Effect of Mixing Ratio of Oppositely Charged Block Copolymers on Polyion Complex Micelles for In Vivo Application
Abstract
:1. Introduction
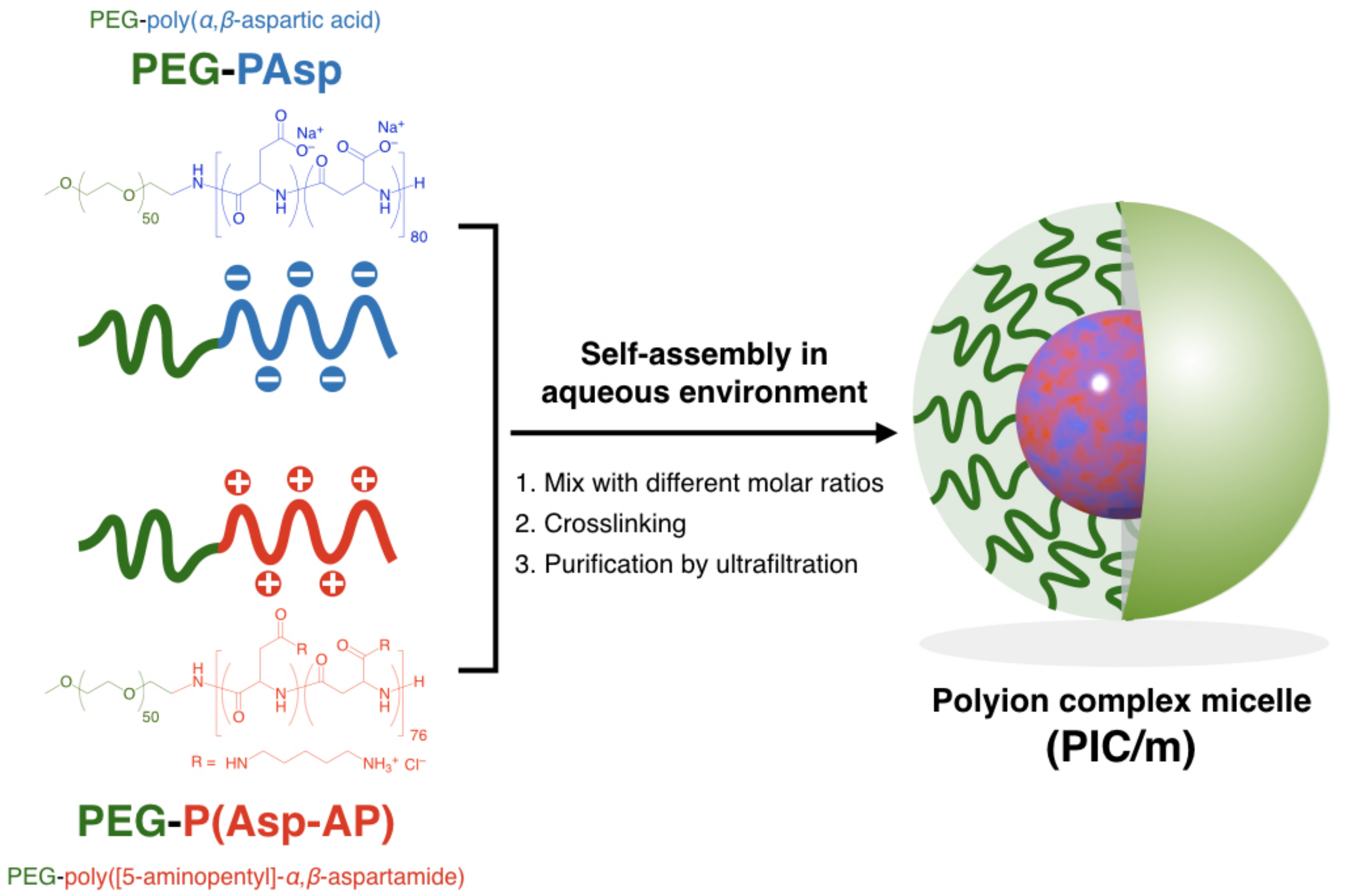
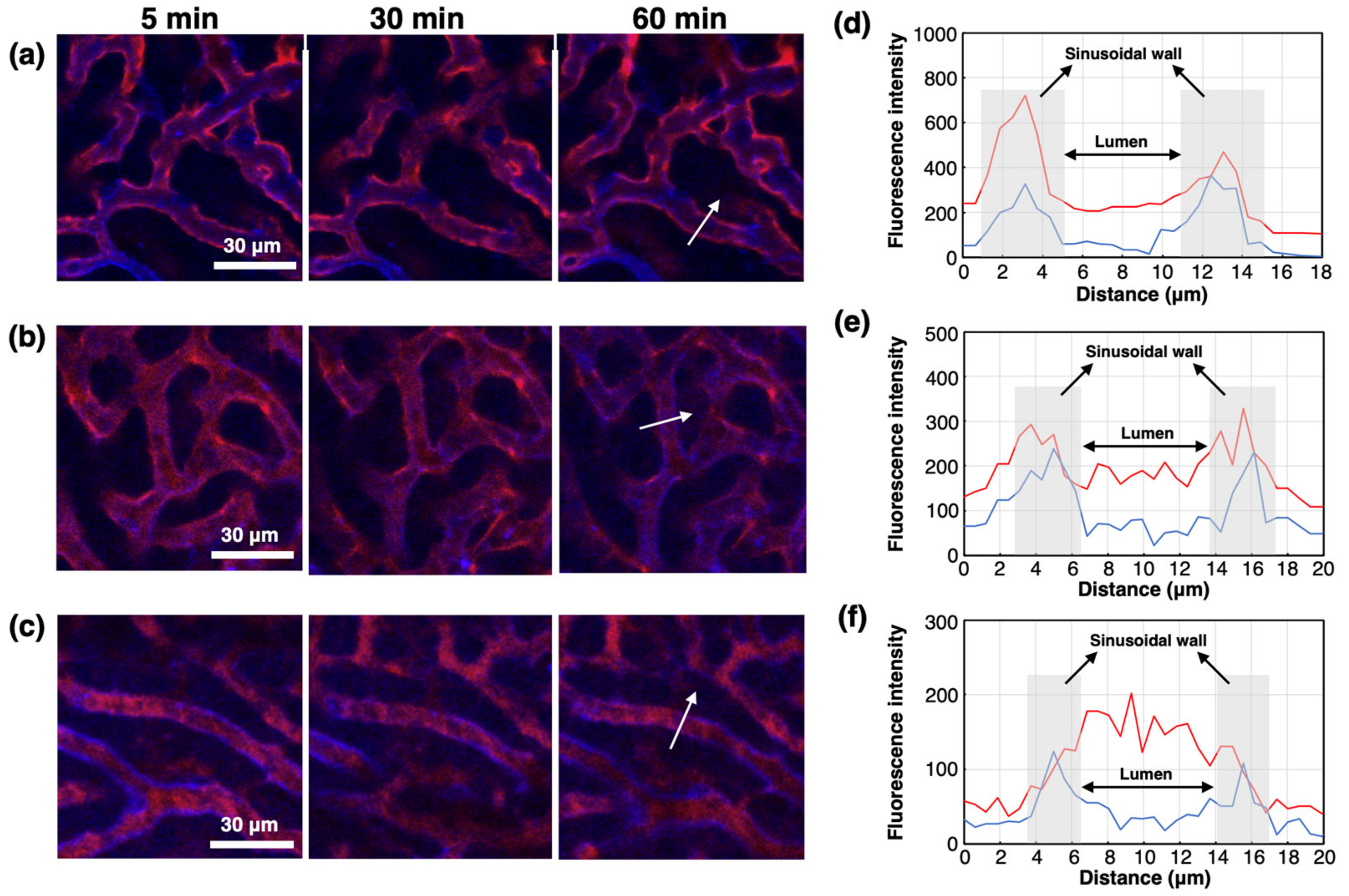
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, G.; Huang, Z.; Xu, Z.; Yan, L.T. Tailoring Interfacial Nanoparticle Organization through Entropy. Acc. Chem. Res. 2018, 51, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Yang, H.B. Construction of Stimuli-Responsive Functional Materials via Hierarchical Self-Assembly Involving Coordination Interactions. Acc. Chem. Res. 2018, 51, 2699–2710. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Lu, J.; Kong, X.; Hyeon, T.; Ling, D. Dynamic Nanoparticle Assemblies for Biomedical Applications. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Magana, J.R.; Sproncken, C.C.M.; Voets, I.K. On Complex Coacervate Core Micelles: Structure-Function Perspectives. Polymers 2020, 12, 1953. [Google Scholar] [CrossRef] [PubMed]
- Palivan, C.G.; Goers, R.; Najer, A.; Zhang, X.; Car, A.; Meier, W. Bioinspired polymer vesicles and membranes for biological and medical applications. Chem. Soc. Rev. 2016, 45, 377–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef]
