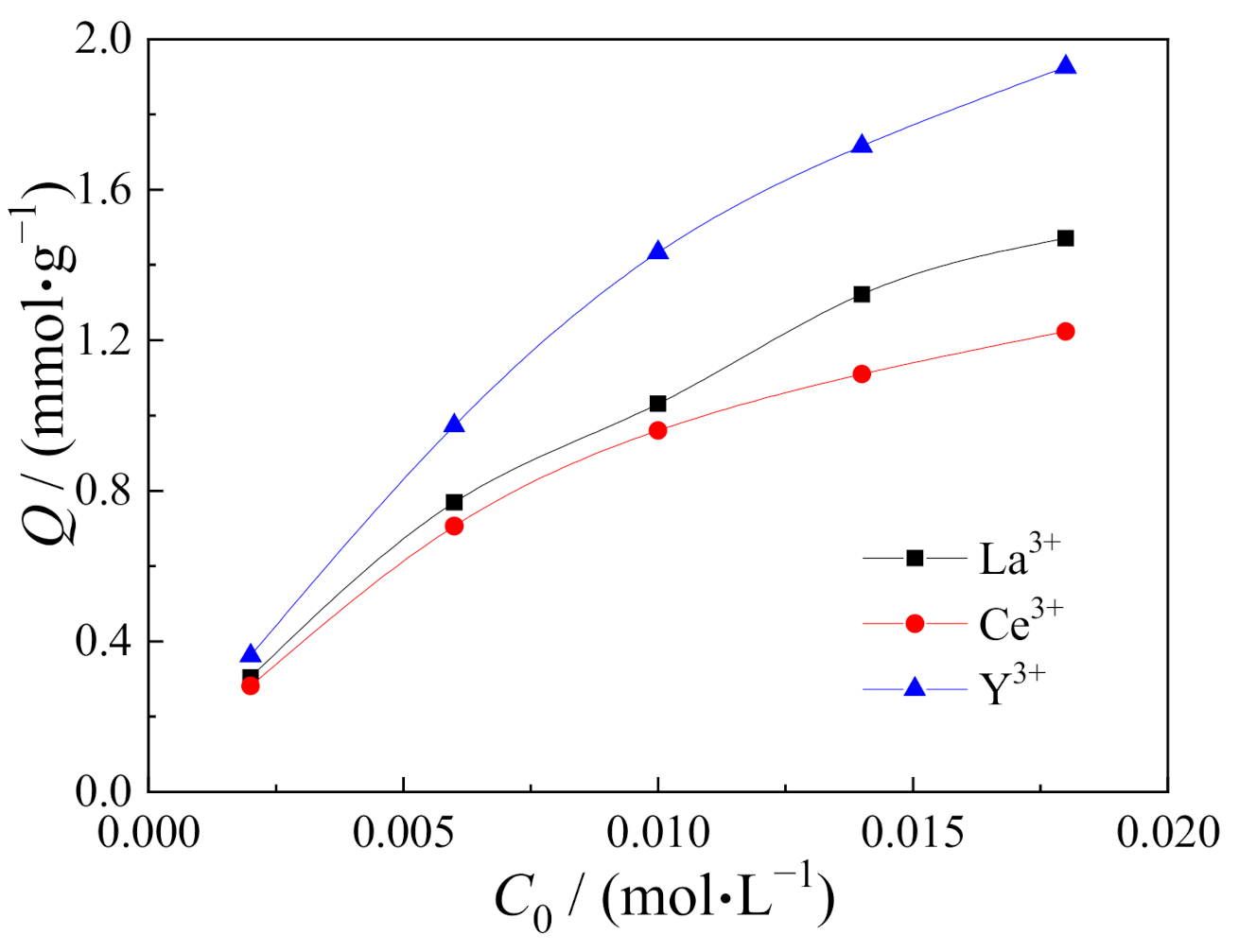
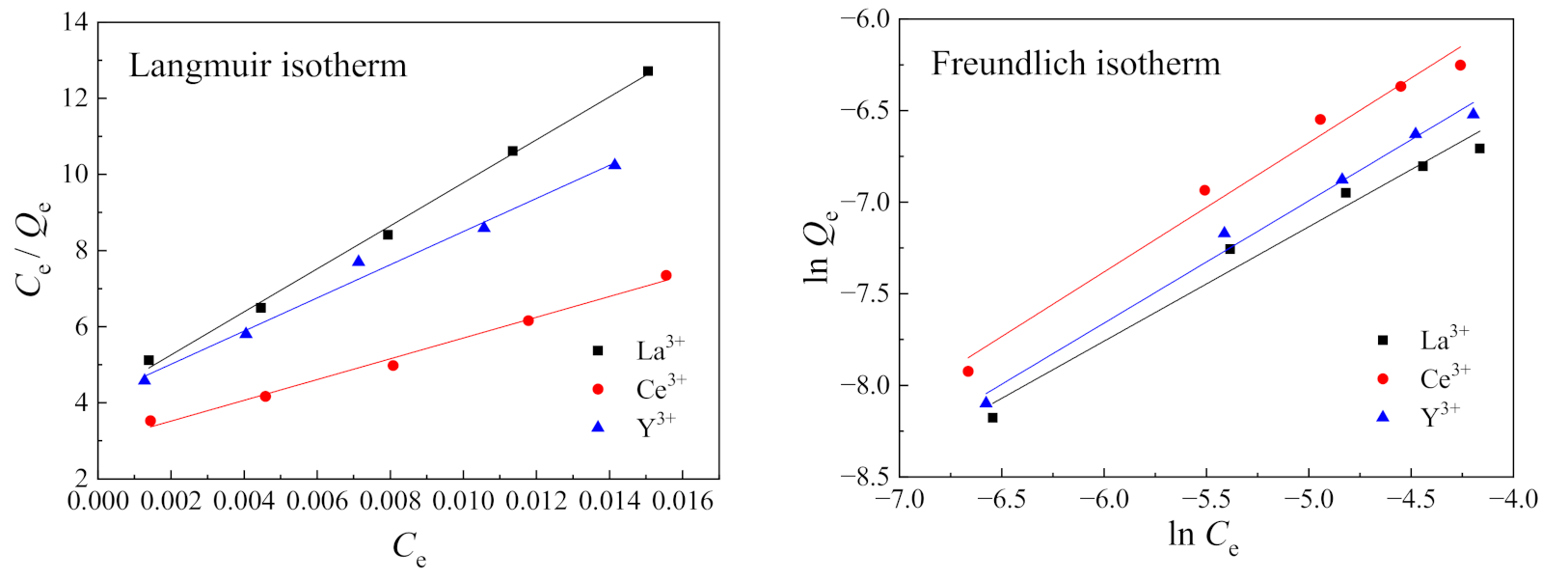
3.6. Isotherm Adsorption
3.7. Thermodynamics Adsorption
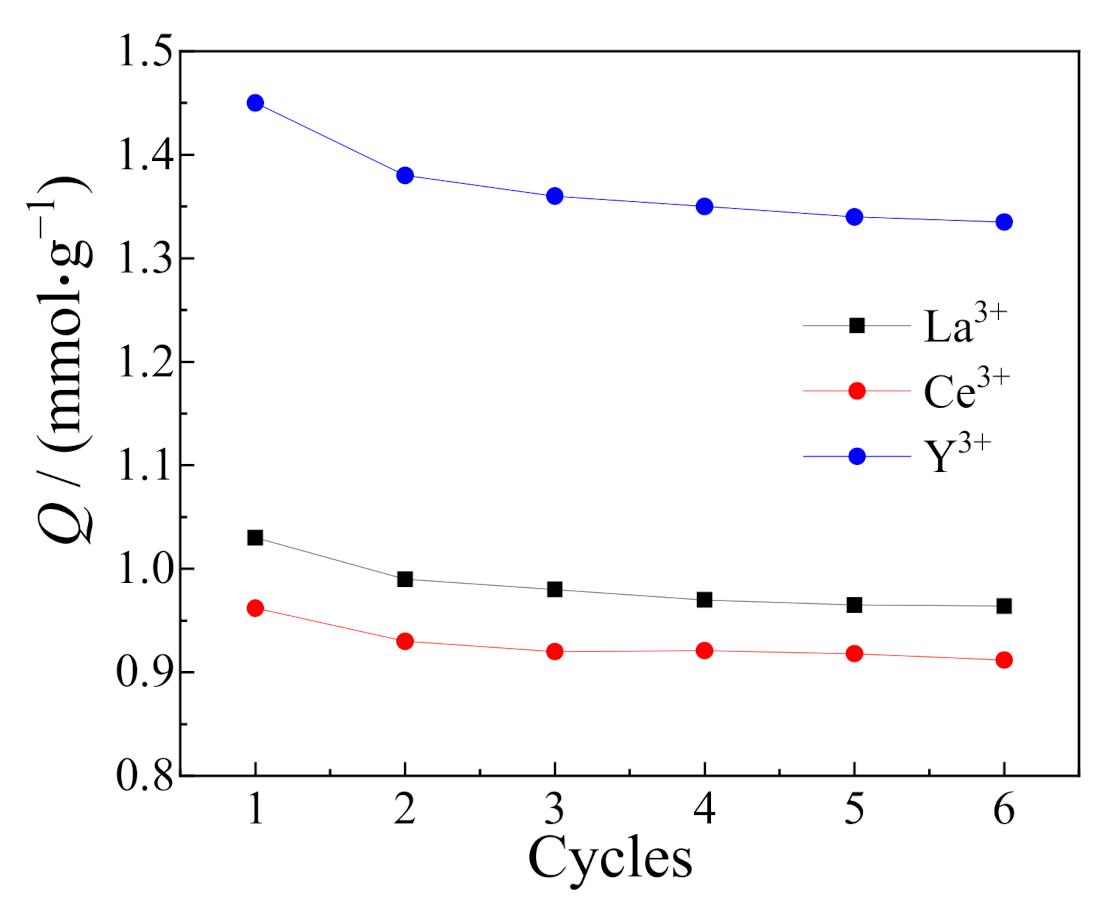
3.8. Desorption Experiments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Li, Y.X.; Cheng, B.W.; Ding, C.K.; Zhang, Y. Synthesis of a starch-based sulfonic ion exchange resin and adsorption of dyestuffs to the resin. Int. J. Biol. Macromol. 2020, 161, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Marin, N.M.; Pascu, L.F.; Demba, A.; Nita-Lazar, M.; Badea1, I.A.; Aboul-Enein, H.Y. Removal of the Acid Orange 10 by ion exchange and microbiological methods. Int. J. Environ. Sci. Technol. 2019, 16, 6357–6366. [Google Scholar] [CrossRef]
- Zhong, W.; Li, X.Y.; Yang, H.; Li, E.H. A novel, effective, and feasible method for deacidifying kiwifruit wine by weakly basic ion exchange resins. J. Food Process Eng. 2019, 42, e12969. [Google Scholar] [CrossRef]
- Chen, X.S.; Li, Q.; He, H.G.; Zhang, J.H.; Mao, Z.G. Effect of ion form of the ion-exchange resin on ε-poly-l -lysine purification from microbial fermentation broth. RSC Adv. 2019, 9, 12174–12181. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Chen, K.; Zhu, J.W.; Wu, Y.Y. Effect of solution polarity and temperature on adsorption separation of erythromycin A and C onto macroporous resin SP825. Sep. Sci. Technol. 2014, 49, 898–906. [Google Scholar] [CrossRef]
- Li, S.; Yu, X.L.; Liu, F.; Deng, F.L.; He, J.W. Synthesis of antibacterial dimethacrylate derived from niacin and its application in preparing antibacterial dental resin system. J. Mech. Behav. Biomed. Mater. 2019, 102, 103521. [Google Scholar] [CrossRef]
