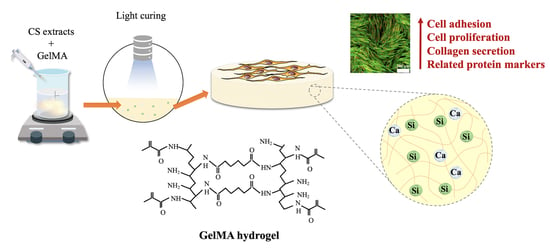
Calcium Silicate-Activated Gelatin Methacrylate Hydrogel for Accelerating Human Dermal Fibroblast Proliferation and Differentiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Extracts of CS Powders
2.2. Preparation of Si-Contained GelMa Hydrogels
2.3. Si-GelMa Hydrogel Characterizations
2.4. In Vitro Immersion Study and Weight Loss
2.5. Cell Proliferation and Morphology
2.6. Western Blot Analysis
2.7. Collagen I Secretion
2.8. Statistical Analyses
3. Results and Discussion
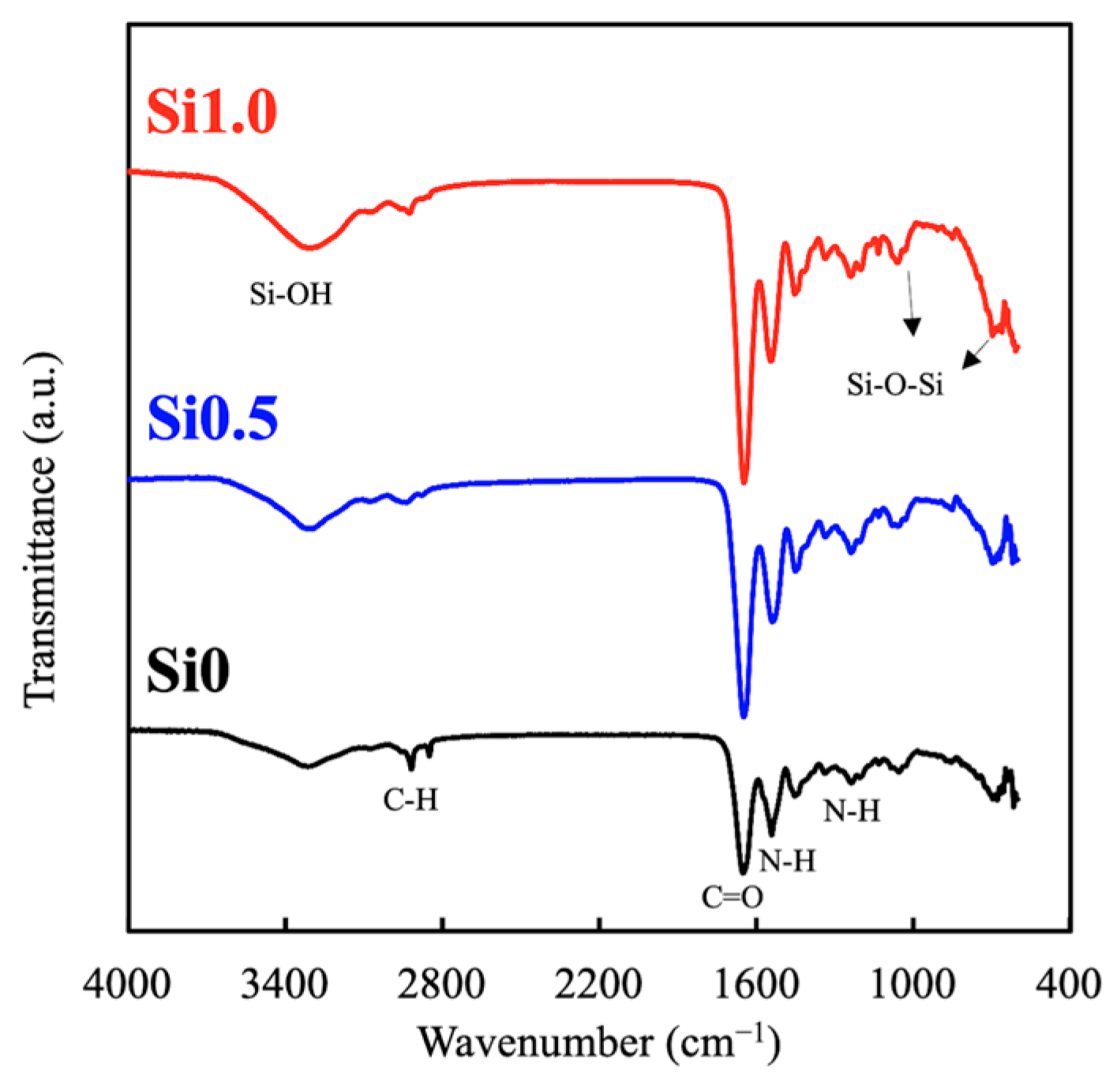
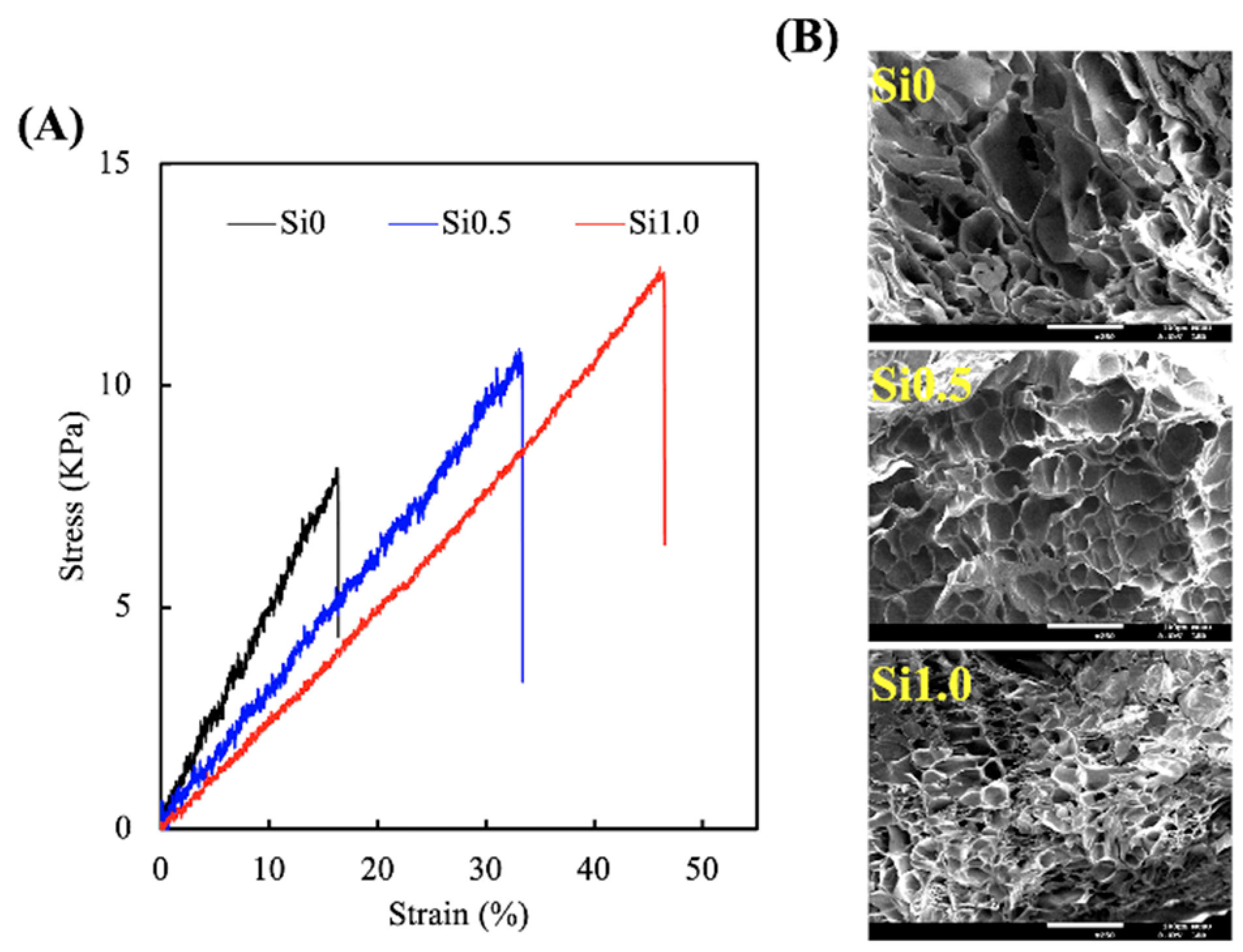
3.1. Characterization of Si-GelMa Hydrogel
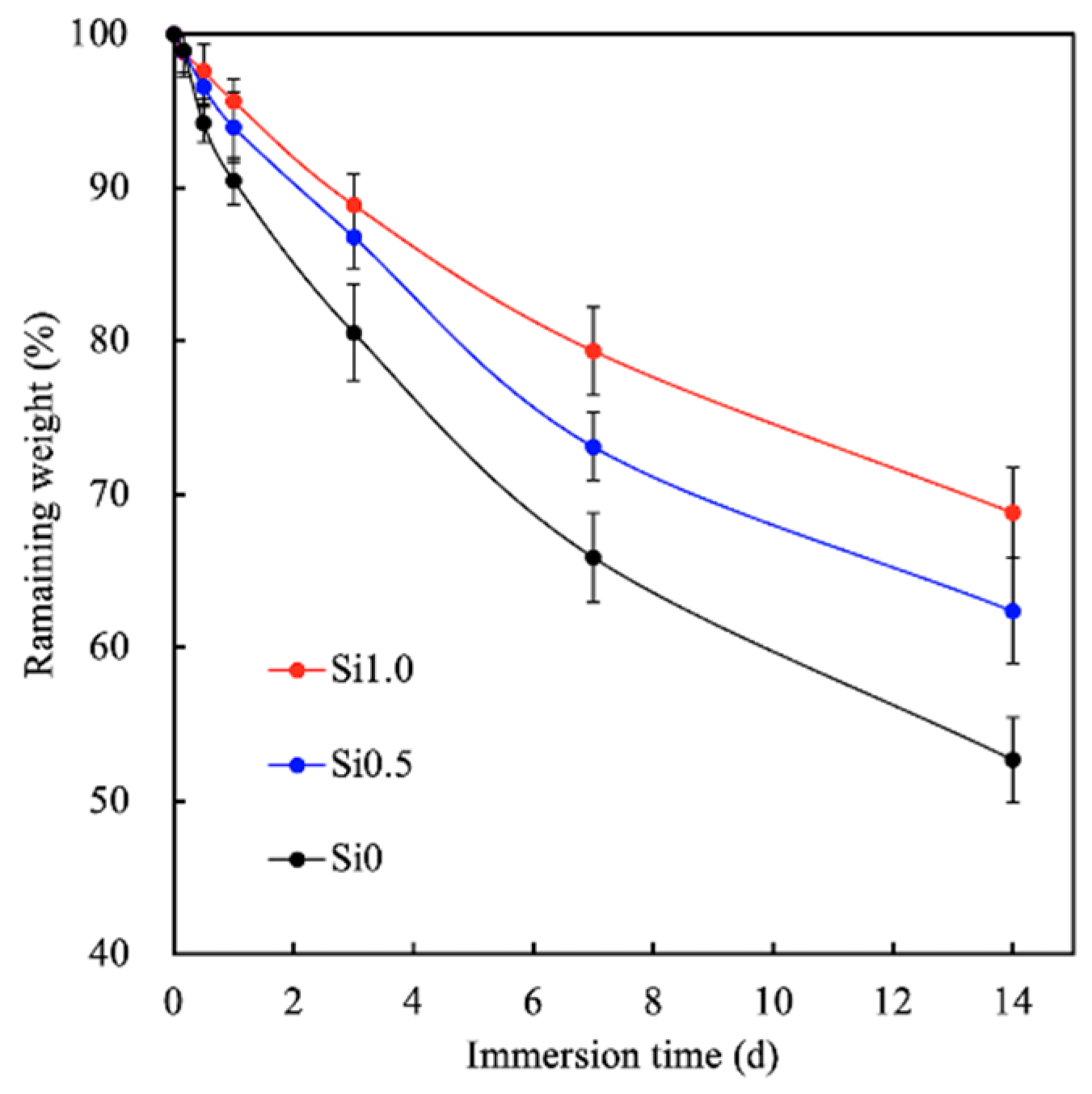
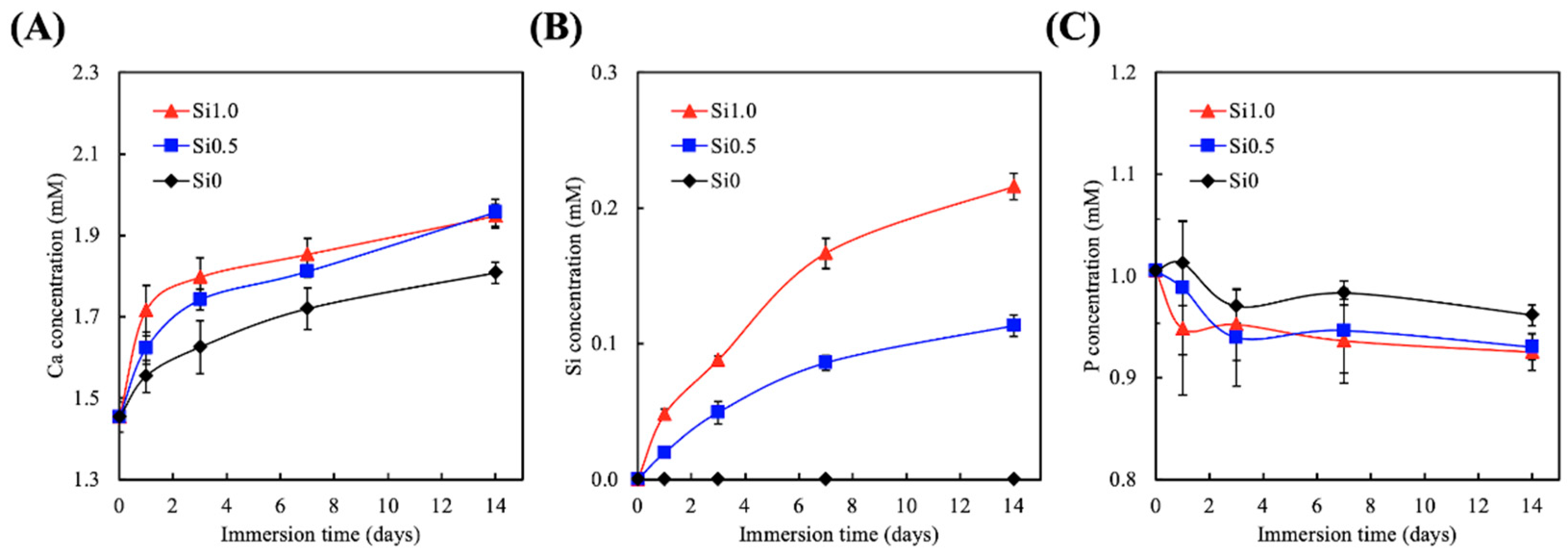
3.2. Degradation and Ion Released Properties of Si-GelMa Hydrogel
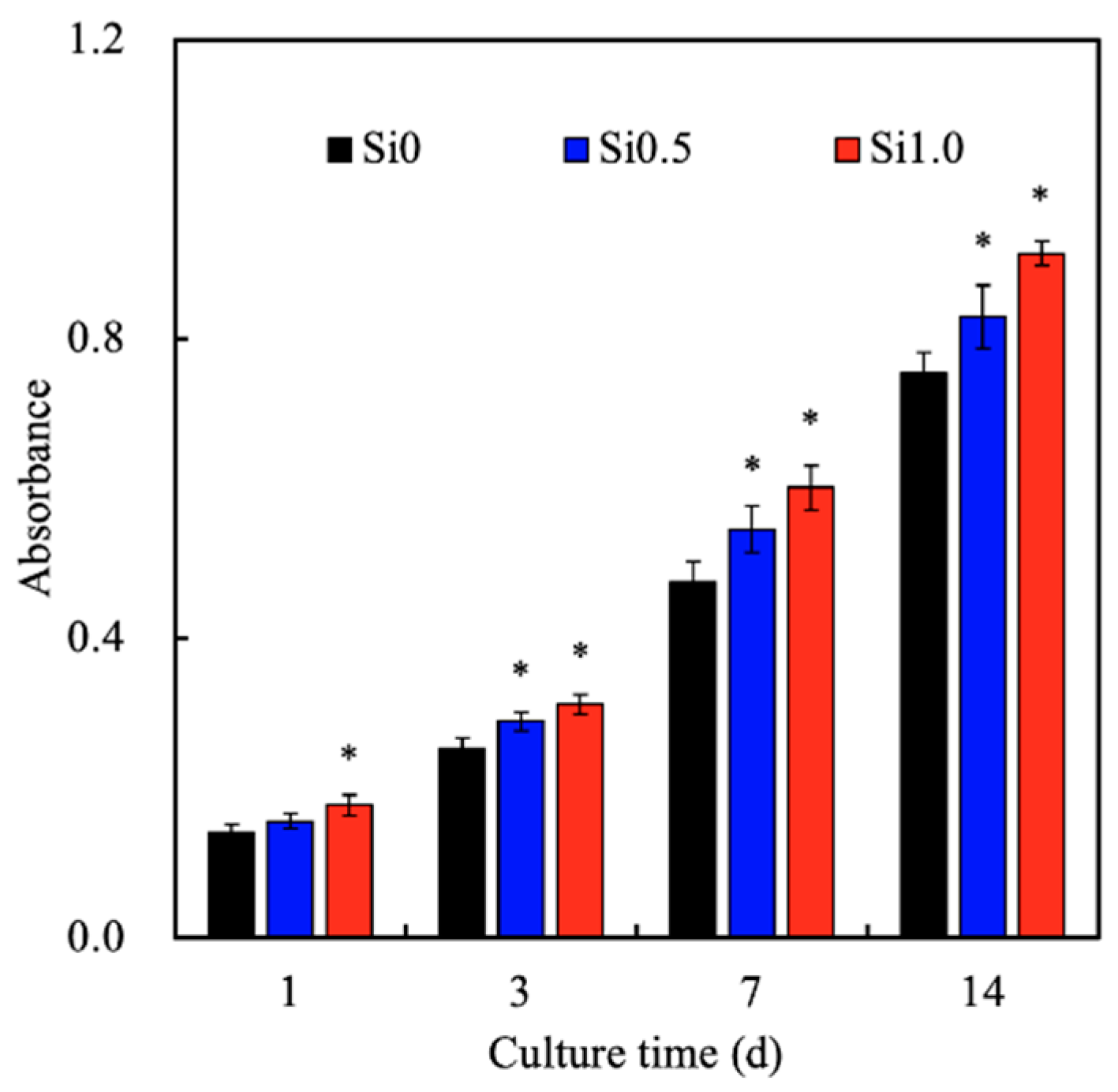
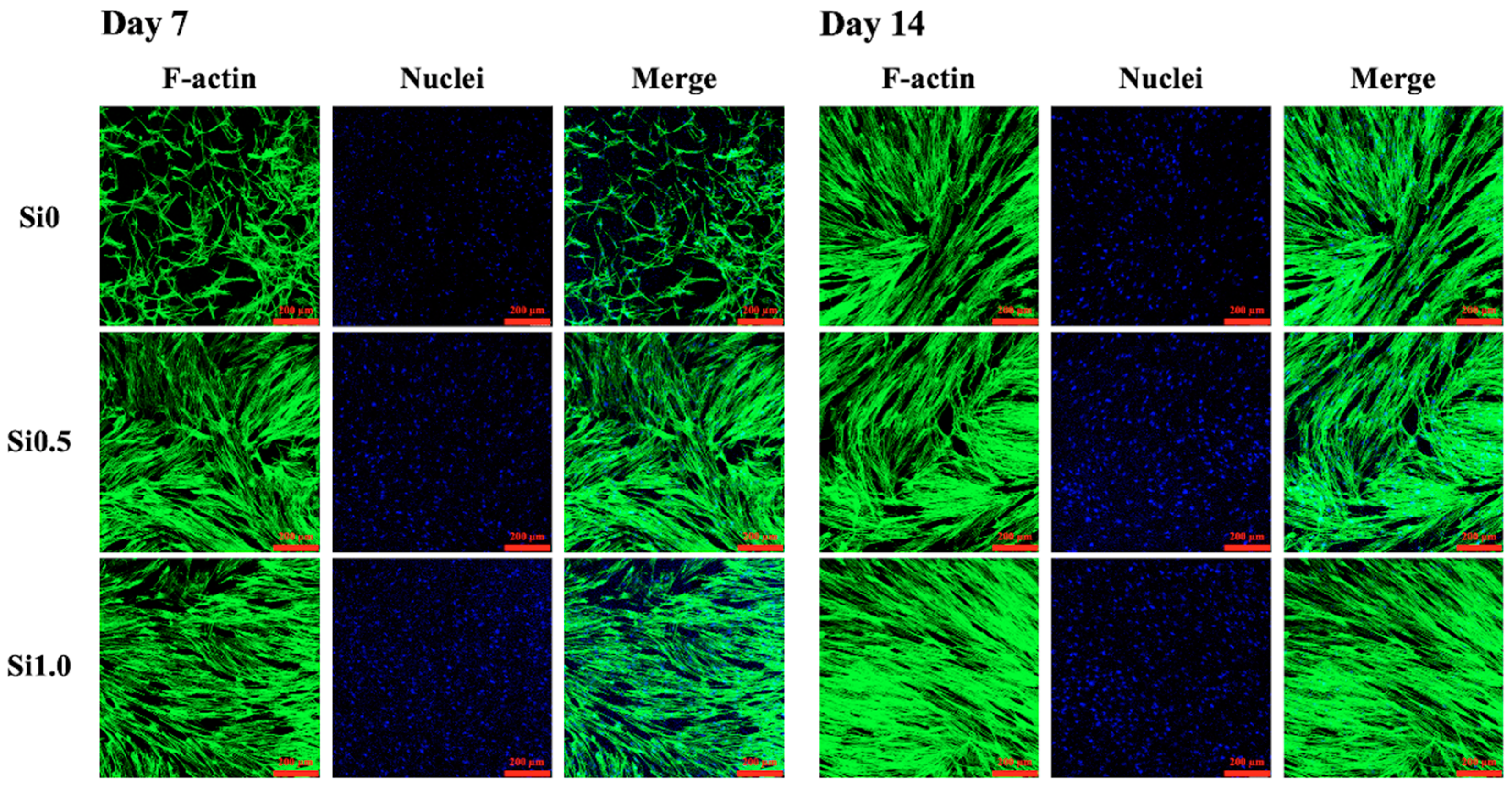
3.3. Cell Adhesion and Proliferation
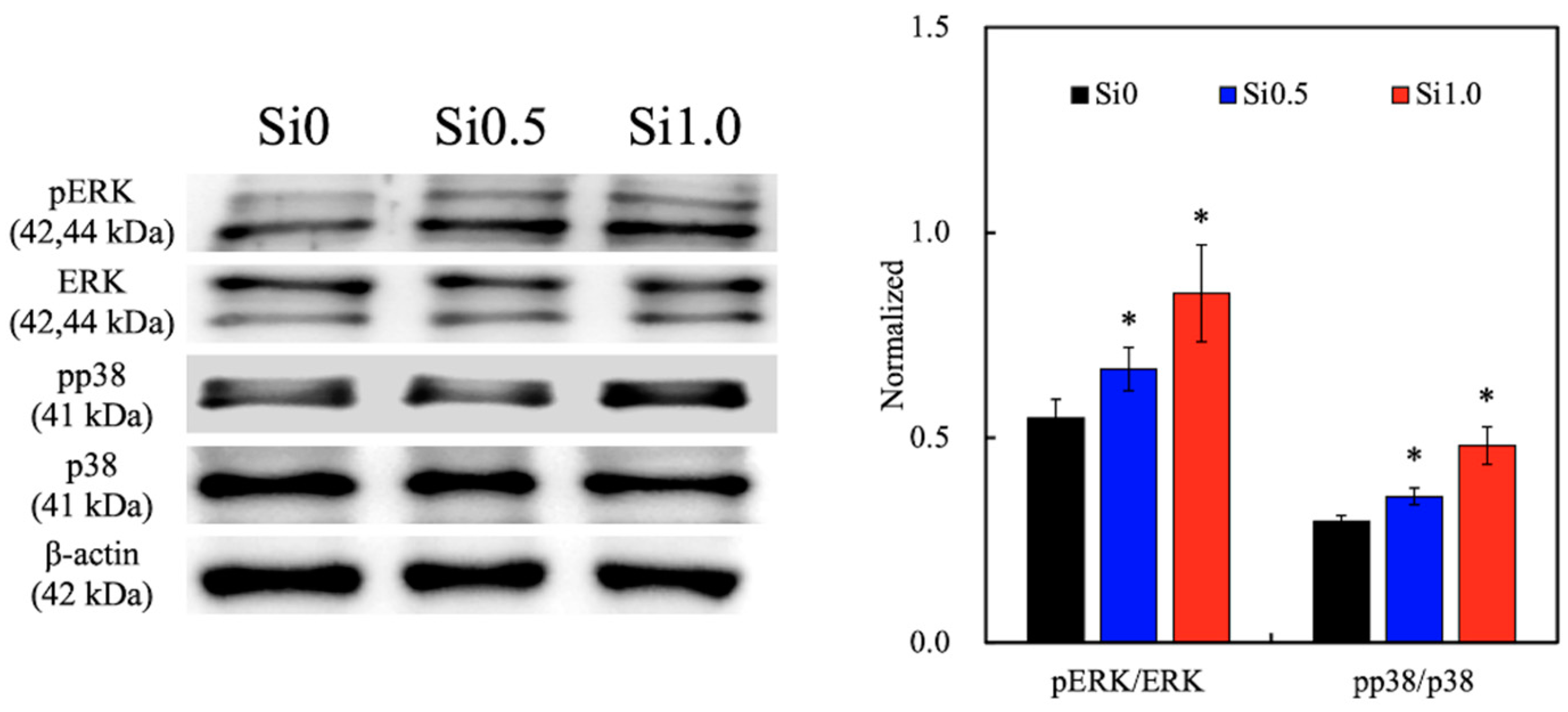
3.4. Mitogen-Activated Protein Kinase (MAPK)
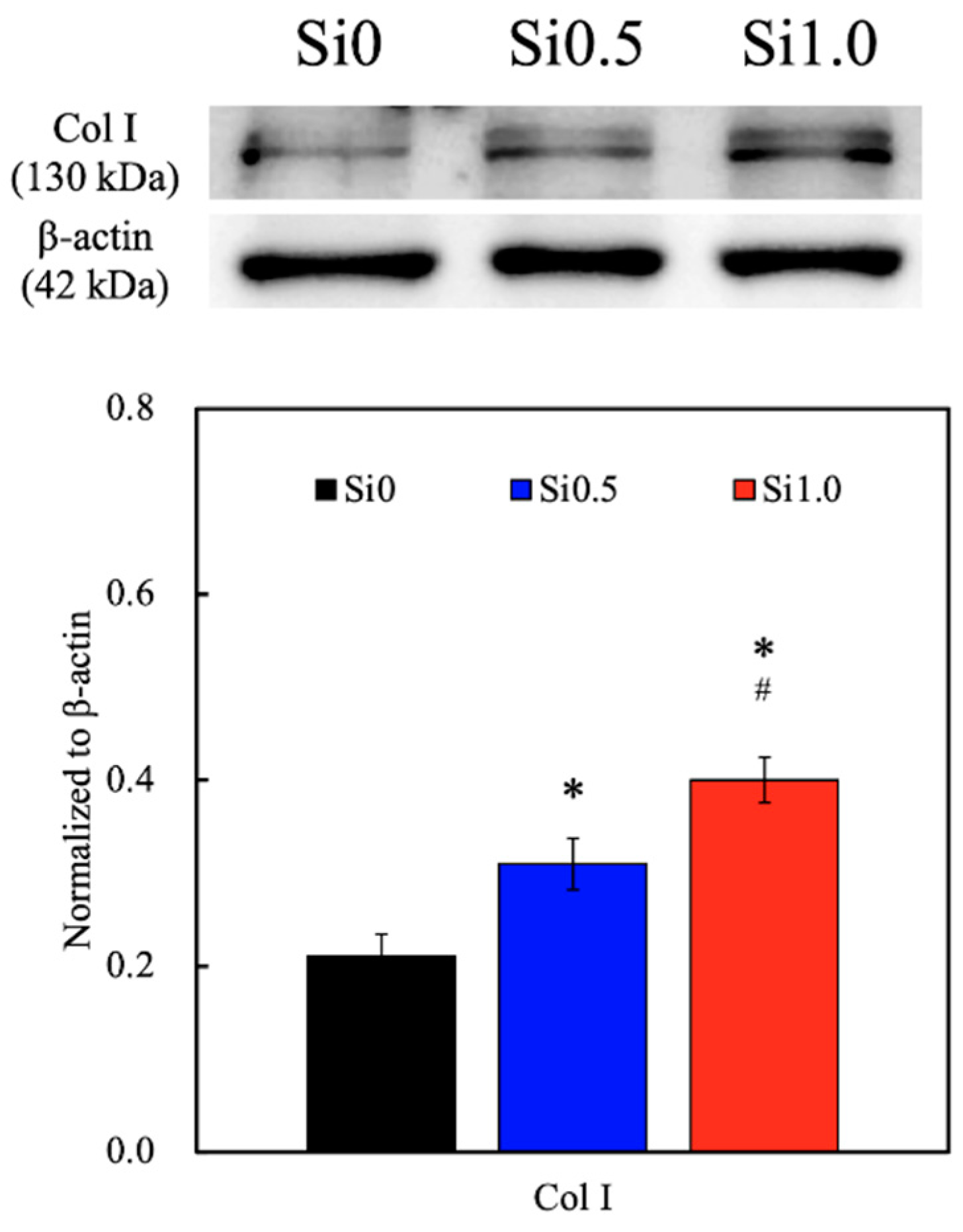
3.5. Collagen I Secretion
3.6. Protein Expression of hDF on Si-GelMa Hydrogel
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, Y.H.; Chuang, T.Y.; Chiang, W.H.; Chen, I.W.P.; Wang, K.; Shie, M.Y.; Chen, Y.W. The synergistic effects of graphene-contained 3D-printed calcium silicate/poly-ε-caprolactone scaffolds promote FGFR-induced osteogenic/angiogenic differentiation of mesenchymal stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109887. [Google Scholar] [CrossRef]
- Elomaa, L.; Keshi, E.; Sauer, I.M.; Weinhart, M. Development of GelMA/PCL and dECM/PCL resins for 3D printing of acellular in vitro tissue scaffolds by stereolithography. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110958. [Google Scholar] [CrossRef]
