Polymer Cold-Flow Improvers for Biodiesel
Abstract
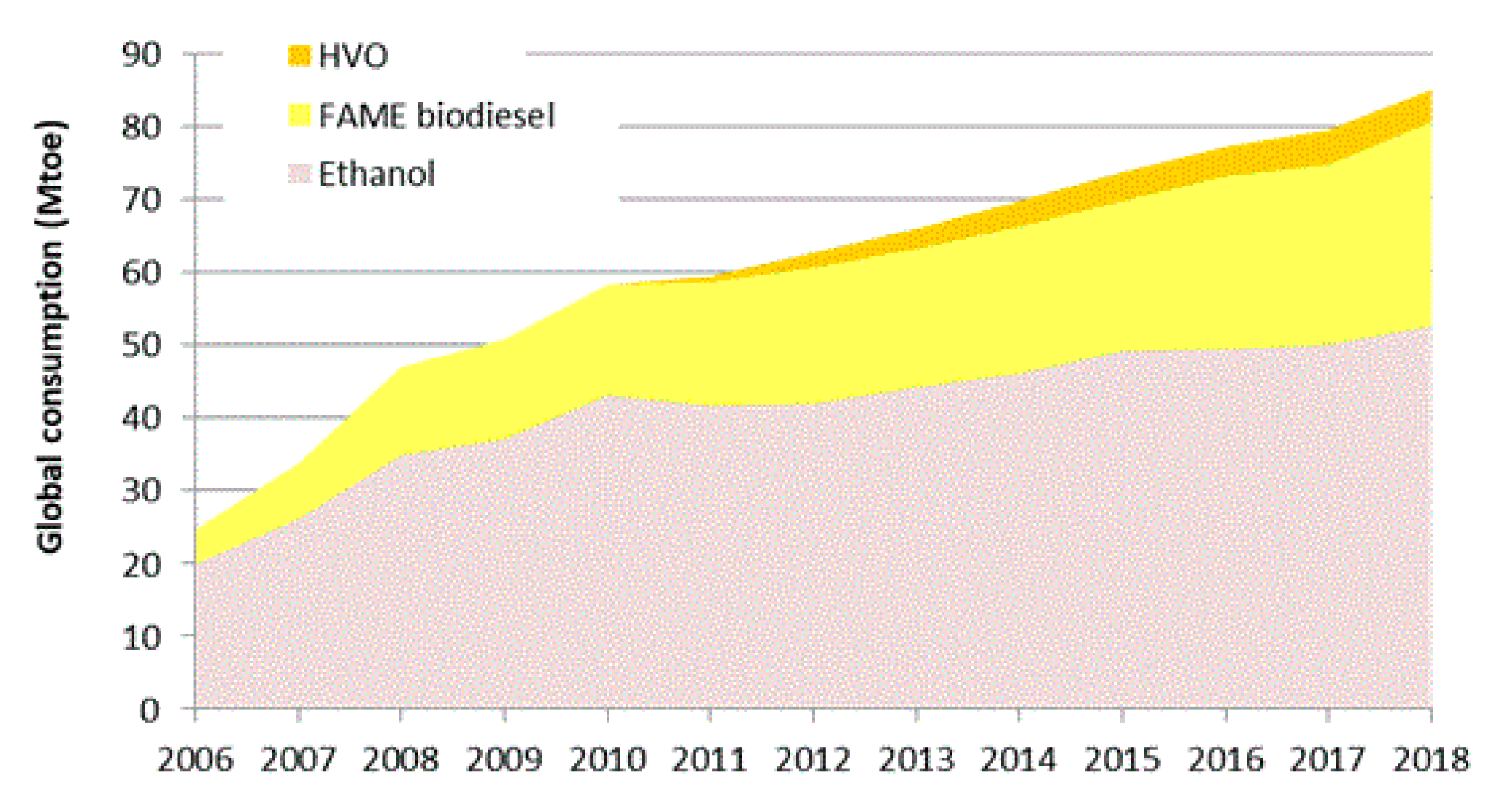
:1. Introduction
2. Sources, Synthesis and Chemical Composition of BD
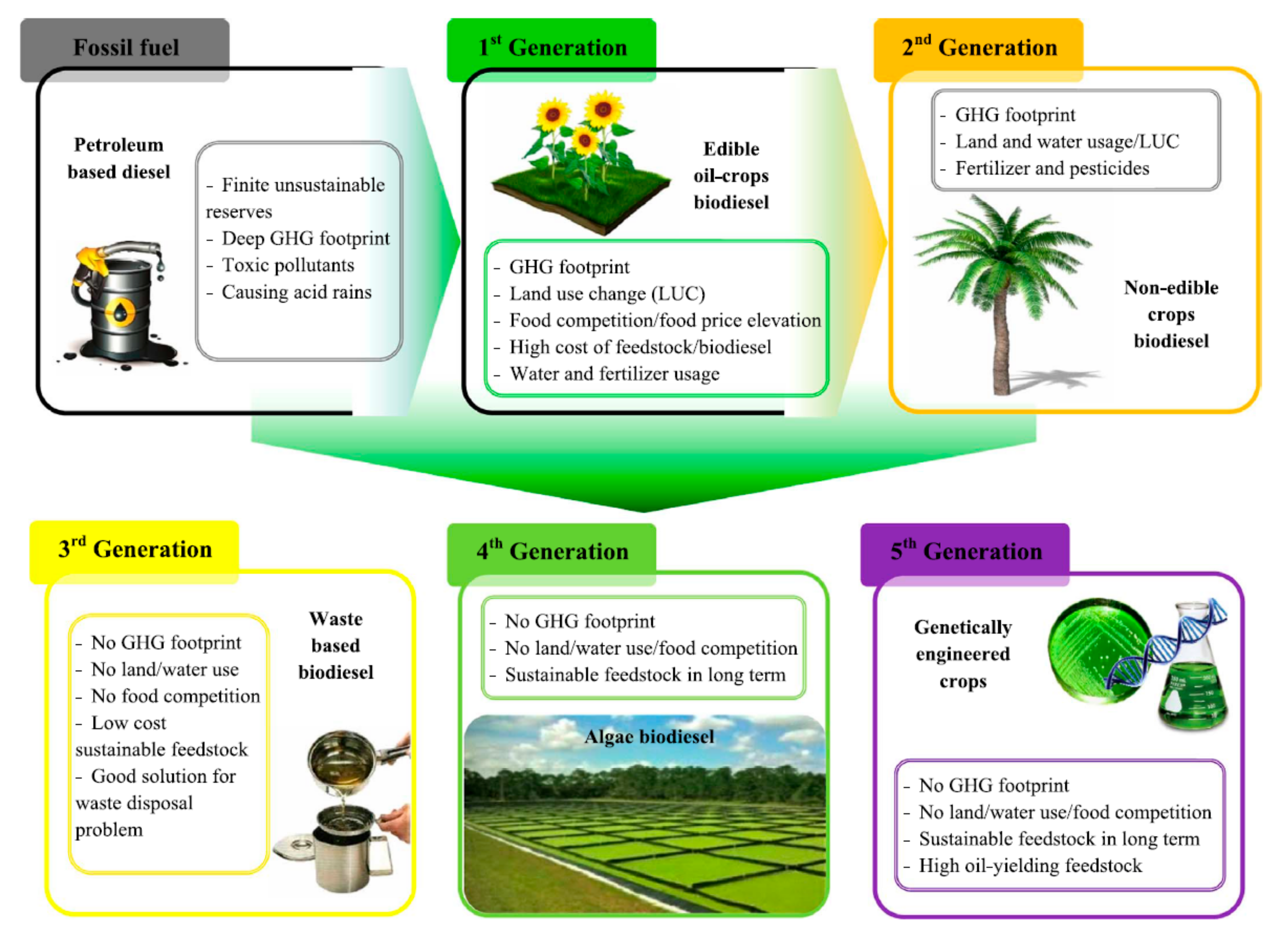
2.1. Oils, as Raw Material for the Production of BD
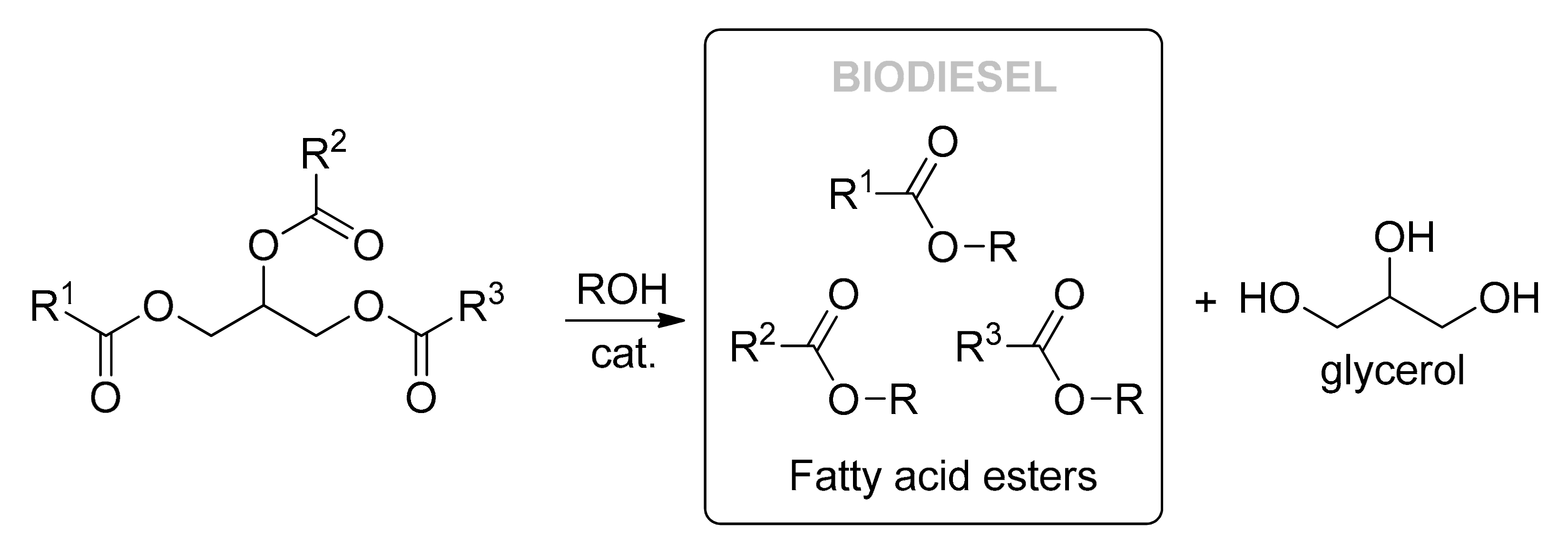
2.2. Synthesis of BD
2.3. Chemical Composition of BD
3. Cold-Flow Properties of BD
3.1. Cold-Flow Properties: Standards and Analysis
3.2. Cold Flow Properties of BDs: FA Composition
3.3. Microimpurities
4. Polymer Additives for BD
4.1. Polyolefins
4.2. Ethylene/Vinyl Acetate Copolymers
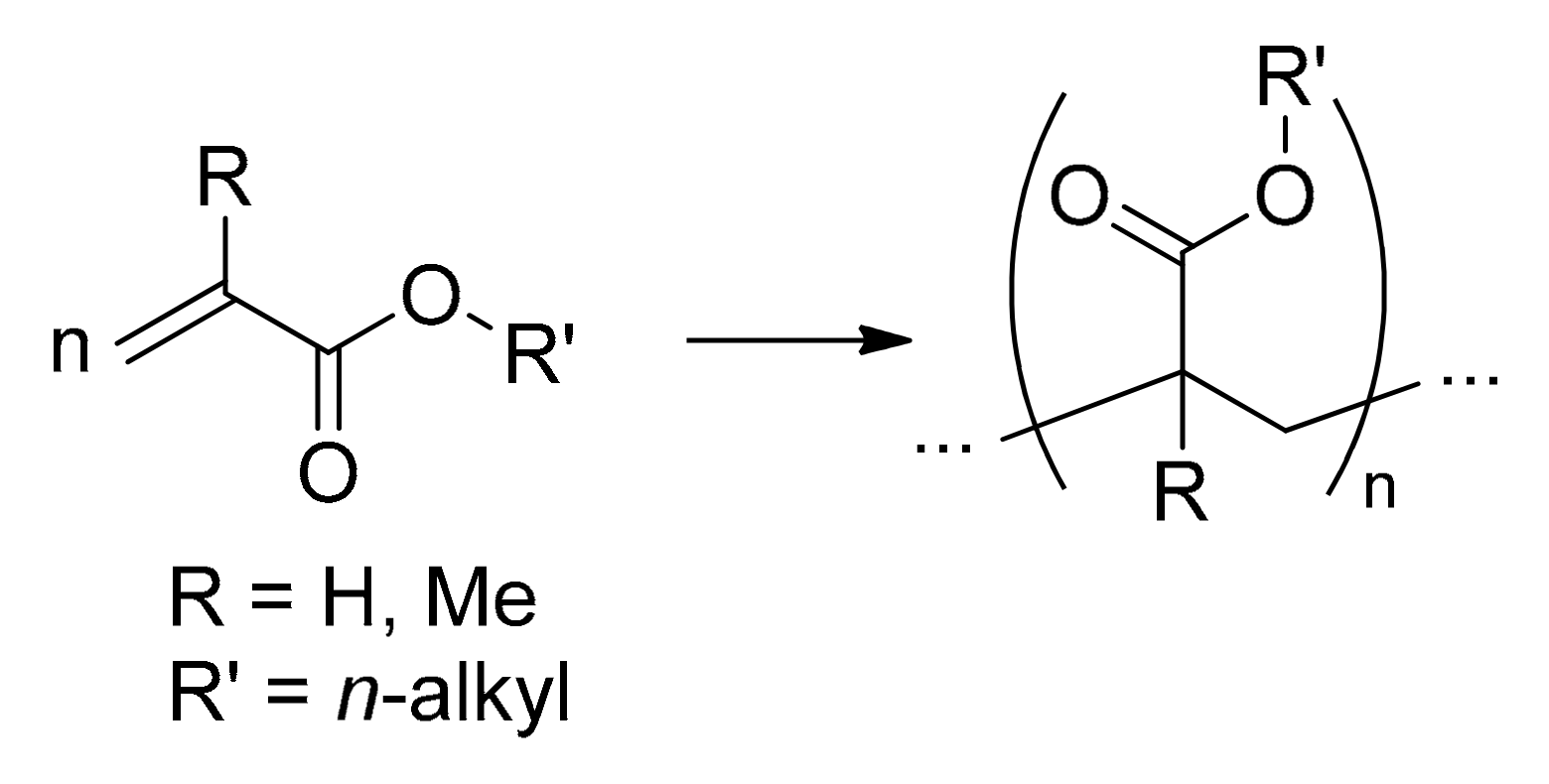
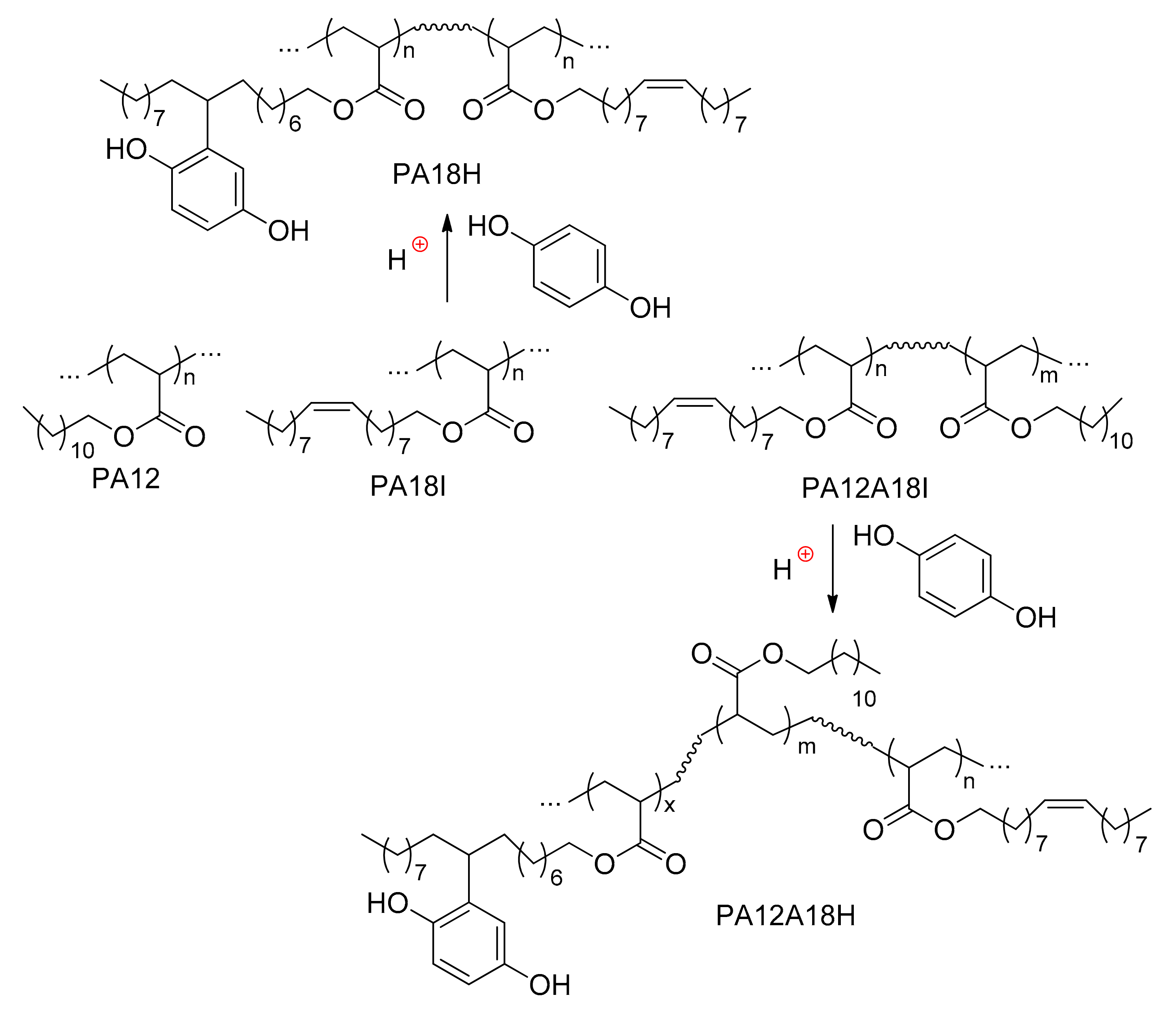