- Xiong, C.H.; Li, Y.L.; Wang, G.T.; Fang, L.; Zhou, S.G.; Yao, C.P.; Chen, Q.; Zheng, X.M.; Qi, D.M.; Fu, Y.Q.; et al. Selective removal of Hg(II) with polyacrylonitrile-2-amino-1,3,4-thiadiazole chelating resin: Batch and column study. Chem. Eng. J. 2015, 259, 257–265. [Google Scholar] [CrossRef]
- Huang, X.P.; Cao, X.Y.; Wang, W.H.; Zhong, H.; Cao, Z.F. Preparation of a novel resin with acyl and thiourea groups and its properties for Cu(II) removal from aqueous solution. J. Environ. Manag. 2017, 204, 264–271. [Google Scholar] [CrossRef]
- Liu, H.Z.; Zhang, B.; Jing, X.J.; Wang, W.; Wang, L.J. Adsorption and desorption properties for rhenium using a kind of weak-base anion resin. Rare Met. 2018, 37, 707–715. [Google Scholar] [CrossRef]
- Wang, S.; Zhong, H.; Liu, G.Y.; Zhang, Q.; Li, T. Synthesis and adsorption properties for Au(III) of alkoxycarbonyl thiourea resin. J. Cent. South Univ. Technol. 2008, 15, 463–468. [Google Scholar] [CrossRef]
- Felipe, E.C.B.; Batista, K.A.; Ladeira, A.C.Q. Recovery of rare earth elements from acid mine drainage by ion exchange. Environ. Technol. 2020, 272, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.P.; Cao, X.Y.; Wang, W.H.; Zhong, H.; Cao, Z.F. Investigation of removal of Ag(I) from aqueous solution by a novel chelating resin containing acyl and thiourea groups. J. Dispers. Sci. Technol. 2019, 40, 477–486. [Google Scholar] [CrossRef]
- Salisbury, C.M.; Cravatt, B.F. Optimization of activity-based probes for proteomic profiling of histone deacetylase complexes. J. Am. Chem. Soc. 2008, 130, 2184–2194. [Google Scholar] [CrossRef] [PubMed]
- Salinas, F.; Jiménez-Arrabal, M.; Durán, M.S. 2-Pyrilideniminobenzohydroxamic acid as analytical reagent for the spectrophotometric determination of vanadium(V). Bull. Soc. Chim. Belg. 2015, 94, 101–109. [Google Scholar] [CrossRef]