- Hussein, K.H.; Abdelhamid, H.N.; Zou, X.; Woo, H.-M. Ultrasonicated graphene oxide enhances bone and skin wound regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Shefa, A.A.; Sultana, T.; Park, M.K.; Lee, S.Y.; Gwon, J.-G.; Lee, B.T. Curcumin incorporation into an oxidized cellulose nanofiber-polyvinyl alcohol hydrogel system promotes wound healing. Mater. Des. 2020, 186, 108313. [Google Scholar] [CrossRef]
- Sundarakrishnan, A.; Zukas, H.; Coburn, J.; Bertini, B.T.; Liu, Z.; Georgakoudi, I.; Baugh, L.; Dasgupta, Q.; Black, L.D.; Kaplan, D.L. Bioengineered in vitro tissue model of fibroblast activation for modeling pulmonary fibrosis. ACS Biomater. Sci. Eng. 2019, 5, 2417–2429. [Google Scholar] [CrossRef]
- Chong, L.H.; Lim, M.M.; Sultana, N. Fabrication and evaluation of polycaprolactone/gelatin-based electrospun nanofibers with antibacterial properties. J. Nanomater. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Mao, Q.; Hoffmann, O.; Yu, K.; Lu, F.; Lan, G.; Dai, F.; Shang, S.; Xie, R. Self-contracting oxidized starch/gelatin hydrogel for noninvasive wound closure and wound healing. Mater. Des. 2020, 194, 108916. [Google Scholar] [CrossRef]
- Thangprasert, A.; Tansakul, C.; Thuaksubun, N.; Meesane, J. Mimicked hybrid hydrogel based on gelatin/PVA for tissue engineering in subchondral bone interface for osteoarthritis surgery. Mater. Des. 2019, 183, 108113. [Google Scholar] [CrossRef]
- Hao, Y.; Zhao, W.; Zhang, L.; Zeng, X.; Sun, Z.; Zhang, D.; Shen, P.; Li, Z.; Han, Y.; Li, P.; et al. Bio-multifunctional alginate/chitosan/fucoidan sponges with enhanced angiogenesis and hair follicle regeneration for promoting full-thickness wound healing. Mater. Des. 2020, 193, 108863. [Google Scholar] [CrossRef]