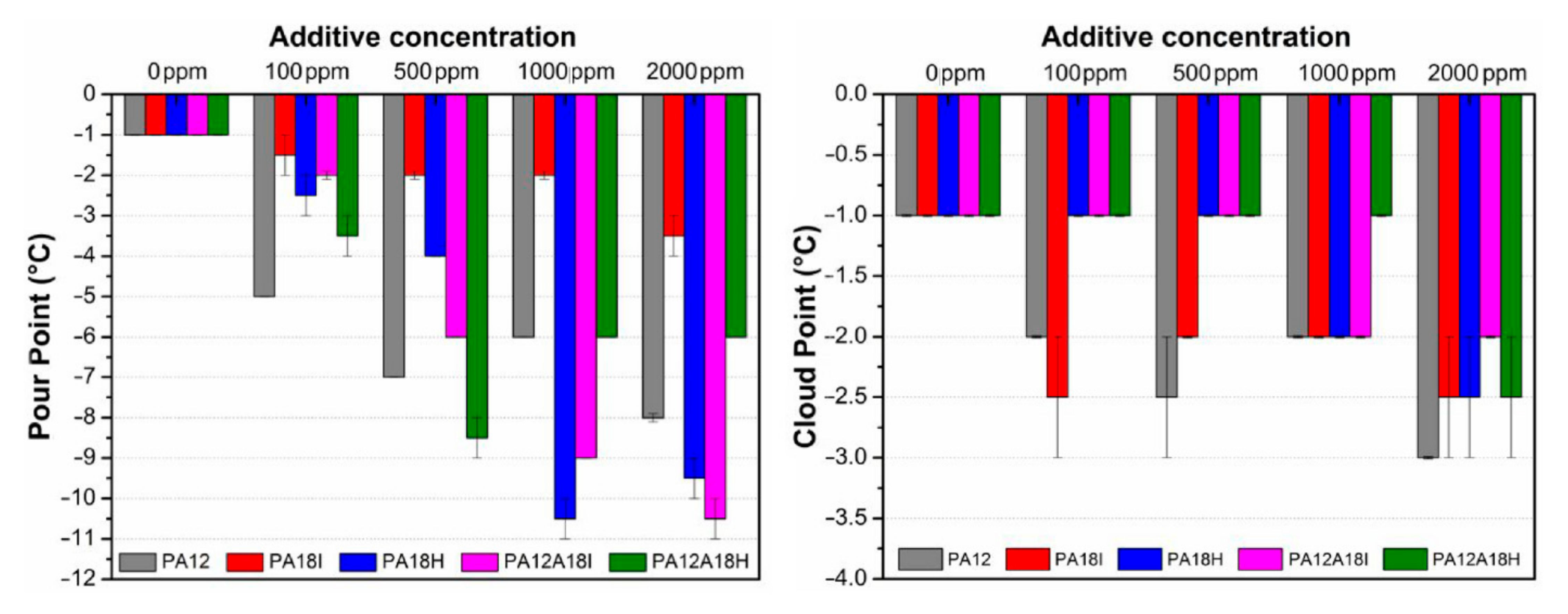
4.3. Polyacrylates and Related Copolymers
4.4. Maleic Anhydride Copolymers and Their Derivatives
4.5. Other Polymeric CFIs
5. Polymer Additives for BD/Petroleum Diesel Blends
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shylesh, S.; Gokhale, A.A.; Ho, C.R.; Bell, A.T. Novel strategies for the production of fuels, lubricants, and chemicals from biomass. Acc. Chem. Res. 2017, 50, 2589–2597. [Google Scholar] [CrossRef] [Green Version]
- Biofuels Dashboard 2020. Available online: https://www.ifpenergiesnouvelles.com/article/biofuels-dashboard-2020 (accessed on 15 April 2021).