- Cao, Z.F.; Qiu, P.; Wang, S.; Zhong, H. Benzohydroxamic acid to improve iron removal from potash feldspar ores. J. Cent. South Univ. 2018, 25, 2190–2198. [Google Scholar] [CrossRef]
- Deng, L.Q.; Zhao, G.; Zhong, H.; Wang, S.; Liu, G.Y. Investigation on the selectivity of N-((hydroxyamino)-alkyl) alkylamide surfactants for scheelite/calcite flotation separation. J. Ind. Eng. Chem. 2016, 33, 131–141. [Google Scholar] [CrossRef]
- Trivedi, U.V.; Menon, S.K.; Agrawal, Y.K. Polymer supported calix[6]arene hydroxamic acid, a novel chelating resin. React. Funct. Polym. 2002, 50, 205–216. [Google Scholar] [CrossRef]
- Zhang, Q. Synthesis of hydroxamic acid resin and its adsorption characteristics for acid mine drainage. Cent. South Univ. 2010, 26–27. [Google Scholar]
- Lee, T.S.; Jeon, D.W.; Kim, J.K.; Hong, S.I. Formation of metal complex in a poly(hydroxamic acid) resin bead. Fibers Polym. 2001, 2, 13–17. [Google Scholar] [CrossRef]
- Cornaz, J.P.; Deuel, H. Selektive ionenaustauscher für Fe3+1. Experientia 1954, 10, 137–138. [Google Scholar] [CrossRef]
- Cornaz, J.P.; Hutschneker, K.; Deuel, H. Saurechloride und hydroxamsauren von carboxyl-ionenaustauschern. 10. Mitteilung über Ionenaustauscher. Helv. Chim. Acta 1957, 40, 2015–2019. [Google Scholar] [CrossRef]
- Vernon, F.; Zin, W.M. Chelating ion-exchangers containing n-substituted hydroxylamine functional groups. Part 6. Sorption and separation gold and silver by a polyhydroxamic acid. Anal. Chim. Acta 1981, 123, 309–313. [Google Scholar] [CrossRef]