- Wu, G.; Ma, X.; Fan, L.; Gao, Y.; Deng, H.; Wang, Y. Accelerating dermal wound healing and mitigating excessive scar formation using LBL modified nanofibrous mats. Mater. Des. 2020, 185, 108265. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Qin, W.; Dai, J.; Zhang, Q.; Lee, K.; Liu, Y. Physicochemical properties of gelatin films containing tea polyphenol-loaded chitosan nanoparticles generated by electrospray. Mater. Des. 2020, 185, 108277. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Suo, H.; Yan, M.; Yin, J.; Fu, J. 3D printing of biomimetic multi-layered GelMA/nHA scaffold for osteochondral defect repair. Mater. Des. 2019, 171, 107708. [Google Scholar] [CrossRef]
- Juncos Bombin, A.D.; Dunne, N.J.; McCarthy, H.O. Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 110994. [Google Scholar] [CrossRef] [PubMed]
- Afjoul, H.; Shamloo, A.; Kamali, A. Freeze-gelled alginate/gelatin scaffolds for wound healing applications: An in vitro, in vivo study. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 113, 110957. [Google Scholar] [CrossRef] [PubMed]
- Shie, M.Y.; Lee, J.J.; Ho, C.C.; Yen, S.Y.; Ng, H.Y.; Chen, Y.W. Effects of gelatin methacrylate bio-ink concentration on mechano-physical properties and human dermal fibroblast behavior. Polymers 2020, 12, 1930. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, F.; Li, Y.; Wang, M.; Chen, X.; Lei, B. 3D-printed photoluminescent bioactive scaffolds with biomimetic elastomeric surface for enhanced bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110153. [Google Scholar] [CrossRef]
- Zou, Q.; Grottkau, B.E.; He, Z.; Shu, L.; Yang, L.; Ma, M.; Ye, C. Biofabrication of valentine-shaped heart with a composite hydrogel and sacrificial material. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110205. [Google Scholar] [CrossRef]