- ExxonMobil. The Outlook for Energy: A View to 2040. Available online: https://corporate.exxonmobil.com/Energy-and-innovation/outlook-for-energy (accessed on 15 April 2021).
- Higher Blending Mandates in Key Markets Support Global Biodiesel and HVO Output in 2020. Available online: https://www.iea.org/reports/renewables-2020/transport-biofuels (accessed on 15 April 2021).
- Dwivedi, G.; Sharma, M.P. Impact of cold flow properties of biodiesel on engine performance. Renew. Sustain. Energy Rev. 2014, 31, 650–656. [Google Scholar] [CrossRef]
- Dwivedi, G.; Sharma, M.P. Cold flow behavior of biodiesel-A review. Int. J. Renew. Energy Resour. 2013, 3, 827–836. [Google Scholar]
- Demirbas, A. Biodiesel fuels from vegetable oils via catalytic and non-catalytic supercritical alcohol transesterifications and other methods: A survey. Energy Convers. Manag. 2003, 44, 2093–2109. [Google Scholar] [CrossRef]
- Gebremariam, S.N.; Marchetti, J.M. Economics of biodiesel production: Review. Energy Convers. Manag. 2018, 168, 74–84. [Google Scholar] [CrossRef]
- Basha, S.A.; Gopal, K.R. A review of the effects of catalyst and additive on biodiesel production, performance, combustion and emission characteristics. Renew. Sustain. Energy Rev. 2012, 16, 711–717. [Google Scholar] [CrossRef]
- Kannan, G.R.; Anand, R. Biodiesel as an alternative fuel for direct injection diesel engines: A review. J. Renew. Sustain. Energy 2012, 4, 012703. [Google Scholar] [CrossRef]
- Bateni, H.; Saraeian, A.; Able, C. A comprehensive review on biodiesel purification and upgrading. Biofuel Res. J. 2017, 4, 668–690. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Sharma, M.P. Selection of potential oils for biodiesel production. Renew. Sustain. Chem. Rev. 2016, 56, 1129–1138. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Knothe, G.; Razon, L.F. Biodiesel fuels. Progr. Energy Combust. Sci. 2017, 58, 36–59. [Google Scholar] [CrossRef]
- Lanjekar, R.D.; Deshmukh, D. A review of the effect of the composition of biodiesel on NOx emission, oxidative stability and cold flow properties. Renew. Sustain. Energy Rev. 2016, 54, 1401–1411. [Google Scholar] [CrossRef]
- Joshi, G.; Pandey, J.K.; Rana, S.; Rawat, D.S. Challenges and opportunities for the application of biofuel. Renew. Sustain. Energy Rev. 2017, 79, 850–866. [Google Scholar] [CrossRef]
- Rodionova, M.V.; Poudyal, R.S.; Tiwari, I.; Voloshin, R.A.; Zharmukhamedov, S.K.; Nam, H.G.; Zayadan, B.K.; Bruce, B.D.; Hou, H.J.M.; Allakhverdiev, S.I. Biofuel production: Challenges and opportunities. Int. J. Hydrog. Energy 2017, 42, 8450–8461. [Google Scholar] [CrossRef]
- Azadi, P.; Malina, R.; Barrett, S.R.H.; Kraft, M. The evolution of the biofuel science. Renew. Sustain. Energy Rev. 2017, 76, 1479–1484. [Google Scholar] [CrossRef]
- Gaurav, N.; Sivasankari, S.; Kiran, G.S.; Ninawe, A.; Selvin, J. Utilization of bioresources for sustainable biofuels: A Review. Renew. Sustain. Energy Rev. 2017, 73, 205–214. [Google Scholar] [CrossRef]
- Acheampong, M.; Ertem, F.C.; Kappler, B.; Neubauer, P. In pursuit of Sustainable Development Goal (SDG) number 7: Will biofuels be reliable? Renew. Sustain. Energy Rev. 2017, 75, 927–937. [Google Scholar] [CrossRef]
- Mahmudul, H.M.; Hagos, F.Y.; Mamat, R.; Adam, A.A.; Ishak, W.F.W.; Alenezi, R. Production, characterization and performance of biodiesel as an alternative fuel in diesel engines–A review. Renew. Sustain. Energy Rev. 2017, 72, 497–509. [Google Scholar] [CrossRef]
- Hajjari, M.; Tabatabaei, M.; Aghbashlo, M.; Ghanavati, H. A review on the prospects of sustainable biodiesel production: A global scenario with an emphasis on waste-oil biodiesel utilization. Renew. Sustain. Energy Rev. 2017, 72, 445–464. [Google Scholar] [CrossRef]
- Mardhiah, H.H.; Ong, H.C.; Masjuki, H.H.; Lim, S.; Lee, H.V. A review on latest developments and future prospects of heterogeneous catalyst in biodiesel production from non-edible oils. Renew. Sustain. Energy Rev. 2017, 67, 1225–1236. [Google Scholar] [CrossRef]
- Gopinath, A.; Sairam, K.; Velraj, R.; Kumaresan, G. Effects of the properties and the structural configurations of fatty acid methyl esters on the properties of biodiesel fuel: A review. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2014, 229, 357–390. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, M.P. Assessment of potential of oils for biodiesel production. Renew. Sustain. Energy Rev. 2015, 44, 814–823. [Google Scholar] [CrossRef]
- Sorate, K.A.; Bhale, P.V. Biodiesel properties and automotive system compatibility issues. Renew. Sustain. Energy Rev. 2015, 41, 777–798. [Google Scholar] [CrossRef]
- Sakthivel, R.; Ramesh, K.; Purnachandran, R.; Shameer, P.M. A review on the properties, performance and emission aspects of the third generation biodiesels. Renew. Sustain. Energy Rev. 2018, 82, 2970–2992. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Kumari, D. Chemical compositions, properties, and standards for different generation biodiesels: A review. Fuel 2019, 253, 60–71. [Google Scholar] [CrossRef]
- Wu, G.; Ge, J.C.; Choi, N.J. A comprehensive review of the application characteristics of biodiesel blends in diesel engines. Appl. Sci. 2020, 10, 8015. [Google Scholar] [CrossRef]
- Ge, J.C.; Yoon, S.K.; Choi, N.J. Using canola oil biodiesel as an alternative fuel in diesel engines: A review. Appl. Sci. 2017, 7, 881. [Google Scholar] [CrossRef]
- Dunn, R.O.; Shockley, M.W.; Bagby, M.O. Improving the low-temperature properties of alternative diesel fuels: Vegetable oil-derived methyl esters. J. Am. Oil Chem. Soc. 1996, 73, 1719–1728. [Google Scholar] [CrossRef]
- Smith, P.C.; Ngothai, Y.; Nguyen, Q.D.; O’Neill, B.K. Improving the low-temperature properties of biodiesel: Methods and consequences. Renew. Energy 2010, 35, 1145–1151. [Google Scholar] [CrossRef]
- Dunn, R.O. Effects of minor constituents on cold flow properties and performance of biodiesel. Progr. Energy Combust. Sci. 2009, 35, 481–489. [Google Scholar] [CrossRef]
- Misra, R.D.; Murthy, M.S. Blending of additives with biodiesels to improve the cold flow properties, combustion and emission performance in a compression ignition engine–A review. Renew. Sustain. Energy Rev. 2011, 15, 2413–2422. [Google Scholar] [CrossRef]
- Ali, O.M.; Mamat, R.; Faizal, C.K.M. Review of the effects of additives on biodiesel properties, performance, and emission features. J. Renew. Sustain. Energy 2013, 5, 012701. [Google Scholar] [CrossRef]
- Monirul, I.M.; Masjuki, H.H.; Kalam, M.A.; Zulkifli, N.W.M.; Rashedul, H.K.; Rashed, M.M.; Imdadula, H.K.; Mosarof, M.H. A comprehensive review on biodiesel cold flow properties and oxidation stability along with their improvement processes. RSC Adv. 2015, 5, 86631–86655. [Google Scholar] [CrossRef]
- Liu, G. Development of low-temperature properties on biodiesel fuel: A review. Int. J. Energy Res. 2015, 39, 1295–1310. [Google Scholar] [CrossRef]
- Sierra-Cantora, J.F.; Guerrero-Fajardo, C.A. Methods for improving the cold flow properties of biodiesel with high saturated fatty acids content: A review. Renew. Sustain. Energy Rev. 2017, 72, 774–790. [Google Scholar] [CrossRef]
- Jing, G.; Yu, H.; Sun, Z.; Zhen, Z. Research progress on biodiesel pour point depressant: A mini-review. Pet. Chem. 2019, 59, 1023–1027. [Google Scholar] [CrossRef]
- Leng, L.; Li, W.; Li, H.; Jiang, S.; Zhou, W. Cold flow properties of biodiesel and the improvement methods: A review. Energy Fuels 2020, 34, 10364–10383. [Google Scholar] [CrossRef]
- Hazrat, M.A.; Rasul, M.G.; Mofijur, M.; Khan, M.M.K.; Djavanroodi, F.; Azad, A.K.; Bhuiya, M.M.K.; Silitonga, A.S. A mini review on the cold flow properties of biodiesel and its blends. Front. Energy Res. 2020, 8, 598651. [Google Scholar] [CrossRef]
- Sia, C.B.; Kansedo, J.; Tan, Y.H.; Lee, K.T. Evaluation on biodiesel cold flow properties, oxidative stability and enhancement strategies: A review. Biocatal. Agric. Biotechnol. 2020, 24, 101514. [Google Scholar] [CrossRef]
- Verma, P.; Sharma, M.P.; Dwivedi, G. Evaluation and enhancement of cold flow properties of palm oil and its biodiesel. Energy Rep. 2016, 2, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Giraldo, S.Y.; Rios, L.A.; Suárez, N. Comparison of glycerol ketals, glycerol acetates and branched alcohol-derived fatty esters as cold-flow improvers for palm biodiesel. Fuel 2013, 108, 709–714. [Google Scholar] [CrossRef]