- Ge, Z.; Liu, S. Functional block copolymer assemblies responsive to tumor and intracellular microenvironments for site-specific drug delivery and enhanced imaging performance. Chem. Soc. Rev. 2013, 42, 7289–7325. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; York-Duran, M.J.; Hosta-Rigau, L. Recent Progress in Micro/Nanoreactors toward the Creation of Artificial Organelles. Adv. Healthc. Mater. 2018, 7, 1700917. [Google Scholar] [CrossRef]
- McQuigg, D.W.; Kaplan, J.I.; Dubin, P.L. Critical conditions for the binding of polyelectrolytes to small oppositely charged micelles. J. Phys. Chem. 1992, 96, 1973–1978. [Google Scholar] [CrossRef]
- Wang, Y.; Kimura, K.; Huang, Q.; Dubin, P.L.; Jaeger, W. Effects of Salt on Polyelectrolyte−Micelle Coacervation. Macromolecules 1999, 32, 7128–7134. [Google Scholar] [CrossRef]
- Chen, J.-X.; Wang, M.; Tian, H.-H.; Chen, J.-H. Hyaluronic acid and polyethylenimine self-assembled polyion complexes as pH-sensitive drug carrier for cancer therapy. Coll. Surf. B Biointerfaces 2015, 134, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Kataoka, K. Effect of Charged Segment Length on Physicochemical Properties of Core−Shell Type Polyion Complex Micelles from Block Ionomers. Macromolecules 2003, 36, 4995–5001. [Google Scholar] [CrossRef]