- Bedir, T.; Ulag, S.; Ustundag, C.B.; Gunduz, O. 3D bioprinting applications in neural tissue engineering for spinal cord injury repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110741. [Google Scholar] [CrossRef]
- Le Duigou, A.; Correa, D.; Ueda, M.; Matsuzaki, R.; Castro, M. A review of 3D and 4D printing of natural fibre biocomposites. Mater. Des. 2020, 194, 108911. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, J.; Liu, Y.; Li, Y.; Zhang, C.; Qi, W.; Yeung, K.W.K.; Wong, T.M.; Zhao, X.; Pan, H. Electrospun chitosan/PVA/bioglass Nanofibrous membrane with spatially designed structure for accelerating chronic wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110083. [Google Scholar] [CrossRef]
- Shie, M.Y.; Ding, S.J. Integrin binding and MAPK signal pathways in primary cell responses to surface chemistry of calcium silicate cements. Biomaterials 2013, 34, 6589–6606. [Google Scholar] [CrossRef] [PubMed]
- Shie, M.Y.; Ding, S.J.; Chang, H.C. The role of silicon in osteoblast-like cell proliferation and apoptosis. Acta Biomater. 2011, 7, 2604–2614. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.G.; Lee, K.X.; Lin, Y.H.; Huang, T.H.; Ho, C.C.; Shie, M.Y. Caffeic acid-coated nanolayer on Mineral Trioxide Aggregate potentiate the host immune responses, angiogenesis, and odontogenesis. J. Endod. 2020, 46, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Shen, Y.F.; Ho, C.C.; Yu, J.; Wu, Y.H.; Wang, K.; Shih, C.T.; Shie, M.Y. Osteogenic and angiogenic potentials of the cell-laden hydrogel/mussel-inspired calcium silicate complex hierarchical porous scaffold fabricated by 3D bioprinting. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 679–687. [Google Scholar] [CrossRef]
- Wang, X.; Chang, J.; Wu, C. Bioactive inorganic/organic nanocomposites for wound healing. Appl. Mater. Today 2018, 11, 308–319. [Google Scholar] [CrossRef]