- Soriano, N.U., Jr.; Migo, V.P.; Matsumura, M. Ozonized vegetable oil as pour point depressant for neat biodiesel. Fuel 2006, 85, 25–31. [Google Scholar] [CrossRef]
- Elias, R.C.; Senra, M.; Soh, L. Cold flow properties of fatty acid methyl ester blends with and without triacetin. Energy Fuels 2016, 30, 7400–7409. [Google Scholar] [CrossRef]
- Leggieri, P.A.; Senra, M.; Soh, L. Cloud point and crystallization in fatty acid ethyl ester biodiesel mixtures with and without additives. Fuel 2018, 222, 243–249. [Google Scholar] [CrossRef]
- De Torres, M.; Jiménez-Osés, G.; Mayoral, J.A.; Pires, E. Fatty acid derivatives and their use as CFPP additives in biodiesel. Bioresour. Technol. 2011, 102, 2590–2594. [Google Scholar] [CrossRef]
- Dunn, R.O.; Wyatt, V.T.; Wagner, K.; Ngo, H.; Hums, M.E. The effect of branched-chain fatty acid alkyl esters on the cold-flow properties of biodiesel. J. Am. Oil Chem. Soc. 2019, 96, 805–823. [Google Scholar] [CrossRef]
- Sugami, Y.; Yoshidomi, S.; Minami, E.; Shisa, N.; Hayashi, H.; Saka, S. The effect of monoglyceride polymorphism on cold-flow properties of biodiesel model fuel. J. Am. Oil Chem. Soc. 2017, 94, 1095–1100. [Google Scholar] [CrossRef] [Green Version]
- Madihalli, C.; Sudhakar, H.; Doble, M. Mannosylerythritol lipid-a as a pour point depressant for enhancing the low-temperature fluidity of biodiesel and hydrocarbon fuels. Energy Fuels 2016, 30, 4118–4125. [Google Scholar] [CrossRef]
- Moser, B.R. Preparation and evaluation of multifunctional branched diesters as fuel property enhancers for biodiesel and petroleum diesel fuels. Energy Fuels 2014, 28, 3262–3270. [Google Scholar] [CrossRef]
- Mohanan, A.; Bouzidi, L.; Narine, S.S. Harnessing the synergies between lipid-based crystallization modifiers and a polymer pour point depressant to improve pour point of biodiesel. Energy 2017, 120, 895–906. [Google Scholar] [CrossRef]
- Bhale, P.V.; Deshpande, N.V.; Thombre, S.B. Improving the low temperature properties of biodiesel fuel. Renew. Energy 2009, 34, 794–800. [Google Scholar] [CrossRef]
- Abe, M.; Hirata, S.; Komatsu, H.; Yamagiwa, K.; Tajima, H. Thermodynamic selection of effective additives to improve the cloud point of biodiesel fuels. Fuel 2016, 171, 94–100. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, Y.; Sjoblom, J.; Li, C.; Paso, K.G. Polymeric wax inhibitors and pour point depressants for waxy crude oils: A critical review. J. Dispers. Sci. Technol. 2015, 36, 213–225. [Google Scholar] [CrossRef]
- Li, N.; Mao, G.-L.; Shi, X.-Z.; Tian, S.-W.; Liu, Y. Advances in the research of polymeric pour point depressant for waxy crude oil. J. Disp. Sci. Technol. 2017, 39, 1165–1171. [Google Scholar] [CrossRef]
- Ivchenko, P.V.; Nifant’ev, I.E. Polymer depressor additives: Synthesis, microstructure, efficiency. Polym. Sci. Ser. A 2018, 60, 577–593. [Google Scholar] [CrossRef]
- Knothe, G. A technical evaluation of biodiesel from vegetable oils vs. algae. Will algae-derived biodiesel perform? Green Chem. 2011, 13, 3048–3065. [Google Scholar] [CrossRef]
- Sims, R.E.H.; Mabee, W.; Saddler, J.N.; Taylor, M. An overview of second generation biofuel technologies. Bioresour. Technol. 2010, 101, 1570–1580. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, P.; Subramanian, K.A.; Dastidar, M.G. Production and characterization of biodiesel from algae. Fuel Proc. Technol. 2014, 120, 79–88. [Google Scholar] [CrossRef]
- Viêgas, C.V.; Hachemi, I.; Freitas, S.P.; Mäki-Arvela, P.; Aho, A.; Hemming, J.; Smeds, A.; Heinmaa, I.; Fontes, F.B.; da Silva Pereira, D.C.; et al. A route to produce renewable diesel from algae: Synthesis and characterization of biodiesel via in situ transesterification of Chlorella alga and its catalytic deoxygenation to renewable diesel. Fuel 2015, 155, 144–154. [Google Scholar] [CrossRef]
- Kligerman, D.C.; Bouwer, E.J. Prospects for biodiesel production from algae-based wastewater treatment in Brazil: A review. Renew. Sustain. Energy Rev. 2015, 52, 1834–1846. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. Composition, properties and challenges of algae biomass for biofuel application: An overview. Fuel 2016, 181, 1–33. [Google Scholar] [CrossRef]
- Piloto-Rodríguez, R.; Sánchez-Borroto, Y.; Melo-Espinosa, E.A.; Verhelst, S. Assessment of diesel engine performance when fueled with biodiesel from algae and microalgae: An overview. Renew. Sustain. Energy Rev. 2017, 69, 833–842. [Google Scholar] [CrossRef]
- Islam, M.A.; Heimann, K.; Brown, R.J. Microalgae biodiesel: Current status and future needs for engine performance and emissions. Renew. Sustain. Energy Rev. 2017, 79, 1160–1170. [Google Scholar] [CrossRef] [Green Version]
- Roveda, A.C.; Comin, M.; Caires, A.R.L.; Ferreira, V.S.; Trindade, M.A.G. Thermal stability enhancement of biodiesel induced by a synergistic effect between conventional antioxidants and an alternative additive. Energy 2016, 109, 260–265. [Google Scholar] [CrossRef]
- Sajjadi, B.; Raman, A.A.A.; Arandiyan, H. A comprehensive review on properties of edible and non-edible vegetable oil-based biodiesel: Composition, specifications and prediction models. Renew. Sustain. Energy Rev. 2016, 63, 62–92. [Google Scholar] [CrossRef]
- De Boer, K.; Bahri, P.A. Supercritical methanol for fatty acid methyl ester production: A review. Biomass Bioenergy 2011, 35, 983–991. [Google Scholar] [CrossRef]
- Aransiola, E.F.; Ojumu, T.V.; Oyekola, O.O.; Madzimbamuto, T.F.; Ikhu-Omoregbe, D.I.O. A review of current technology for biodiesel production: State of the art. Biomass Bioenergy 2014, 61, 276–297. [Google Scholar] [CrossRef]
- Saka, S.; Kusdiana, D. Biodiesel fuel from rapeseed oil as prepared in supercritical methanol. Fuel 2001, 80, 225–231. [Google Scholar] [CrossRef]
- Karki, S.; Sanjel, N.; Poudel, J.; Choi, J.H.; Oh, S.C. Supercritical transesterification of waste vegetable oil: Characteristic comparison of ethanol and methanol as solvents. Appl. Sci. 2017, 7, 632. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.F.; Bennett, J.A.; Manayila, J.C.; Wilson, K. Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chem. Soc. Rev. 2014, 43, 7887–7916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.F.; Wilson, K. Recent developments in heterogeneous catalysis for the sustainable production of biodiesel. Catal. Today 2015, 242, 3–18. [Google Scholar] [CrossRef]
- Guldhe, A.; Singh, B.; Mutanda, T.; Permaul, K.; Bux, F. Advances in synthesis of biodiesel via enzyme catalysis: Novel and sustainable approaches. Renew. Sustain. Energy Rev. 2015, 41, 1447–1464. [Google Scholar] [CrossRef]
- Knothe, G. Analyzing biodiesel: Standards and other methods. J. Am. Oil. Chem. Soc. 2006, 83, 823–833. [Google Scholar] [CrossRef]
- Monteiro, M.R.; Ambrozin, A.R.P.; Lião, L.M.; Ferreira, A.G. Critical review on analytical methods for biodiesel characterization. Talanta 2008, 77, 593–605. [Google Scholar] [CrossRef]
- Nimal Ratnayake, W.M.; Hansen, S.L.; Kennedy, M.P. Evaluation of the CP-Sil 88 and SP-2560 GC columns used in the recently approved AOCS official method Ce 1h-05: Determination of cis-, trans-, saturated, monounsaturated, and polyunsaturated fatty acids in vegetable or non-ruminant animal oils and fats by capillary GLC method. J. Am. Oil Chem. Soc. 2006, 83, 475–488. [Google Scholar] [CrossRef]
- Pauls, R.E. A review of chromatographic characterization techniques for biodiesel and biodiesel blends. J. Chrom. Sci. 2011, 49, 384–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tariq, M.; Ali, S.; Khali, N. Activity of homogeneous and heterogeneous catalysts, spectroscopic and chromatographic characterization of biodiesel: A review. Renew. Sustain. Energy Rev. 2012, 16, 6303–6316. [Google Scholar] [CrossRef]
- Flores, I.S.; Godinho, M.S.; de Oliveira, A.E.; Alcantara, G.B.; Monteiro, M.R.; Menezes, S.M.C.; Lião, L.M. Discrimination of biodiesel blends with 1H NMR spectroscopy and principal component analyses. Fuel 2012, 99, 40–44. [Google Scholar] [CrossRef]
- ASTM Updates Biodiesel Specifications for Measuring Cloud Point. Biodiesel Magasine. Available online: http://biodieselmagazine.com/articles/3420/astm-updates-biodiesel-specifications-for-measuring-cloud-point/ (accessed on 9 May 2021).