- Harada, A.; Kataoka, K. Formation of Polyion Complex Micelles in an Aqueous Milieu from a Pair of Oppositely-Charged Block Copolymers with Poly(ethylene glycol) Segments. Macromolecules 1995, 28, 5294–5299. [Google Scholar] [CrossRef]
- Abe, K.; Ohno, H.; Tsuchida, E. Phase changes of polyion complex between poly(methacrylic acid) and a polycation carrying charges in the chain backbone. Die Makromol. Chem. 1977, 178, 2285–2293. [Google Scholar] [CrossRef]
- Anraku, Y.; Kishimura, A.; Kobayashi, A.; Oba, M.; Kataoka, K. Size-controlled long-circulating PICsome as a ruler to measure critical cut-off disposition size into normal and tumor tissues. Chem. Commun. 2011, 47, 6054–6056. [Google Scholar] [CrossRef]
- Harada, A.; Kataoka, K. Chain length recognition: Core-shell supramolecular assembly from oppositely charged block copolymers. Science 1999, 283, 65–67. [Google Scholar] [CrossRef]
- Miyata, K.; Nishiyama, N.; Kataoka, K. Rational design of smart supramolecular assemblies for gene delivery: Chemical challenges in the creation of artificial viruses. Chem. Soc. Rev. 2012, 41, 2562–2574. [Google Scholar] [CrossRef]