- Gao, L.; Zhou, Y.; Peng, J.; Xu, C.; Xu, Q.; Xing, M.; Chang, J. A novel dual-adhesive and bioactive hydrogel activated by bioglass for wound healing. NPG Asia Mater. 2019, 11, 66. [Google Scholar] [CrossRef]
- Chiu, Y.C.; Shie, M.Y.; Lin, Y.H.; Lee, K.X.; Chen, Y.W. Effect of strontium substitution on the physicochemical properties and bone regeneration potential of 3D printed calcium silicate scaffolds. Int. J. Mol. Sci. 2019, 20, 2729. [Google Scholar] [CrossRef]
- Shaker, M.A.; Doré, J.J.E.; Younes, H.M. Synthesis, characterization and cytocompatibility of a poly(diol-tricarballylate) visible light photo-cross-linked biodegradable elastomer. J. Biomat. Sci. Polym. E 2010, 21, 507–528. [Google Scholar] [CrossRef]
- Sujan, M.I.; Sarkar, S.D.; Sultana, S.; Bushra, L.; Tareq, R.; Roy, C.K.; Azam, M.S. Bi-functional silica nanoparticles for simultaneous enhancement of mechanical strength and swelling capacity of hydrogels. RSC Adv. 2020, 10, 6213–6222. [Google Scholar] [CrossRef]
- Liu, Y.; Pharr, M.; Salvatore, G.A. Lab-on-skin: A review of flexible and stretchable electronics for wearable health monitoring. ACS Nano 2017, 11, 9614–9635. [Google Scholar] [CrossRef]
- Trappmann, B.; Gautrot, J.E.; Connelly, J.T.; Strange, D.G.T.; Li, Y.; Oyen, M.L.; Cohen Stuart, M.A.; Boehm, H.; Li, B.; Vogel, V.; et al. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 2012, 11, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Bittner, S.M.; Smith, B.T.; Diaz-Gomez, L.; Hudgins, C.D.; Melchiorri, A.J.; Scott, D.W.; Fisher, J.P.; Mikos, A.G. Fabrication and mechanical characterization of 3D printed vertical uniform and gradient scaffolds for bone and osteochondral tissue engineering. Acta Biomater. 