- Santos, N.A.; Rosenhaim, R.; Dantas, M.B.; Bicudo, T.C.; Cavalcanti, E.H.S.; Barro, A.K.; Santos, I.M.G.; Souza, A.G. Rheology and MT-DSC studies of the flow properties of ethyl and methyl babassu biodiesel and blends. J. Therm. Anal. Calorim. 2011, 106, 501–506. [Google Scholar] [CrossRef]
- Dunn, R.O. Cold flow properties of biodiesel: A guide to getting an accurate analysis. Biofuels 2015, 6, 115–128. [Google Scholar] [CrossRef]
- Chupka, G.M.; Fouts, L.; Lennon, J.A.; Alleman, T.L.; Daniels, D.A.; McCormick, R.L. Saturated monoglyceride effects on low-temperature performance of biodiesel blends. Fuel Proc. Technol. 2014, 118, 302–309. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Chen, J. Crystallization behaviors of biodiesel in relation to its rheological properties. Fuel 2016, 171, 178–185. [Google Scholar] [CrossRef]
- Alicke, A.A.; Leopércio, B.C.; Marchesini, F.H.; de Souza Mendes, P.R. Guidelines for the rheological characterization of biodiesel. Fuel 2015, 140, 446–452. [Google Scholar] [CrossRef]
- Knothe, G.; Dunn, R.O. A comprehensive evaluation of the melting points of fatty acids and esters determined by differential scanning calorimetry. J. Am. Oil Chem. Soc. 2009, 86, 843–856. [Google Scholar] [CrossRef]
- Kerschbaum, S.; Rinke, G.; Schubert, K. Winterization of biodiesel by micro process engineering. Fuel 2008, 87, 2590–2597. [Google Scholar] [CrossRef]
- Yuan, M.-H.; Chen, Y.-H.; Chen, J.-H.; Luo, Y.-M. Dependence of cold filter plugging point on saturated fatty acid profile of biodiesel blends derived from different feedstocks. Fuel 2017, 195, 59–68. [Google Scholar] [CrossRef]
- Liu, X.; Gibbs, A.S.; Nichol, G.S.; Tang, C.C.; Knight, K.S.; Dowding, P.J.; Mored, I.; Pulham, C.R. Temperature-induced polymorphism in methyl stearate. CrystEngComm 2018, 20, 6885–6893. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Bull, C.L.; Kleppe, A.K.; Dowding, P.J.; Lewtas, K.; Pulham, C.R. High-pressure crystallisation studies of biodiesel and methyl stearate. CrystEngComm 2019, 21, 4427–4436. [Google Scholar] [CrossRef]
- Prathapa, S.J.; Slabbert, C.; Fernandes, M.A.; Lemmerer, A. Structure determination of fatty acid ester biofuels via in situ cryocrystallisation and single crystal X-ray diffraction. CrystEngComm 2018, 21, 41–52. [Google Scholar] [CrossRef]
- Lacoste, F.; Dejean, F.; Griffon, H.; Rouquette, C. Quantification of free and esterified steryl glucosides in vegetable oils and biodiesel. Eur. J. Lipid Sci. Technol. 2009, 111, 822–828. [Google Scholar] [CrossRef]
- Chupka, G.M.; Yanowitz, J.; Chiu, G.; Alleman, T.L.; McCormick, R.L. Effect of Saturated Monoglyceride Polymorphism on Low-Temperature Performance of Biodiesel. Energy Fuels 2011, 25, 398–405. [Google Scholar] [CrossRef]
- Chiu, C.-W.; Schumacher, L.G.; Suppes, G.J. Impact of cold flow improvers on soybean biodiesel blend. Biomass Bioenergy 2004, 27, 485–491. [Google Scholar] [CrossRef]
- Wang, J.; Cao, L.; Han, S. Effect of polymeric cold flow improvers on flow properties of biodiesel from waste cooking oil. Fuel 2014, 117, 876–881. [Google Scholar] [CrossRef]
- Echim, C.; Maes, J.; De Greyt, W. Improvement of cold filter plugging point of biodiesel from alternative feedstocks. Fuel 2012, 93, 642–648. [Google Scholar] [CrossRef]
- Chastek, T.Q. Improving cold flow properties of canola-based biodiesel. Biomass Bioenergy 2011, 35, 600–607. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, Z.; Yin, C. The interaction of waxes with pour point depressants. Fuel 2010, 89, 1127–1132. [Google Scholar] [CrossRef]
- Xue, Y.; Zhao, Z.; Xu, G.; Lian, X.; Yang, C.; Zhao, W.; Ma, P.; Lin, H.; Han, S. Effect of poly-alpha-olefin pour point depressant on cold flow properties of waste cooking oil biodiesel blends. Fuel 2016, 184, 110–117. [Google Scholar] [CrossRef]
- Cao, L.; Wang, J.; Liu, C.; Chen, Y.; Liu, K.; Han, S. Ethylene vinyl acetate copolymer: A bio-based cold flow improver for waste cooking oil derived biodiesel blends. Appl. Energy 2014, 132, 163–167. [Google Scholar] [CrossRef]
- Zhou, M.; He, Y.; Lin, H.; Han, S. Effect of MC–MA polymer pour point depressants on the flow properties of biodiesel. Energy Sources Part A Rec. Util. Environ. Eff. 2016, 38, 1962–1968. [Google Scholar] [CrossRef]
- Sern, C.H.; May, C.Y.; Zakaria, Z.; Daik, R.; Foon, C.S. The effect of polymers and surfactants on the pour point of palm oil methyl esters. Eur. J. Lipid Sci. Technol. 2007, 109, 440–444. [Google Scholar] [CrossRef]
- Boshui, C.; Yuqiu, S.; Jianhua, F.; Jiu, W.; Jiang, W. Effect of cold flow improvers on flow properties of soybean biodiesel. Biomass Bioenergy 2010, 34, 1309–1313. [Google Scholar] [CrossRef]
- Muniz, A.S.; Vlnieska, V.; Ferraz, F.A.; dos Santos Oliveira, A.R.; Ramos, L.P.; César-Oliveira, M.A.F. Polymer Additives as Cold Flow Improvers for Palm Oil Methyl Esters. Macromol. Symp. 2019, 383, 1800026. [Google Scholar] [CrossRef]
- Nie, S.; Cao, L. Effect of mixed commercial cold flow improvers on flow properties of biodiesel from waste cooking oil. Processes 2020, 8, 1094. [Google Scholar] [CrossRef]
- Xue, Y.; Yin, S.; Jin, D.; Zhu, X.; Yang, T.; Yuan, M.; Li, X.; Lin, H.; Han, S. Nitrogen-Containing Ternary Polymer Biodiesel Pour Point Depressant, Preparation Method and Application Thereof. Patent CN111826222, 27 October 2020. [Google Scholar]
- Jawak, D.A.; Mitra, P.; Kariparambil, S.K. A Pour Point Depressant Polymer Composition. Patent Application WO2009/047786, 16 April 2009. [Google Scholar]
- Lin, H.; Yuan, M.; Yin, S.; Li, X.; Su, B.; Yang, T.; Wu, J.; Xue, Y.; Han, S. Biodiesel Pour Point Depressant Composition as Well as Preparation and Application Thereof. Patent CN111676072, 19 September 2020. [Google Scholar]