- Vernon, F. Chelating ion exchangers-the synthesis and uses of poly(hydroxamic acid) resins. Pure Appl. Chem. 1982, 54, 2151–2158. [Google Scholar] [CrossRef]
- Agrawal, Y.K.; Kaur, H.; Menon, S.K. Poly(styrene-p-hydroxamic acids): Synthesis, and ion exchange separation of rare earths. React. Funct. Polym. 1999, 39, 155–164. [Google Scholar] [CrossRef]
- Haron, M.J.; Yunus, W.M.Z.W.; Tan, W.C.; Kassim, A. Sorption removal of arsenic (V) by Sn-loaded poly(hydroxamic) acid chelating resin. J. Ion Exch. 2007, 18, 240–245. [Google Scholar] [CrossRef]
- Kumar, S.A.; Pandey, S.P.; Shenoy, N.S.; Kumar, S.D. Matrix separation and preconcentration of rare earth elements from seawater by poly hydroxamic acid cartridge followed by determination using ICP-MS. Desalination 2011, 281, 49–54. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, J.N.; Yang, X. Preparation of novel chelating sponge as an adsorbent for the removal of Cu2+ from water. Chin. Chem. Lett. 2013, 24, 997–1000. [Google Scholar] [CrossRef]
- Rahman, M.L.; Mandal, B.H.; Sarkar, S.M.; Wahab, N.A.A.; Yusoff, M.M.; Arshad, S.E.; Musta, B. Synthesis of poly(hydroxamic acid) ligand from polymer grafted khaya cellulose for transition metals extraction. Fiber. Polym. 2016, 4, 521–532. [Google Scholar] [CrossRef] [Green Version]
- Jiao, C.L.; Zhang, Z.F.; Tao, J.; Zhang, D.S.; Chen, Y.Y.; Lin, H. Synthesis of a poly(amidoxime-hydroxamic acid) cellulose derivative and its application in heavy metal ion removal. RSC Adv. 2017, 7, 27787–27795. [Google Scholar] [CrossRef] [Green Version]
- Barcelos, D.C.T.; Carlos, D.S.M.G.; Adeodato, V.M.G. Recovery of rare-earth metals from aqueous solutions by bio/adsorption using non-conventional materials: A review with recent studies and promising approaches in column applications. J. Rare Earths 2020, 38, 339–355. [Google Scholar]