2019, 90, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Hsu, T.T.; Wang, K.; Shie, M.Y. Preparation of the fast setting and degrading Ca-Si-Mg cement with both odontogenesis and angiogenesis differentiation of human periodontal ligament cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.T.; Wang, F.M.; Liu, Y.T.; Ng, H.Y.; Jhong, Y.R.; Hung, C.H.; Chen, Y.W. Effect of bone morphogenic protein-2 loaded mesoporous strontium substitution calcium silicate/recycled fish gelatin 3D cell-laden scaffold for bone tissue engineering. Processes 2020, 8, 493. [Google Scholar] [CrossRef]
- Zhang, J.; Yun, S.; Du, Y.; Zannettino, A.; Zhang, H. Hydrogel-based preparation of cell aggregates for biomedical applications. Appl. Mater. Today 2020, 20, 100747. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Iezzi, G.; Piattelli, A.; Prati, C.; Scarano, A. Osteoinductive potential and bone-bonding ability of ProRoot MTA, MTA Plus and Biodentine in rabbit intramedullary model: Microchemical characterization and histological analysis. Dent. Mater. 2017, 33, 221–238. [Google Scholar] [CrossRef]
- Yu, H.; Peng, J.; Xu, Y.; Chang, J.; Li, H. Bioglass activated skin tissue engineering constructs for wound healing. ACS Appl. Mater. Interfaces 2016, 8, 703–715. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, X.; Bellido, T.; Helms, J.A. A correlation between Wnt/beta-catenin signaling and the rate of dentin secretion. J. Endod. 2019, 45, 1357–1364. [Google Scholar] [CrossRef]
- Chen, Y.W.; Wang, K.; Ho, C.C.; Kao, C.T.; Ng, H.Y.; Shie, M.Y. Cyclic tensile stimulation enrichment of Schwann cell-laden auxetic hydrogel scaffolds towards peripheral nerve tissue engineering. Mater. Des. 2020, 195, 108982. [Google Scholar] [CrossRef]
- Kao, C.T.; Chiu, Y.C.; Lee, K.X.; Lin, Y.H.; Huang, T.H.; Liu, Y.C.; Shie, M.Y. The synergistic effects of Xu Duan combined Sr-contained calcium silicate/poly-ε-caprolactone scaffolds for the promotion of osteogenesis marker expression and the induction of bone regeneration in osteoporosis. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111629. [Google Scholar] [CrossRef]