- Yadav, K.; Kumar, N.; Chaudhary, R. A review on cold flow properties of biodiesel and their improvement. IOP Conf. Ser. Mater. Sci. Eng. 2020, 804, 012027. [Google Scholar] [CrossRef]
- Leube, W.; Monkenbusch, M.; Schneiders, D.; Richter, D.; Adamson, D.; Fetters, L.; Dounis, P.; Lovegrove, R. Wax-crystal modification for fuel oils by self-aggregating partially crystallizable hydrocarbon block copolymers. Energy Fuels 2000, 14, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Monkenbusch, M.; Schneiders, D.; Richter, D.; Willner, L.; Leube, W.; Fetters, L.J.; Huang, J.S.; Lin, M. Aggregation behaviour of PE-PEP copolymers and the winterization of diesel fuel. Phys. B Condens. Matter 2000, 276–278, 941–943. [Google Scholar] [CrossRef]
- Ashbaugh, H.S.; Fetters, L.J.; Adamson, D.H.; Prud’homme, R.K. Flow improvement of waxy oils mediated by self-aggregating partially crystallizable diblock copolymers. J. Rheol. 2002, 46, 763–776. [Google Scholar] [CrossRef]
- Schwahn, D.; Richter, D.; Wright, P.J.; Symon, C.; Fetters, L.J.; Lin, M. Self-assembling behavior in decane solution of potential wax crystal nucleators based on poly(co-olefins). Macromolecules 2002, 35, 861–870. [Google Scholar] [CrossRef] [Green Version]
- Schwahn, D.; Richter, D.; Lin, M.; Fetters, L.J. Cocrystallization of a poly(ethylene-butene) random copolymer with C24 in n-decane. Macromolecules 2002, 35, 3762–3768. [Google Scholar] [CrossRef]
- Guo, X.; Pethica, B.A.; Huang, J.S.; Prud’homme, R.K.; Adamson, D.H.; Fetters, L.J. Crystallization of mixed paraffin from model waxy oils and the influence of micro-crystalline poly(ethylene-butene) random copolymers. Energy Fuels 2004, 18, 930–937. [Google Scholar] [CrossRef]
- Guo, X.; Pethica, B.A.; Huang, J.S.; Adamson, D.H.; Prud’homme, R.K. Effect of cooling rate on crystallization of model waxy oils with microcrystalline poly(ethylene butene). Energy Fuels 2006, 20, 250–256. [Google Scholar] [CrossRef]
- Tinsley, J.F.; Prud’homme, R.K.; Guo, X.; Adamson, D.H.; Callahan, S.; Amin, D.; Shao, S.; Kriegel, R.M.; Saini, R. Novel laboratory cell for fundamental studies of the effect of polymer additives on wax deposition from model crude oils. Energy Fuels 2007, 21, 1301–1308. [Google Scholar] [CrossRef]
- Ilnycky, S.J.; Rupar, C.B. Ethylene-Vinyl Ester Pour Depressant for Middle Distillates. U.S. Patent 3048479, 7 August 1962. [Google Scholar]
- Chen, J.C. Cold Flow Improver. U.S. Patent 4512775, 23 April 1985. [Google Scholar]
- Botros, M.G. Ethylene Vinyl Acetate and Isobutylene Terpolymer as a Cold Flow Improver for Distillate Fuel Compositions. U.S. Patent 5681359, 28 October 1997. [Google Scholar]
- Machado, A.L.C.; Lucas, E.F.; Gonzalez, G. Poly(ethylene-co-vinyl acetate) (EVA) as wax inhibitor of a Brazilian crude oil: Oil viscosity, pour point and phase behavior of organic solutions. J. Petrol. Sci. Eng. 2001, 32, 159–165. [Google Scholar] [CrossRef]
- Jafari Ansaroudi, H.R.; Vafaie-Sefti, M.; Masoudi, S.; Jafari Behbahani, T.; Jafari, H. Study of the morphology of wax crystals in the presence of ethylene-co-vinyl acetate copolymer. Pet. Sci. Technol. 2013, 31, 643–651. [Google Scholar] [CrossRef]
- Mähling, F.-O.; Sondjaja, R.; Hess, B.; Couet, J.; Thong, D. Cold Flow Improver with Broad Applicability in Mineral Diesel, Biodiesel and Blends. U.S. Patent Application 2015344801, 3 December 2015. [Google Scholar]
- Hamada, H.; Kato, H.; Ito, N.; Takase, Y.; Nanbu, H.; Mishima, S.; Sakaki, H.; Sato, K. Effects of polyglycerol esters of fatty acids and ethylene-vinyl acetate co-polymer on crystallization behavior of biodiesel. Eur. J. Lipid Sci. Technol. 2010, 112, 1323–1330. [Google Scholar] [CrossRef]
- Bartl, H.; Hardt, D. Graft Polymerization of Vinyl Compounds on Ethylene-Vinyl Acetate Copolymers. In Advances in Chemistry; Platzer, N., Ed.; American Chemical Society: Washington, DC, USA, 1969; Volume 91, pp. 477–488. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Su, B.-Y. Substantial modifications of ethylene/vinyl acetate copolymers: Saponification and graft copolymerization. Angew. Makromol. Chem. 1993, 212, 77–91. [Google Scholar] [CrossRef]
- Ren, Y.; Fang, L.; Chen, Z.; Du, H.; Zhang, X. Synthesis and evaluation of grafted EVAL as pour point depressant for waxy crude oil. Ind. Eng. Chem. Res. 2018, 57, 8612–8619. [Google Scholar] [CrossRef]
- Sweeney, W.M. Polyacrylates and Waxy Residual Fuel Compositions Thereof. U.S. Patent US3904385, 9 September 1975. [Google Scholar]
- Soldi, R.A.; Oliveira, A.R.S.; Barbosa, R.V.; Cesar-Oliveira, M.A.F. Polymethacrylates: Pour point depressants in diesel oil. Eur. Polym. J. 2007, 43, 3671–3678. [Google Scholar] [CrossRef]
- Ma, P.; Xue, Y.; Zhao, W.; Lan, G.; Hang, Z.; Liu, F.; Han, S. Study on the performance mechanism of methacrylate pour point depressant in soybean biodiesel blends. RSC Adv. 2015, 5, 90144–90149. [Google Scholar] [CrossRef]
- Xue, Y.; Yang, C.; Xu, G.; Zhao, Z.; Lian, X.; Sheng, H. The influence of polymethyl acrylate as a pour point depressant for biodiesel. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 17–22. [Google Scholar] [CrossRef]
- Roy, M.M.; Calder, J.; Wang, W.; Mangad, A.; Diniz, F.C.M. Emission analysis of a modern Tier 4 DI diesel engine fueled by biodiesel-diesel blends with a cold flow improver (Wintron Synergy) at multiple idling conditions. Appl. Energy 2016, 179, 45–54. [Google Scholar] [CrossRef]
- Martyak, N.M.; Masy, N.E.; Gernon, M.D.; Schmidt, S.C.; Dowling, C.M. Acrylic Polymer Low Temperature Flow Modifiers in Bio-Derived Fuels. U.S. Patent US8236069, 7 August 2012. [Google Scholar]
- Muniz, A.S.; Inoue, M.H.; Oliveira, D.C.; Oliveira, A.R.S.; Ramos, L.P.; Mittelbach, M.; César-Oliveira, M.A.F. Bifunctional additives to improve the cold flow properties and oxidation stability of soybean oil biodiesel. Energy Fuels 2020, 34, 5907–5916. [Google Scholar] [CrossRef]