- Cao, X.Y.; Wang, Q.; Wang, S.; Man, R.L. Preparation of a novel polystyrene-poly(hydroxamic acid) copolymer and its adsorption properties for rare earth metal ions. Polymers 2020, 12, 1905. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.Y.; Wang, Q.; Wang, S.; Man, R.L. A novel polystyrene-poly(hydroxamic acid) interpenetrating polymer networks and its adsorption towards rare earth ions. J. Rare Earths 2020. [Google Scholar] [CrossRef]
- Huang, J.P.; Zhong, H.; Qiu, X.Y.; Wang, S.; Zhao, G.; Gao, Y.D.; Dai, Z.L.; Liu, G.Y. Flotation behavior and adsorption mechanism of cyclohexyl hydroxamic acid to wolframite. Chin. J. Nonferr. Met. 2013, 23, 2023–2030. [Google Scholar]
- Rahman, M.L.; Biswas, T.K.; Sarkar, S.M.; Yusoff, M.M.; Sarjad, M.S.; Arshad, S.E.; Musta, B. Adsorption of rare earth metals from water using a kenaf cellulose-based poly(hydroxamic acid) ligand. J. Mol. Liq. 2017, 243, 616–623. [Google Scholar] [CrossRef] [Green Version]
- Xiong, C.H.; Zheng, Z.W. Evaluation of D113 cation exchange resin for the removal of Eu from aqueous solution. J. Rare Earths 2010, 28, 862. [Google Scholar] [CrossRef]
- Cui, L.M.; Wang, Y.G.; Gao, L.; Hu, L.H.; Yan, L.G.; Wei, Q.; Du, B. EDTA functionalized magnetic graphene oxide for removal of Pb(II), Hg(II) and Cu(II) in water treatment: Adsorption mechanism and separation property. Chem. Eng. J. 2015, 281, 1–10. [Google Scholar] [CrossRef]
- Wen, Z.Q.; Huang, K.H.; Niu, Y.Q.; Yao, Y.X.; Wang, S.; Cao, Z.F.; Zhong, H. Kinetic study of ultrasonic-assisted uranium adsorption by anion exchange resin. Colloids Surf. A 2020, 585, 124021. [Google Scholar] [CrossRef]