- Shie, M.Y.; Chang, H.C.; Ding, S.J. Composition-dependent protein secretion and integrin level of osteoblastic cell on calcium silicate cements. J. Biomed. Mater. Res. Part A 2014, 102, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Ding, S.J.; Hsu, T.C.; Kao, C.T. Effects of mineral trioxide aggregate (MTA) extracts on mitogen-activated protein kinase activity in human osteosarcoma cell line (U2OS). Biomaterials 2003, 24, 3909–3913. [Google Scholar] [CrossRef]
- Chiu, Y.C.; Fang, H.Y.; Hsu, T.T.; Lin, C.Y.; Shie, M.Y. The characteristics of Mineral Trioxide Aggregate/polycaprolactone 3-dimensional scaffold with osteogenesis properties for tissue regeneration. J. Endod. 2017, 43, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Mavrogonatou, E.; Kletsas, D. Differential response of nucleus pulposus intervertebral disc cells to high salt, sorbitol, and urea. J. Cell. Physiol. 2011, 227, 1179–1187. [Google Scholar] [CrossRef]
- Uhlik, M.T.; Abell, A.N.; Johnson, N.L.; Sun, W.; Cuevas, B.D.; Lobel-Rice, K.E.; Horne, E.A.; Dell’Acqua, M.L.; Johnson, G.L. Rac–MEKK3–MKK3 scaffolding for p38 MAPK activation during hyperosmotic shock. Nat. Cell Biol. 2003, 5, 1104–1110. [Google Scholar] [CrossRef]
- Ma, H.; Feng, C.; Chang, J.; Wu, C. 3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta Biomater. 2018, 79, 37–59. [Google Scholar] [CrossRef]
- Huang, S.; Fu, X. Naturally derived materials-based cell and drug delivery systems in skin regeneration. J. Control. Release 2010, 142, 149–159. [Google Scholar] [CrossRef]