- Lutz, J.-F. Sequence-controlled polymerizations: The next Holy Grail in polymer science? Polym. Chem. 2010, 1, 55–62. [Google Scholar] [CrossRef]
- Lappalainen, E.; Koskimies, S. Preparation of alternating olefine maleic anhydride copolymers from C4-C6 hydrocarbon mixtures formed in petrochemical cracking units. J. Polym. Sci. Part C Polym. Lett. 1986, 24, 17–23. [Google Scholar] [CrossRef]
- Davis, F.; Hodge, P.; Towns, C.R.; Ali-Adib, Z. Langmuir and Langmuir-Blodgett films of derivatives of alternating copolymers of straight-chain α-olefins and maleic anhydride. Macromolecules 1991, 24, 5695–5703. [Google Scholar] [CrossRef]
- Martínez, F.; Uribe, E.; Olea, A.F. Copolymerization of maleic anhydride with styrene and α-olefins. Molecular and thermal characterization. J. Macromol. Sci. Part A Pure Appl. Chem. 2005, 42, 1063–1072. [Google Scholar] [CrossRef]
- Davies, M.C.; Dawkins, J.V.; Hourston, D.J.; Meehan, E. Molar mass determination of poly(octadecene-alt-maleic anhydride) copolymers by size exclusion chromatography and dilute solution viscometry. Polymer 2002, 43, 4311–4314. [Google Scholar] [CrossRef]
- Li, L.; Xu, J.; Tinsley, J.; Adamson, D.H.; Pethica, B.A.; Huang, J.S.; Prud’homme, R.K.; Guo, X. Improvement of oil flowability by assembly of comb-type copolymers with paraffin and asphaltene. AIChE J. 2012, 58, 2254–2261. [Google Scholar] [CrossRef]
- Mazi, H.; Kibarer, G.; Emregul, E.; Rzaev, Z.M.O. Bioengineering Functional Copolymers. IX. Poly[(maleic anhydride-co-hexene-1)-g-poly(ethylene oxide)]. Macromol. Biosci. 2006, 6, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Nifant’ev, I.; Vinogradov, A.; Vinogradov, A.; Ivchenko, P. DFT modeling of the alternating radical copolymerization and alder-ene reaction between maleic anhydride and olefins. Polymers 2020, 12, 744. [Google Scholar] [CrossRef] [Green Version]
- Soni, H.P.; Kiranbala, A.K.S.; Nagar, A.; Bharambe, D.P. Designing maleic anhydride-a-olifin copolymeric combs as wax crystal growth nucleators. Fuel Proc. Technol. 2010, 91, 997–1004. [Google Scholar] [CrossRef]
- Ke-Jian, L.; Yuchin, Z. A study of three kinds of alkohols esterified copolymer of maleic anhydride and olefins as pour-point depressant for diesels. Pet. Sci. Technol. 1998, 16, 971–977. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Bondarenko, G.N.; Korchagina, S.A.; Shlyakhtin, A.V.; Roznyatovsky, V.A.; Ivchenko, P.V. Copolymers of maleic anhydride and methylene alkanes: Synthesis, modification, and pour point depressant properties. Polym. Sci. Ser. B 2018, 60, 469–480. [Google Scholar] [CrossRef]
- Usta, N.; Aydoğan, B.; Çon, A.H.; Uğuzdoğan, E.; Özkal, S.G. Properties and quality verification of biodiesel produced from tobacco seed oil. Energy Convers. Manag. 2011, 52, 2031–2039. [Google Scholar] [CrossRef]
- Li, X.H.; Soriano, N.U.; Bode, H. Cold Flow Additive for Middle Distillate Fuels. Patent Application WO2020014189, 16 January 2020. [Google Scholar]
- Tasić, I.; Tomić, M.D.; Aleksić, A.L.J.; Đurišić-Mladenović, N.; Martinović, F.L.; Mićić, R.D. Improvement of low-temperature characteristics of biodiesel by additivation. Hemijska Ind. 2019, 73, 103–114. [Google Scholar] [CrossRef]
- Vinogradov, A.A.; Nifant’ev, I.E.; Vinogradov, A.A.; Borisov, R.S.; Ivchenko, P.V. Precision rheological study of the effectiveness of polymer cold flow improvers for corn oil based biodiesel. Mendeleev Commun. accepted.
- Tesfaye, M.; Katiyar, V. Microwave assisted synthesis of biodiesel from soybean oil: Effect of poly (lactic acid)-oligomer on cold flow properties, IC engine performance and emission characteristics. Fuel 2016, 170, 107–114. [Google Scholar] [CrossRef]
- Abe, M.; Komatsu, H.; Yamagiwa, K.; Tajima, H. Evaluation of the separation of saturated fatty acid methyl esters obtained from additive winterization using a nonionic surfactant. Fuel 2018, 214, 607–613. [Google Scholar] [CrossRef]
- Davies, B.W.; Lewtas, K.; Lombardi, A. Oil Additives and Compositions. U.S. Patent US5743923, 28 April 1998. [Google Scholar]
- Monirul, I.M.; Kalam, M.A.; Masjuki, H.H.; Zulkifli, N.W.M.; Shahir, S.A.; Mosarof, M.H.; Ruhul, A.M. Influence of poly(methyl acrylate) additive on cold flow properties of coconut biodiesel blends and exhaust gas emissions. Renew. Energy 2017, 101, 702–712. [Google Scholar] [CrossRef]
- Monirul, I.M.; Masjuki, H.H.; Kalam, M.A.; Zulkifli, N.W.M.; Shancita, I. Influence of polymethyl acrylate additive on the formation of particulate matter and NOX emission of a biodiesel–diesel-fueled engine. Environ. Sci. Pollut. Res. 2017, 24, 18479–18493. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Hassan, M.H.; Kalam, M.A.; binti Mohd Zulkifli, N.W.; Habibullah, M.; Hossain, M.M. Improvement of cold flow properties of Cocos nucifera and Calophyllum inophyllum biodiesel blends using polymethyl acrylate additive. J. Clean. Product. 2016, 137, 322–329. [Google Scholar] [CrossRef]
- Stohr, T.; Schnabel, J.; Janssen, D.; Muller, M. Motor Fuel Composition Comprising Renewable Raw Materials. U.S. Patent Application US2009064568, 12 March 2009. [Google Scholar]
- Lin, H.; Xie, M.; Yin, S.; Yang, T.; Su, B.; Chen, F.; Han, S.; Xue, Y. Influence of methacrylate-benzyl methacrylate-N-vinyl-2-pyrrolidone as pour point depression on cold flow properties of diesel fuel. Energy Fuels 2020, 34, 1514–1523. [Google Scholar] [CrossRef]
- Su, B.; Wang, L.; Xue, Y.; Yan, J.; Dong, Z.; Lin, H.; Han, S. Effect of pour point depressants combined with dispersants on the cold flow properties of biodiesel-diesel blends. J. Am. Oil Chem. Soc. 2021, 98, 163–172. [Google Scholar] [CrossRef]
- Lai, Y.; Chen, X.; Yuan, Y. Improving the cold flow properties of biodiesel derived from palm. Adv. Mater. Res. 2011, 236-238, 164–168. [Google Scholar] [CrossRef]