- Mutaf, O.F.; Kishimura, A.; Mochida, Y.; Kim, A.; Kataoka, K. Induction of Secondary Structure through Micellization of an Oppositely Charged Pair of Homochiral Block- and Homopolypeptides in an Aqueous Medium. Macromol. Rapid Commun. 2015, 36, 1958–1964. [Google Scholar] [CrossRef]
- Wibowo, A.; Osada, K.; Matsuda, H.; Anraku, Y.; Hirose, H.; Kishimura, A.; Kataoka, K. Morphology Control in Water of Polyion Complex Nanoarchitectures of Double-Hydrophilic Charged Block Copolymers through Composition Tuning and Thermal Treatment. Macromolecules 2014, 47, 3086–3092. [Google Scholar] [CrossRef]
- Anraku, Y.; Kishimura, A.; Oba, M.; Yamasaki, Y.; Kataoka, K. Spontaneous formation of nanosized unilamellar polyion complex vesicles with tunable size and properties. J. Am. Chem. Soc. 2010, 132, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Kataoka, K. Formation of Stable and Monodispersive Polyion Complex Micelles in Aqueous Medium from Poly(L-lysine) And Poly(Ethylene Glycol)-Poly(Aspartic Acid) Block Copolymer. J. Macromol. Sci. Part A 1997, 34, 2119–2133. [Google Scholar] [CrossRef]
- Zahr, A.S.; Davis, C.A.; Pishko, M.V. Macrophage Uptake of Core−Shell Nanoparticles Surface Modified with Poly(ethylene glycol). Langmuir 2006, 22, 8178–8185. [Google Scholar] [CrossRef] [PubMed]