- Wang, Z.W.; Zhang, J.; Wu, Q.; Han, X.X.; Zhang, M.N.; Liu, W.; Yao, X.D.; Feng, J.L.; Dong, S.Y.; Sun, J.H. Magnetic supramolecular polymer: Ultrahigh and highly selective Pb(II) capture from aqueous solution and battery wastewater. Chemosphere 2020, 248, 126042. [Google Scholar] [CrossRef]
- Tang, N.; Niu, C.G.; Li, X.T.; Liang, C.; Guo, H.; Lin, L.S.; Zheng, C.W.; Zeng, G.M. Efficient removal of Cd2+ and Pb2+ from aqueous solution with amino-and thiol-functionalized activated carbon: Isotherm and kinetics modeling. Sci. Total Environ. 2018, 635, 1331–1344. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Zhao, R.; Li, Y.; Li, C.; Zhang, C.L. Adsorption of Pb(II) on activated carbon prepared from Polygonum orientale Linn.: Kinetics, isotherms, pH, and ionic strength studies. Bioresour. Technol. 2010, 101, 5808–5814. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Xiong, J.Q.; Jiao, C.L.; Zhang, D.S.; Lin, H.; Chen, Y.Y. Hybrid mesoporous silica based on hyperbranch-substrate nanonetwork as highly efficient adsorbent for water treatment. ACS Sustain. Chem. Eng. 2016, 4, 60–68. [Google Scholar] [CrossRef]
- Wang, J.L.; Guo, X. Adsorption isotherm models Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases of plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385. [Google Scholar]
- Zhang, N.; Zhang, H.H.; Li, R.; Xing, Y.J. Preparation and adsorption properties of citrate-crosslinked chitosan salt microspheres by microwave assisted method. Int. J. Biol. Macromol. 2020, 152, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.S.; Cao, Z.F.; Wen, X.; Wang, S.; Zhong, H.; Wu, Z.K. Preparation of a novel nano-Fe3O4 /triethanolamine/GO composites to enhance Pb2+/Cu2+ ions removal. Environ. Sci. Pollut. Res. 2019, 10, 10174–10187. [Google Scholar] [CrossRef]