- Lv, P.; Cheng, Y.; Yang, L.; Yuan, Z.; Li, H.; Luo, W. Improving the low temperature flow properties of palm oil biodiesel: Addition of cold flow improver. Fuel Proc. Technol. 2013, 110, 61–64. [Google Scholar] [CrossRef]
- Shrestha, D.S.; Van Gerpen, J.; Thompson, J. Effectiveness of cold flow additives on various biodiesels, diesel, and their blends. Trans. ASABE 2008, 51, 1365–1370. [Google Scholar] [CrossRef]





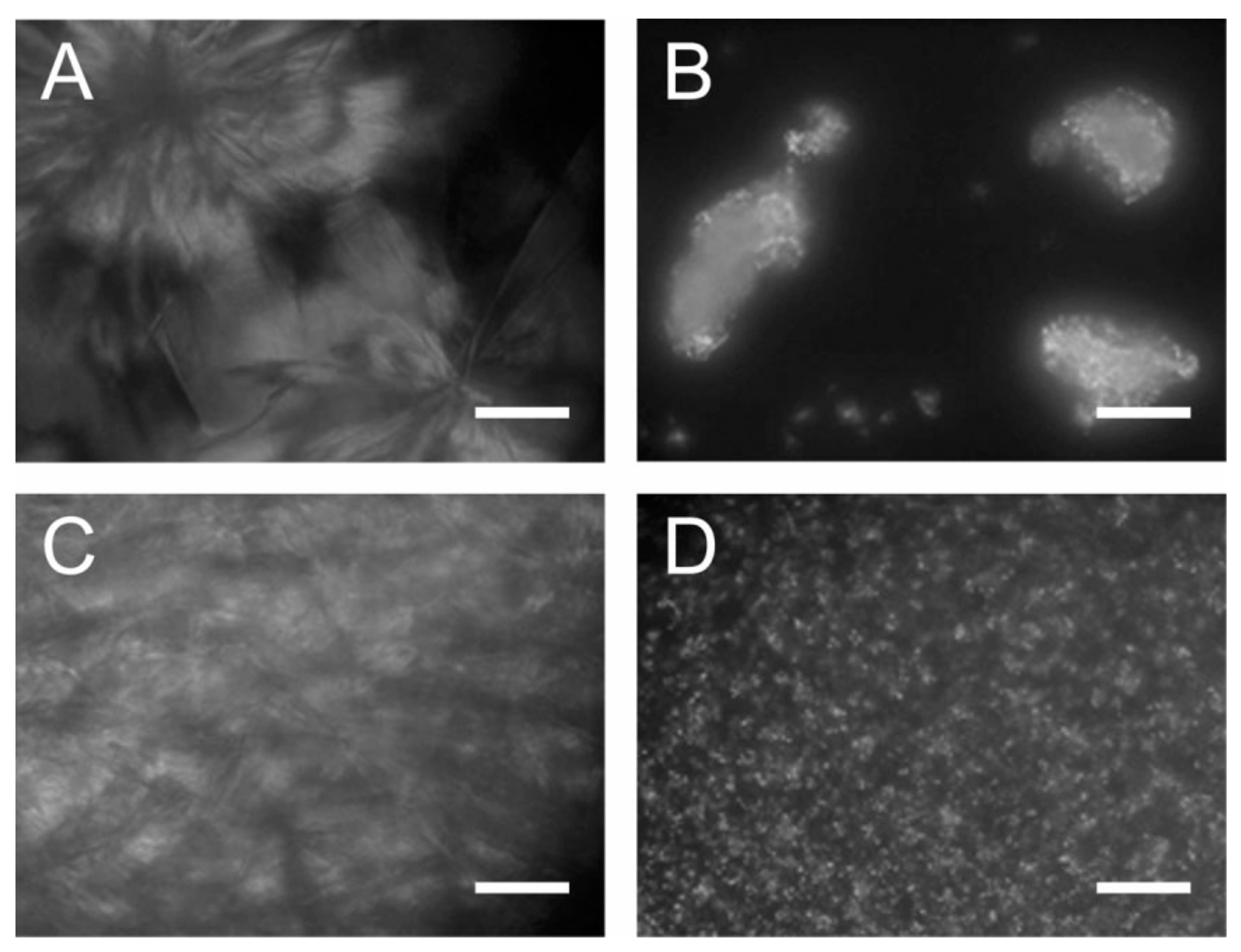




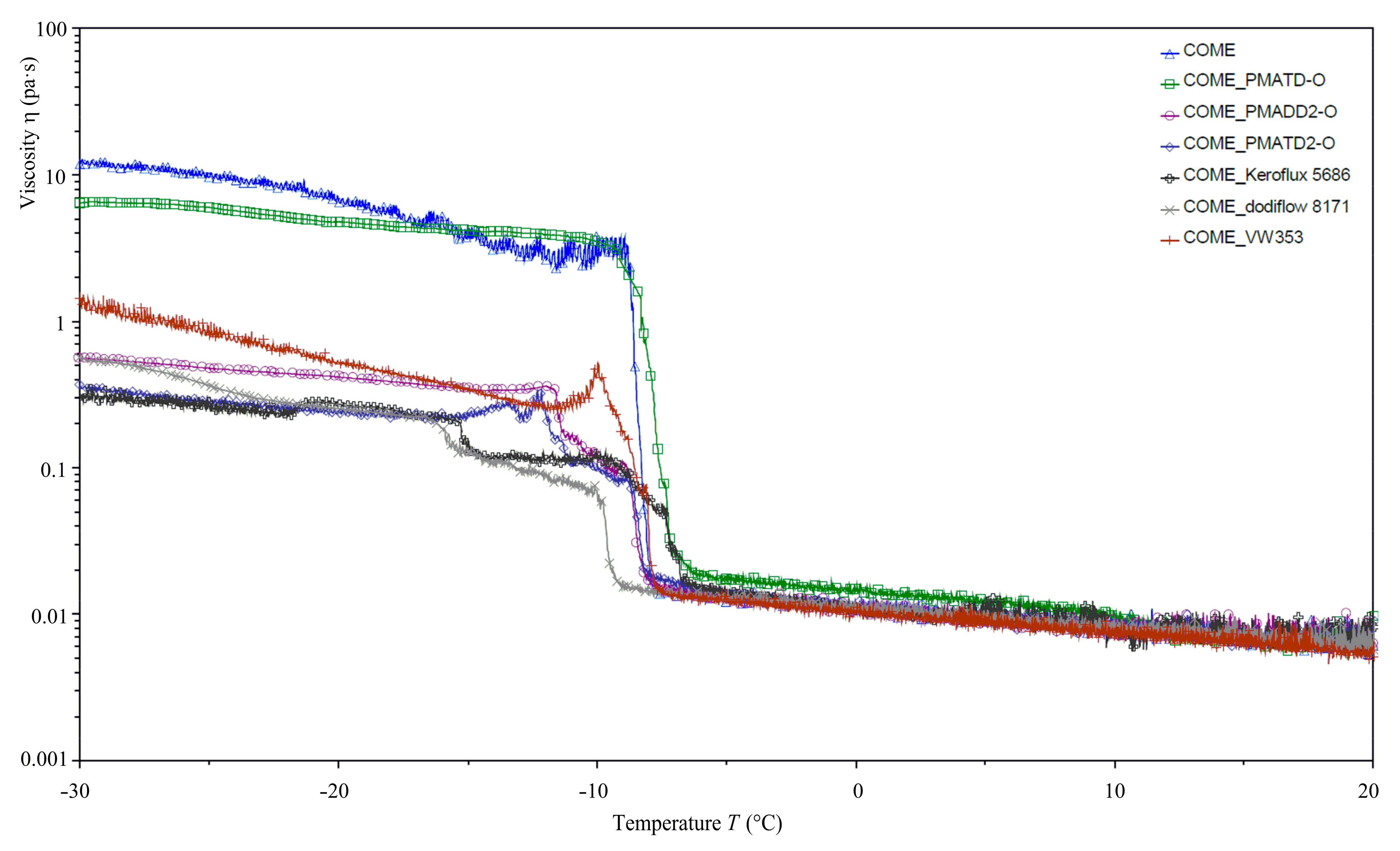

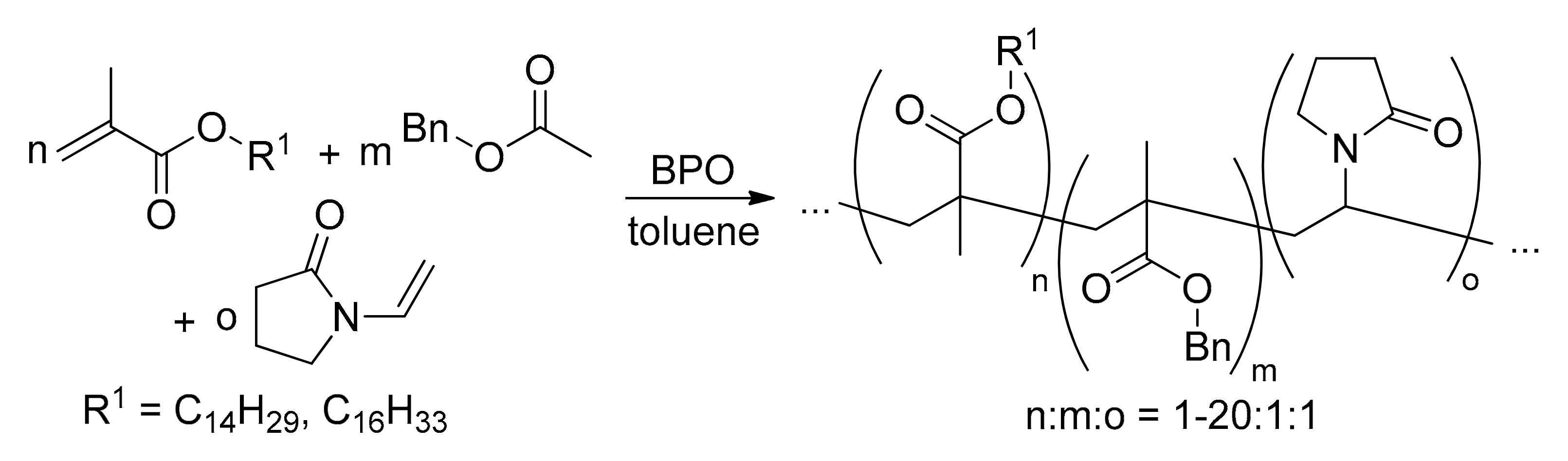
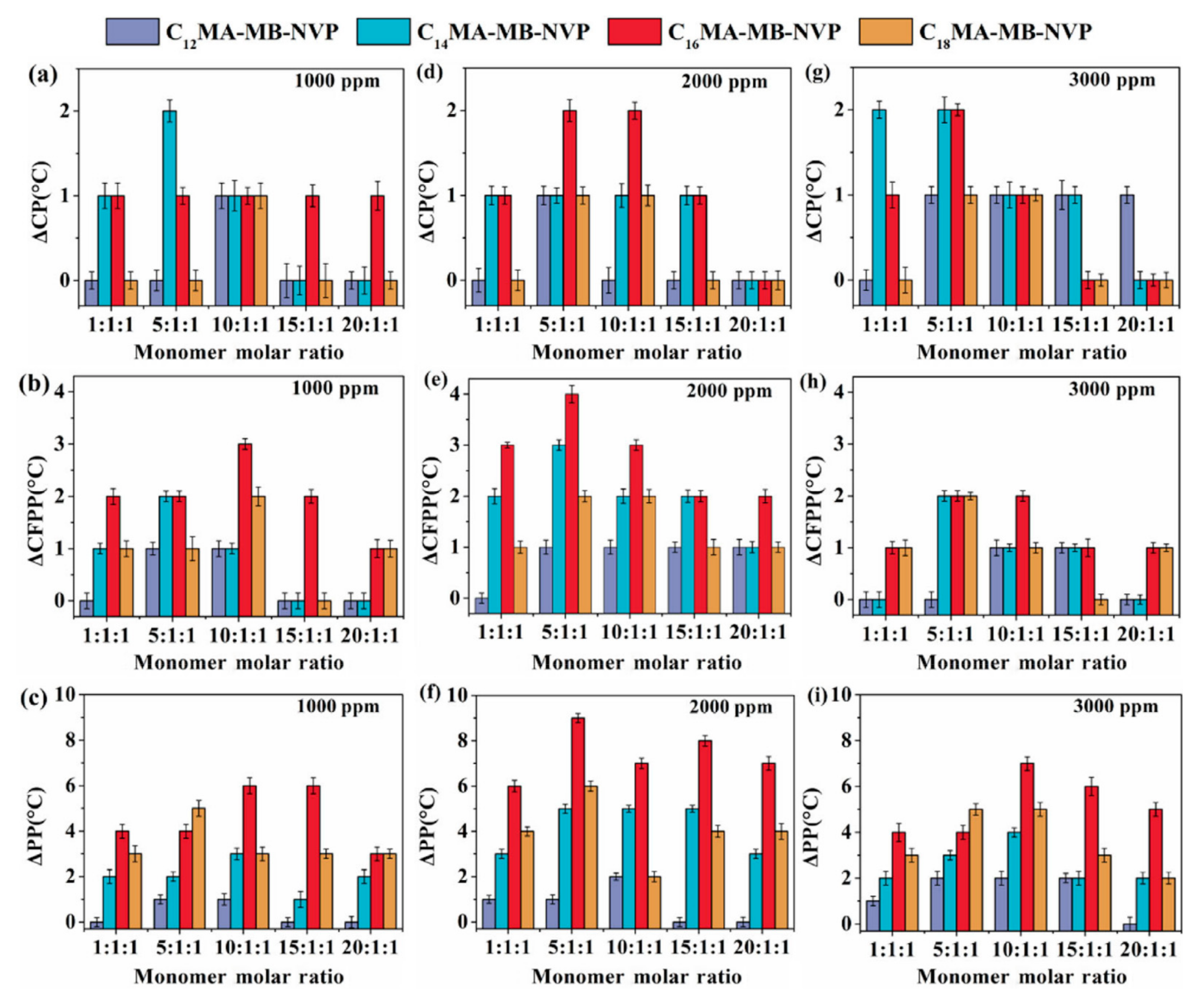
| Acid Name | Percent Composition in Oil 1 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Common | Lipid | RS | CA | CR | SU | SB | P | PK | OL | PN | CO | SA | TL | AlC 2 |
| Caproic | 6:0 | – | – | – | – | – | – | tr–1.5 | – | – | 0–0.8 | – | – | – |
| Caprylic | 8:0 | – | – | – | – | – | tr | 3–5 | – | – | 5–9 | – | – | – |
| Capric | 10:0 | – | – | – | – | tr | tr | 3–7 | – | – | 6–10 | – | – | – |
| Lauric | 12:0 | – | – | – | – | tr | tr | 40–52 | – | – | 44–52 | – | tr–0.2 | – |
| Myristic | 14:0 | – | – | tr–1.7 | – | tr | 0.5–6 | 14–18 | 0.1–1.2 | tr–1 | 13–19 | tr | 2–8 | 2.0 |
| Palmitic | 16:0 | 1–3 | 4 | 8–12 | 3–6 | 7–11 | 32–45 | 7–9 | 7–16 | 6–9 | 8–11 | 3–6 | 24–37 | 19.6 |
| Stearic | 18:0 | 0.4–3.5 | 2 | 2–5 | 1–3 | 2–6 | 2–7 | 1–3 | 1–3 | 3–6 | 1–3 | 1–4 | 14–29 | 3.3 |
| Arachidic | 20:0 | 0.5–2.4 | – | tr | 0.6–4 | 0.3–3 | tr | tr–1 | 0.1–0.3 | 2–4 | 0–0.4 | tr–0.2 | tr–1.2 | – |
| Behenic | 22:0 | 0.6–2.1 | – | tr | tr–0.8 | tr | – | – | – | 1–3 | – | – | – | – |
| Palmitoleic | 16:1 | 0.2–3 | – | 0.2–1.6 | tr | tr | 0.8–1.8 | tr–1 | tr | tr–1.7 | 0–1 | – | 1.9–2.7 | 0.8 |
| Oleic | 18:1 | 12–24 | 61 | 19–49 | 14–43 | 15–33 | 38–52 | 11–19 | 65–85 | 53–71 | 5–8 | 13–21 | 40–50 | 5.7 |
| Gadoleic | 20:1 | 4–12 | – | – | – | – | – | – | – | – | – | – | – | 0.1 |
| Erucic | 22:1 | 40–55 | tr | – | – | – | – | – | – | – | – | – | – | – |
| Linoleic | 18:2 | 12–16 | 21 | 34–62 | 44–75 | 43–56 | 5–11 | 0.5–2 | 4–15 | 13–27 | tr–2.5 | 73–79 | 1–5 | 11.8 |
| Linolenic | 18:3 | 7–10 | 9–12 | tr | tr | 5–11 | tr | – | tr–1 | tr | – | tr | – | 22.3 |
| Timnodonic | 20:5 | – | – | – | – | – | – | – | – | – | – | – | – | 1.3 |
| M. P. (°C) | M. P. (°C) | ||||
|---|---|---|---|---|---|
| Saturated Esters | Me | Et | Unsaturated Esters | Me | Et |
| 8:0 | −37.4 | –44.7 | 16:1 Δ9c | −34.1 | –36.7 |
| 10:0 | −13.5 | –20.4 | 18:1 Δ6c | −1.0 | –7.7 |
| 12:0 | 4.3 | –1.8 | 18:1 Δ9c | –20.2 | –20.3 |
| 14:0 | 18.1 | 12.5 | 18:1 Δ9t | 9.9 | 4.2 |
| 16:0 | 28.5 | 23.2 | 18:1 Δ11c | −24.3 | –36.5 |
| 18:0 | 37.7 | 32.9 | 18:2 Δ9c, Δ12c | −43.1 | –56.7 |
| 20:0 | 46.4 | 41.3 | 18:3 Δ9c, Δ12c, Δ15c | –52 | –61.7 |
| 22:0 | 53.2 | 48.6 | 20:1 Δ11c | −7.8 | –8.8 |
| 24:0 | 58.6 | 55.9 | 22:1 Δ13c | −3.1 | –10.5 |
| Biodiesel | Polymer CFIs Added | ΔCP, °C | ΔPP, °C | ΔCFPP, °C | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|
| Name | Saturated FAMEs wt% | Type | Composition/ MW/ÐM | Concentration, wt% | No. | Rating 1 | |||
| Canola | 6.2 | EVA | – 2/–/– | 1 | 0 | 11 | – | 100 | Q1 |
| Canola | 6.2 | poly(acrylate) | +/+/+ | 0.5–1 | – | 30 | – | 100 | Q1 |
| Canola | – | poly(acrylate) | –/–/– | 2 | 5 | – | – | 135 | Q1 |
| Canola | – | MA/C18OCH=CH2 | +/+/– | 1 | – | 3 | – | 100 | Q1 |
| Rapeseed | – | MA/olefin/acrylate | –/–/– | 0.5 | – | 2 | 4 | 151 | Q3 |
| Rapeseed | ~10 | poly(acrylate) | +/+/+ | 0.3–0.5 | – | 9 | 6 | 126 | P |
| Rapeseed/soybean | 10.0 | EVA-g-acrylate | +/+/+ | 0.5 | – | 15 | 5 | 126 | P |
| Rapeseed/soybean | 10.0 | EVA-acrylate | +/+/+ | 0.3 | 2 | – | 9 | 126 | P |
| Soybean | 14.3 | EVA | –/–/– | 0.01 | – | 2 | 1 | 106 | Q1 |
| Soybean | 17.4 | poly(acrylate) | –/–/– | 0–1 | – | – | 0 | 133 | Q1 |
| Soybean | – | poly(acrylate) | –/–/– | 0.5–1 | – | 12 | – | 110 | P |
| Soybean | – | poly(acrylate) | +/+/+ | 0.1 | – | 5 | – | 136 | P |
| Soybean | – | poly(acrylate) | +/+/+ | 0.2 | 3 | 9 | – | 137 | Q1 |
| Soybean | 14.5 | poly(acrylate) | –/–/– | 0.5 | – | 30 | – | 24 | Q1 |
| Palm | 43.6 | polyolefin | 1-decene/–/– | 0–2 | 0 | 0 | 0 | 105 | Q1 |
| Palm | 46 | EVA | –/–/– | 1 | – | 6 | 127 | Q1 | |
| Palm | – | poly(acrylate) | +/+/+ | 0.01–0.1 | – | 0 | – | 107 | Q3 |
| Palm | – | poly(acrylate) | –/–/– | 0.5–1 | – | 9 | – | 110 | P |
| Palm | 36.9 | poly(acrylate) | +/–/– | 0–2 | 0 | 0 | 0 | 105 | Q1 |
| Palm | 36.9 | MA/C18 | +/–/– | 2 | – | 6 | – | 105 | Q1 |
| Tobacco seed | 12 | MA/C18 | +/–/– | 1 | – | – | 7 | 149 | Q1 |
| Waste cooking | – | polyolefin | –/–/– | 0.1 | – | 2–3 | 2–3 | 108 | Q2 |
| Waste cooking | – | polyolefin | –/–/– | 0.02–0.08 | 1 | 1 | 3 | 98 | Q1 |
| Waste cooking | – | EVA | –/–/– | 0.02–0.08 | 2 | 6 | 2 | 98 | Q1 |
| Waste cooking | 43.5 | EVA | –/–/– | 0.02–0.08 | 0 | 3 | 1 | 103 | Q1 |
| Waste cooking | – | poly(acrylate) | –/–/– | 0.02–0.08 | 0 | 8 | 6 | 98 | Q1 |
| Waste cooking | 32 | poly(acrylate) | –/–/– | 0.5 | – | – | 7 | 134 | Q3 |
| Waste cooking | – | MA/acrylate/C14N | +/–/– | 0.05 | – | 13 | – | 104 | Q3 |
| – | – | MA/C18/ON 3 | +/+/+ | 0.01 | 5 | – | – | 150 | P |
| – | – | acrylate/acrylamide | –/–/– | 0.5 | – | <5 | <5 | 109 | P |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nifant’ev, I.; Ivchenko, P. Polymer Cold-Flow Improvers for Biodiesel. Polymers 2021, 13, 1580. https://doi.org/10.3390/polym13101580
Nifant’ev I, Ivchenko P. Polymer Cold-Flow Improvers for Biodiesel. Polymers. 2021; 13(10):1580. https://doi.org/10.3390/polym13101580
Chicago/Turabian StyleNifant’ev, Ilya, and Pavel Ivchenko. 2021. "Polymer Cold-Flow Improvers for Biodiesel" Polymers 13, no. 10: 1580. https://doi.org/10.3390/polym13101580
APA StyleNifant’ev, I., & Ivchenko, P. (2021). Polymer Cold-Flow Improvers for Biodiesel. Polymers, 13(10), 1580. https://doi.org/10.3390/polym13101580