- Shen, J.; Ji, Y.; Xie, M.; Zhao, H.; Xuan, W.; Yin, L.; Yu, X.; Xu, F.; Su, S.; Nie, J.; et al. Cell-modified bioprinted microspheres for vascular regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110896. [Google Scholar] [CrossRef]
- Lazurko, C.; Khatoon, Z.; Goel, K.; Sedlakova, V.; Eren Cimenci, C.; Ahumada, M.; Zhang, L.; Mah, T.F.; Franco, W.; Suuronen, E.J.; et al. Multifunctional nano and collagen-based therapeutic materials for skin repair. ACS Biomater. Sci. Eng. 2019, 6, 1124–1134. [Google Scholar] [CrossRef]
- Abalymov, A.; Parakhonskiy, B.; Skirtach, A.G. Polymer- and hybrid-based biomaterials for interstitial, connective, vascular, nerve, visceral and musculoskeletal tissue engineering. Polymers 2020, 12, 620. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, F.-S.; Lee, J.-J.; Lee, A.K.-X.; Ho, C.-C.; Liu, Y.-T.; Shie, M.-Y. Calcium Silicate-Activated Gelatin Methacrylate Hydrogel for Accelerating Human Dermal Fibroblast Proliferation and Differentiation. Polymers 2021, 13, 70. https://doi.org/10.3390/polym13010070
Lin F-S, Lee J-J, Lee AK-X, Ho C-C, Liu Y-T, Shie M-Y. Calcium Silicate-Activated Gelatin Methacrylate Hydrogel for Accelerating Human Dermal Fibroblast Proliferation and Differentiation. Polymers. 2021; 13(1):70. https://doi.org/10.3390/polym13010070
Chicago/Turabian StyleLin, Fong-Sian, Jian-Jr Lee, Alvin Kai-Xing Lee, Chia-Che Ho, Yen-Ting Liu, and Ming-You Shie. 2021. "Calcium Silicate-Activated Gelatin Methacrylate Hydrogel for Accelerating Human Dermal Fibroblast Proliferation and Differentiation" Polymers 13, no. 1: 70. https://doi.org/10.3390/polym13010070
APA StyleLin, F.-S., Lee, J.-J., Lee, A. K.-X., Ho, C.-C., Liu, Y.-T., & Shie, M.-Y. (2021). Calcium Silicate-Activated Gelatin Methacrylate Hydrogel for Accelerating Human Dermal Fibroblast Proliferation and Differentiation. Polymers, 13(1), 70. https://doi.org/10.3390/polym13010070