- Chandran, T.; Katragadda, U.; Teng, Q.; Tan, C. Design and evaluation of micellar nanocarriers for 17-allyamino-17-demethoxygeldanamycin (17-AAG). Int. J. Pharm. 2010, 392, 170–177. [Google Scholar] [CrossRef]
- Endres, T.K.; Beck-Broichsitter, M.; Samsonova, O.; Renette, T.; Kissel, T.H. Self-assembled biodegradable amphiphilic PEG–PCL–lPEI triblock copolymers at the borderline between micelles and nanoparticles designed for drug and gene delivery. BioMaterials 2011, 32, 7721–7731. [Google Scholar] [CrossRef]
- Tao, A.; Huang, G.L.; Igarashi, K.; Hong, T.; Liao, S.; Stellacci, F.; Matsumoto, Y.; Yamasoba, T.; Kataoka, K.; Cabral, H. Polymeric Micelles Loading Proteins through Concurrent Ion Complexation and pH-Cleavable Covalent Bonding for In Vivo Delivery. Macromol. Biosci. 2020, 20, e1900161. [Google Scholar] [CrossRef]
- Mutaf, O.F.; Anraku, Y.; Kishimura, A.; Kataoka, K. Unilamellar polyion complex vesicles (PICsomes) with tunable permeabilities for macromolecular solutes with different shapes and sizes. Polymer 2017, 133, 1–7. [Google Scholar] [CrossRef]