- Mallakpour, S.; Tabesh, F. Tragacanth gum based hydrogel nanocomposites for the adsorption of methylene blue: Comparison of linear and non-linear forms of different adsorption isotherm and kinetics models. Int. J. Biol. Macromol. 2019, 133, 745–766. [Google Scholar] [CrossRef]

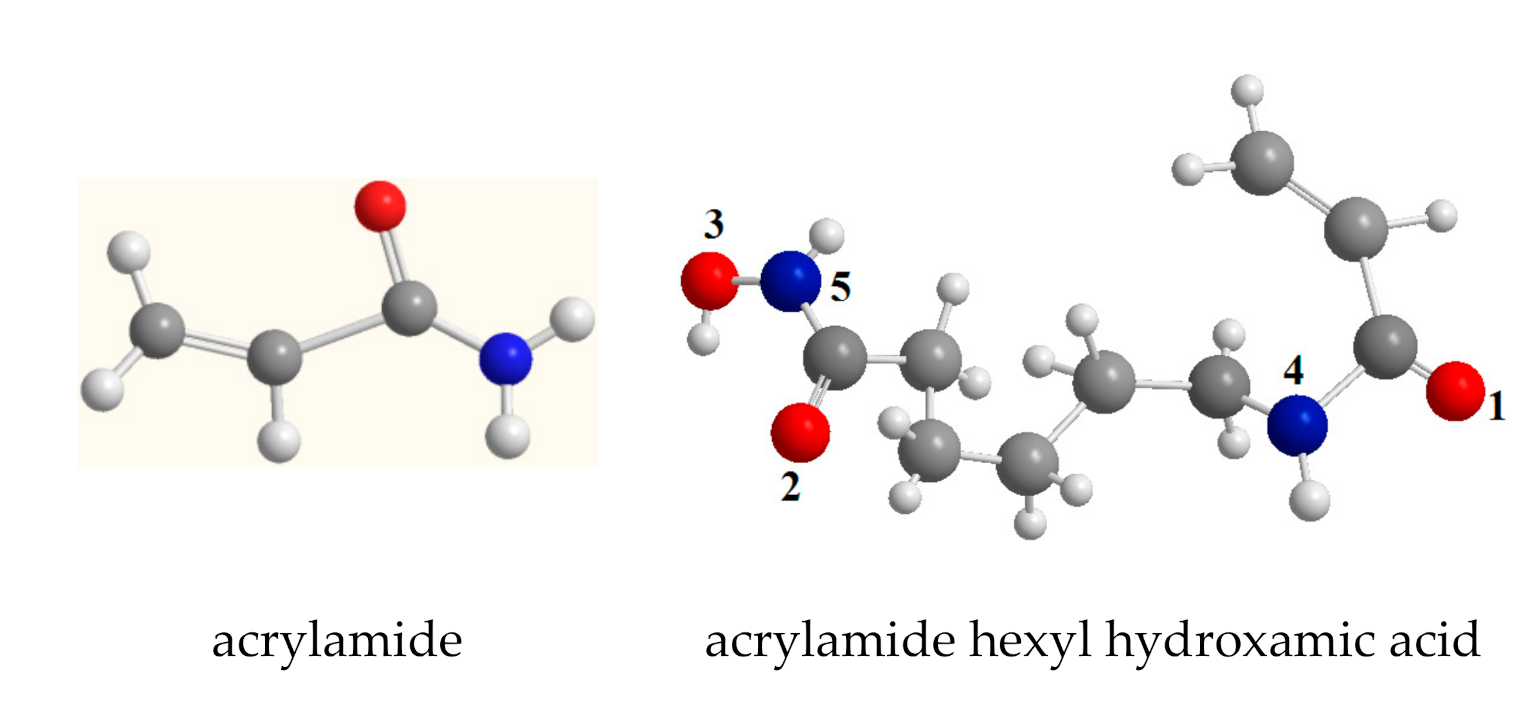




| Molecule | Energy/(a.u.) | HOMO/(a.u.) | LUMO/(a.u.) |
|---|---|---|---|
| acrylamide | −247.307621563 | −0.26592 | −0.05158 |
| acrylamide hexyl hydroxamic acid | −687.53675764 | −0.26051 | −0.05516 |
| Atom | Charge | Atom | Charge |
|---|---|---|---|
| O1 | −0.483 | O6 | −0.388 |
| O2 | −0.562 | O7 | −0.387 |
| O3 | −0.223 | O8 | −0.557 |
| N4 | −0.402 | O9 | −0.217 |
| N5 | −0.517 | N10 | −0.132 |
| N11 | −0.520 |
| Wavenumber/cm−1 | Functional Group | |||
|---|---|---|---|---|
| PAMHA | PAMHA-La(III) | PAMHA-Ce(III) | PAMHA-Y(III) | |
| 3419 | 3440 | 3443 | 3446 | N–H, O–H |
| 2963 | 2913 | 2925 | 2923 | C–H |
| 1741 | 1701 | 1701 | 1707 | C=O |
| 1435 | 1456 | 1456 | 1460 | –C=N |
| 1158 | 1160 | 1155 | 1161 | C–C |
| Species | Binding Energy/eV | |||||
|---|---|---|---|---|---|---|
| C 1s | O 1s | N 1s | La 3d | Ce 3d | Y 3d | |
| PAMHA | 284.82 | 531.98 | 400.87 | — | — | — |
| PAMHA-La(III) | 284.80 | 531.99 | 400.66 | 837.80 | — | — |
| PAMHA-Ce(III) | 284.80 | 532.01 | 400.12 | — | 885.67 | — |
| PAMHA-Y(III) | 284.80 | 532.03 | 400.32 | — | — | 158.32 |
| Resin | Adsorption Capacity/(mmol·g−1) | ||
|---|---|---|---|
| La3+ | Ce3+ | Y3+ | |
| PAMHA | 1.030 | 0.962 | 1.450 |
| D113 | 0.681 | 0.778 | 0.442 |
| Rare Earth Ions | Pseudo-First-Order | Pseudo-Second-Order | Elovich | ||||||
|---|---|---|---|---|---|---|---|---|---|
| k1 | Q1 | R2 | k2 | Q2 | R2 | α | β | R2 | |
| La3+ | 0.7752 | 1.391 | 0.9732 | 0.1824 | 1.731 | 0.9886 | 1.226 | 2.710 | 0.9876 |
| Ce3+ | 0.7796 | 1.245 | 0.9834 | 0.2453 | 1.515 | 0.9902 | 1.219 | 2.979 | 0.9891 |
| Y3+ | 0.8284 | 1.798 | 0.9929 | 0.2371 | 2.077 | 0.9881 | 2.095 | 2.070 | 0.9887 |
| Adsorbate | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| Qm/(mmol·g−1) | KL/(L·mol−1) | R2 | n | KF | R2 | |
| La3+ | 1.770 | 136.83 | 0.9960 | 1.602 | 0.0181 | 0.9675 |
| Ce3+ | 3.664 | 91.89 | 0.9879 | 1.417 | 0.0431 | 0.9760 |
| Y3+ | 2.295 | 105.24 | 0.9830 | 1.498 | 0.0259 | 0.9859 |
| Adsorbate | Fitting Equation | ΔG/(kJ·mol−1) | ΔH/(kJ·mol−1) | ΔS/(J·mol−1·K−1) |
|---|---|---|---|---|
| La3+ | lnD = 11.3519 − 2.0791/T | −12.727 | 17.286 | 94.380 |
| Ce3+ | lnD = 13.8713 − 2.7363/T | −13.924 | 22.750 | 115.326 |
| Y3+ | lnD = 12.1424 − 2.3203/T | −12.812 | 19.291 | 100.952 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Zhou, C.; Wang, S.; Man, R. Adsorption Properties for La(III), Ce(III), and Y(III) with Poly(6-acryloylamino-hexyl hydroxamic acid) Resin. Polymers 2021, 13, 3. https://doi.org/10.3390/polym13010003
Cao X, Zhou C, Wang S, Man R. Adsorption Properties for La(III), Ce(III), and Y(III) with Poly(6-acryloylamino-hexyl hydroxamic acid) Resin. Polymers. 2021; 13(1):3. https://doi.org/10.3390/polym13010003
Chicago/Turabian StyleCao, Xiaoyan, Chunjie Zhou, Shuai Wang, and Ruilin Man. 2021. "Adsorption Properties for La(III), Ce(III), and Y(III) with Poly(6-acryloylamino-hexyl hydroxamic acid) Resin" Polymers 13, no. 1: 3. https://doi.org/10.3390/polym13010003