- Watanabe, S.; Hayashi, K.; Toh, K.; Kim, H.J.; Liu, X.; Chaya, H.; Fukushima, S.; Katsushima, K.; Kondo, Y.; Uchida, S.; et al. In vivo rendezvous of small nucleic acid drugs with charge-matched block catiomers to target cancers. Nat. Commun. 2019, 10, 1894. [Google Scholar] [CrossRef]
- Dirisala, A.; Uchida, S.; Toh, K.; Li, J.; Osawa, S.; Tockary, T.A.; Liu, X.; Abbasi, S.; Hayashi, K.; Mochida, Y.; et al. Transient stealth coating of liver sinusoidal wall by anchoring two-armed PEG for retargeting nanomedicines. Sci. Adv. 2020, 6, eabb8133. [Google Scholar] [CrossRef]
- Kishimura, A.; Liamsuwan, S.; Matsuda, H.; Dong, W.-F.; Osada, K.; Yamasaki, Y.; Kataoka, K. pH-dependent permeability change and reversible structural transition of PEGylated polyion complex vesicles (PICsomes) in aqueous media. Soft Matter. 2009, 5, 529–532. [Google Scholar] [CrossRef]
- Lindberg, S.; Regberg, J.; Eriksson, J.; Helmfors, H.; Muñoz-Alarcón, A.; Srimanee, A.; Figueroa, R.A.; Hallberg, E.; Ezzat, K.; Langel, Ü. A convergent uptake route for peptide- and polymer-based nucleotide delivery systems. J. Control. Release 2015, 206, 58–66. [Google Scholar] [CrossRef] [PubMed]
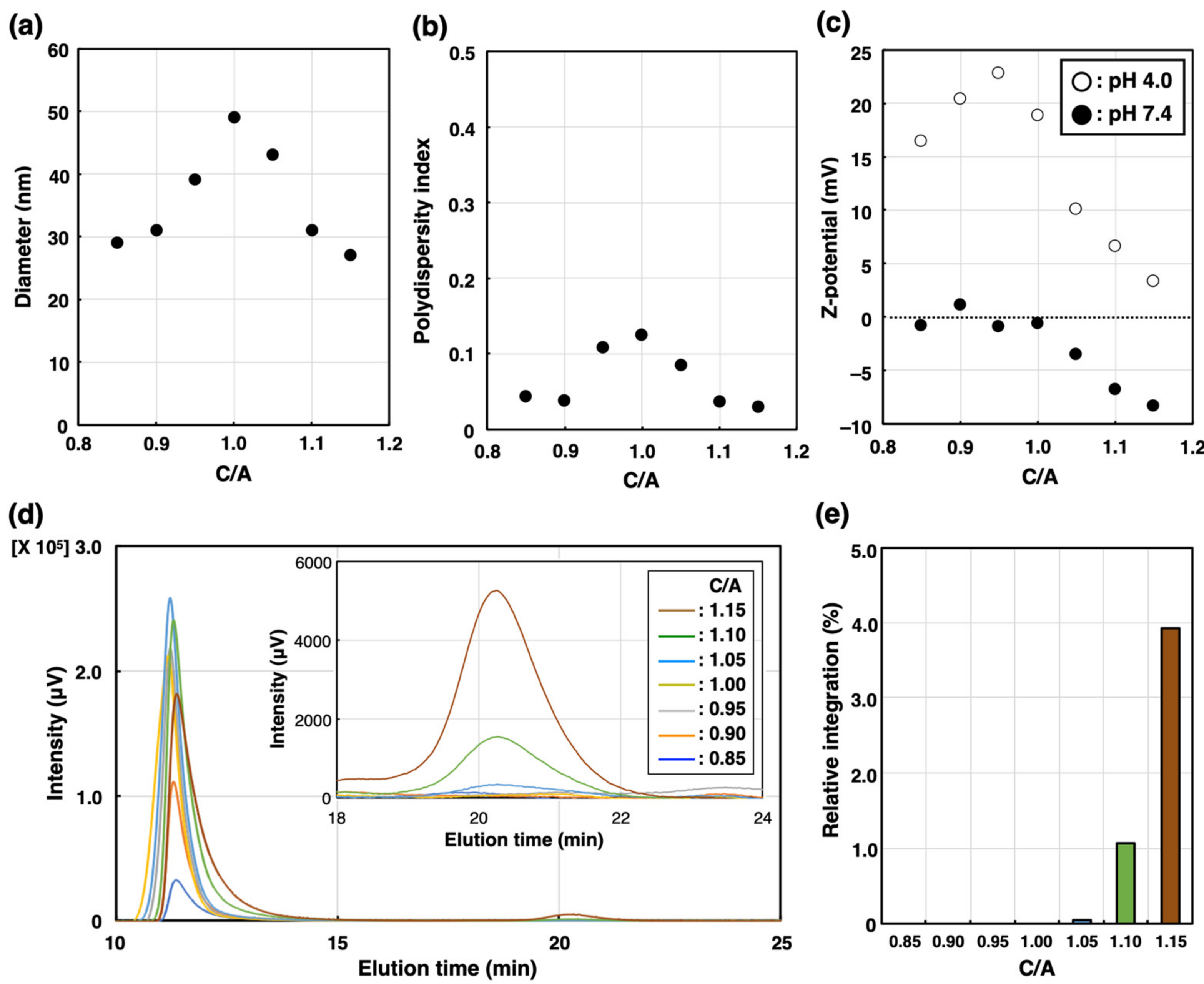
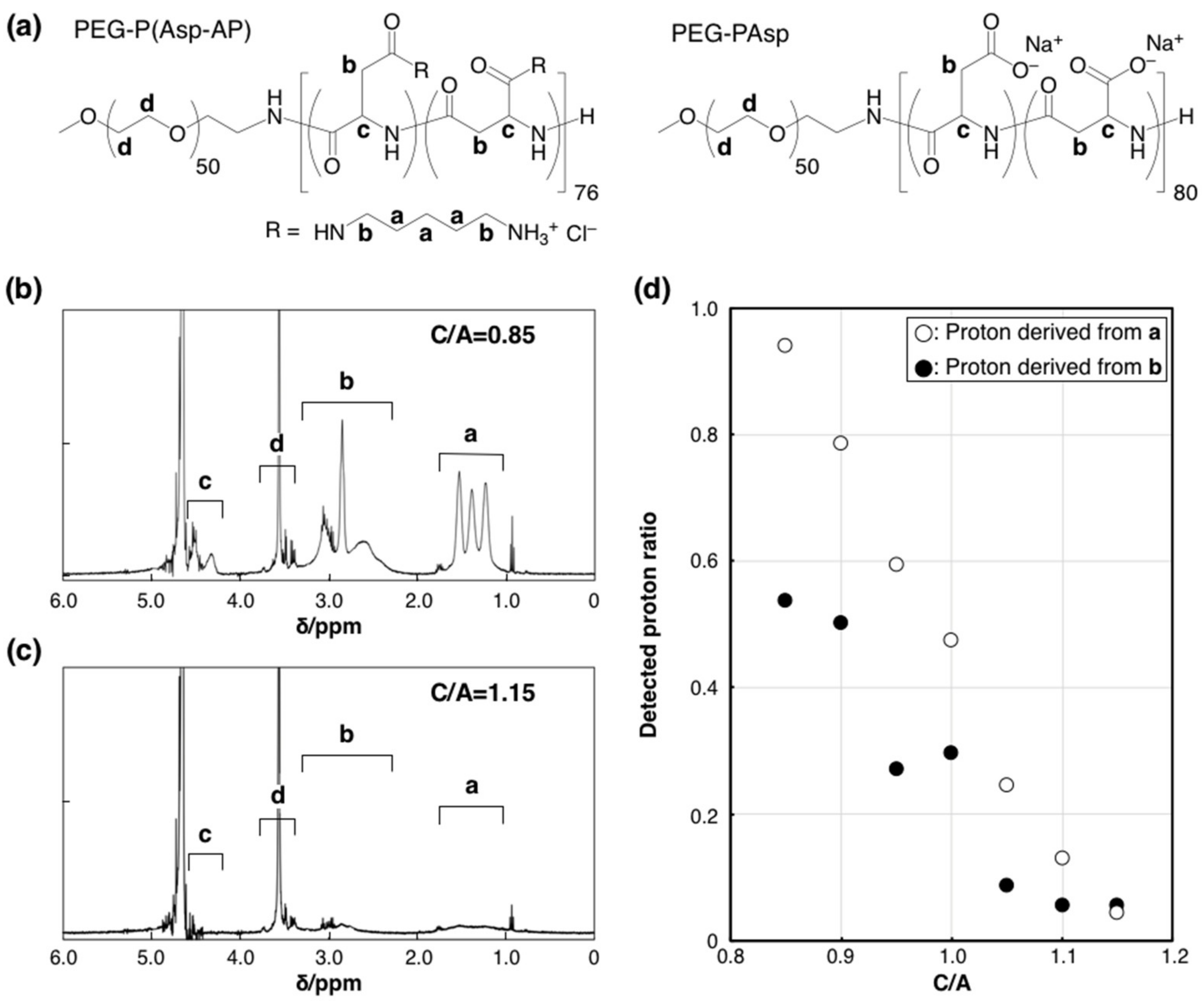
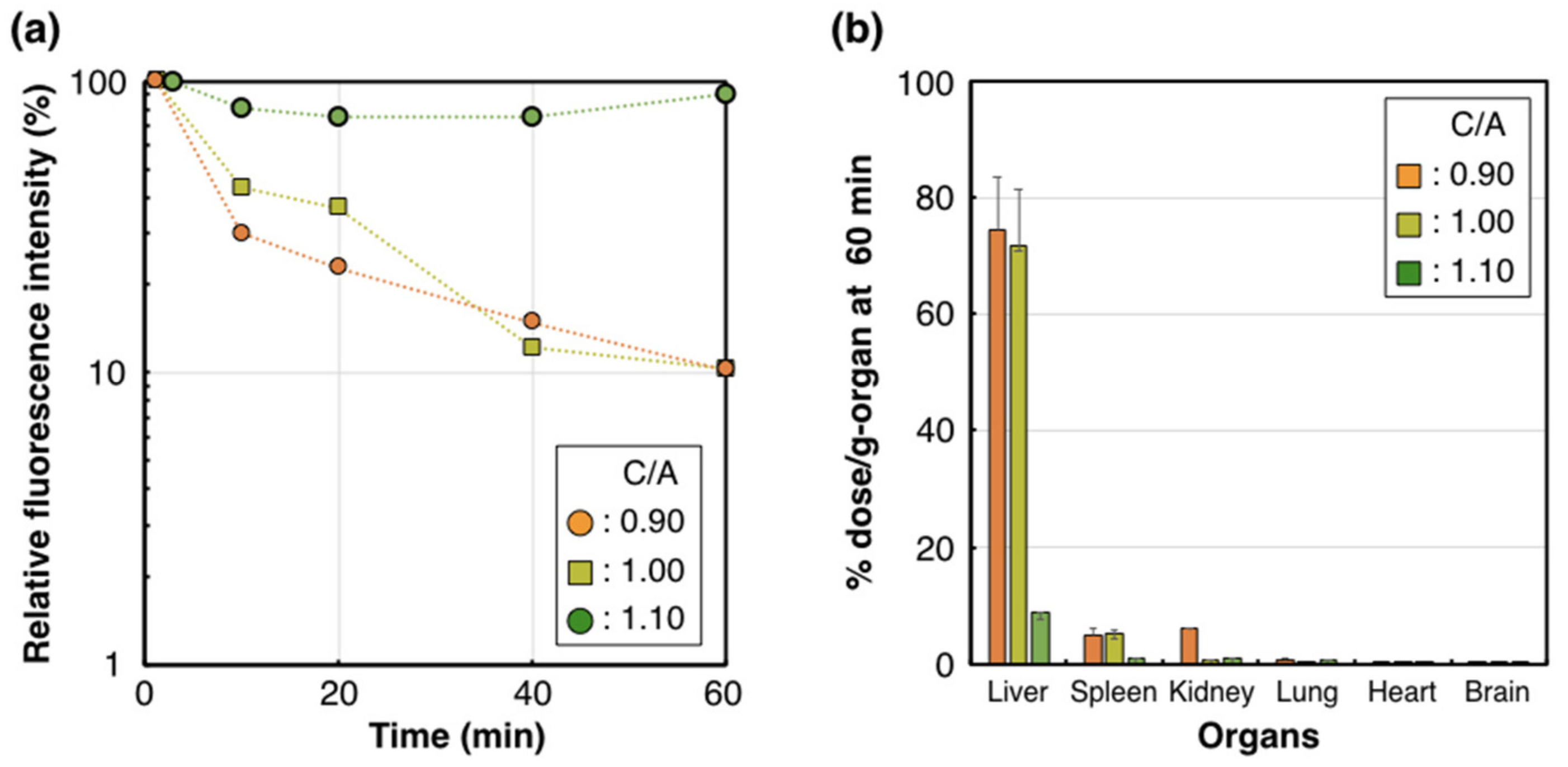
| C/A | 0.90 | 0.95 | 1.00 | 1.05 | 1.10 |
|---|---|---|---|---|---|
| Diameter (nm) * | 31 | 39 | 49 | 43 | 31 |
| Micellar association number ** | 97 | 286 | 400 | 185 | 177 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, N.; Mochida, Y.; Toh, K.; Fukushima, S.; Cabral, H.; Anraku, Y. Effect of Mixing Ratio of Oppositely Charged Block Copolymers on Polyion Complex Micelles for In Vivo Application. Polymers 2021, 13, 5. https://doi.org/10.3390/polym13010005
Nakamura N, Mochida Y, Toh K, Fukushima S, Cabral H, Anraku Y. Effect of Mixing Ratio of Oppositely Charged Block Copolymers on Polyion Complex Micelles for In Vivo Application. Polymers. 2021; 13(1):5. https://doi.org/10.3390/polym13010005
Chicago/Turabian StyleNakamura, Noriko, Yuki Mochida, Kazuko Toh, Shigeto Fukushima, Horacio Cabral, and Yasutaka Anraku. 2021. "Effect of Mixing Ratio of Oppositely Charged Block Copolymers on Polyion Complex Micelles for In Vivo Application" Polymers 13, no. 1: 5. https://doi.org/10.3390/polym13010005
APA StyleNakamura, N., Mochida, Y., Toh, K., Fukushima, S., Cabral, H., & Anraku, Y. (2021). Effect of Mixing Ratio of Oppositely Charged Block Copolymers on Polyion Complex Micelles for In Vivo Application. Polymers, 13(1), 5. https://doi.org/10.3390/polym13010005







