Intumescent Flame Retardant Mechanism of Lignosulfonate as a Char Forming Agent in Rigid Polyurethane Foam
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Rigid Polyurethane (RPU) Foam and IFR Formulations
2.2.1. Preparation of RPU Foam
2.2.2. The Ratio of IFR Formulations
2.2.3. The Best Ratio of LS as Char Forming Agent in IFR Formulation
2.3. Characterizations
3. Results and Discussion
3.1. The Effect of LS as a Char Forming Agent in IFR Formulation
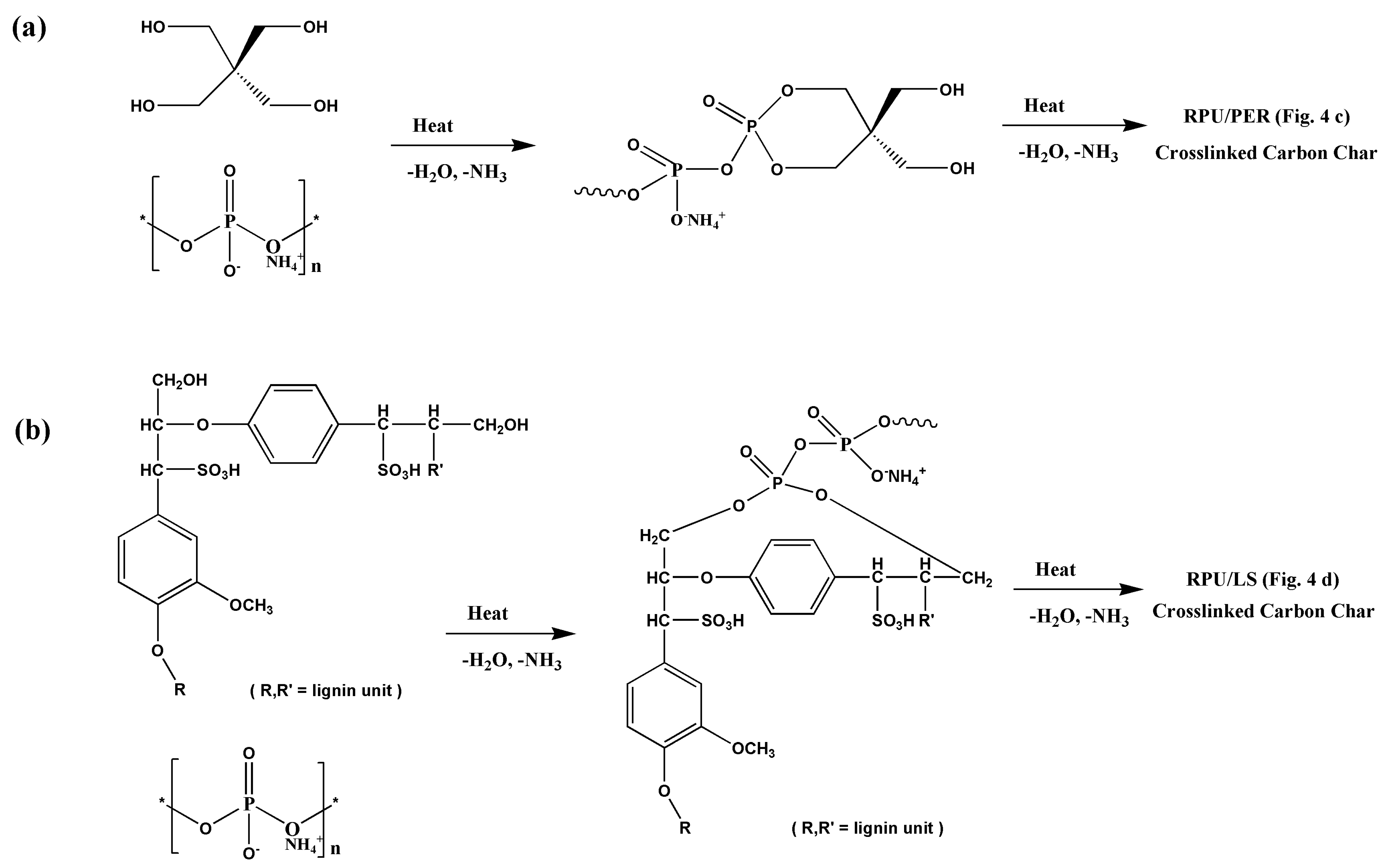
3.2. The Flame Retardant Mechanism of LS as a Char Forming Agent in IFR Formulation
3.3. The Best Ratio of LS as a Char Forming Agent in IFR Formulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, F.; Wu, K.; Lu, M. Enhanced thermal stability and flame retardancy of polyurethane foam composites with polybenzoxazine modified ammonium polyphosphates. RSC Adv. 2016, 6, 13418–13425. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. Thermal decomposition, combustion and fire-retardancy of polyurethanes—A review of the recent literature. Polym. Int. 2004, 53, 1585–1610. [Google Scholar] [CrossRef]
- Doğan, M.; Yılmaz, A.; Bayramlı, E. Synergistic effect of boron containing substances on flame retardancy and thermal stability of intumescent polypropylene composites. Polym. Degrad. Stab. 2010, 95, 2584–2588. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Nando, G.; Naik, Y.; Singha, N.K. Halogen-free flame retardant PUF: Effect of melamine compounds on mechanical, thermal and flame retardant properties. Polym. Degrad. Stab. 2010, 95, 1138–1145. [Google Scholar] [CrossRef]
- Wu, K.; Shen, M.-M.; Hu, Y. Synthesis of a novel intumescent flame retardant and its flame retardancy in polypropylene. J. Polym. Res. 2011, 18, 425–433. [Google Scholar] [CrossRef]
- Grexa, O.; Poutch, F.; Maníková, D.; Martvonova, H.; Barteková, A. Intumescence in fire retardancy of lignocellulosic panels. Polym. Degrad. Stab. 2003, 82, 373–377. [Google Scholar] [CrossRef]
- Ni, J.; Tai, Q.; Lu, H.; Hu, Y.; Song, L. Microencapsulated ammonium polyphosphate with polyurethane shell: Preparation, characterization, and its flame retardance in polyurethane. Polym. Adv. Technol. 2009, 21, 392–400. [Google Scholar] [CrossRef]
- Yang, L.; Cheng, W.; Zhou, J.; Li, H.; Wang, X.; Chen, X.; Zhang, Z. Effects of microencapsulated APP-II on the microstructure and flame retardancy of PP/APP–II/PER composites. Polym. Degrad. Stab. 2014, 105, 150–159. [Google Scholar] [CrossRef]
- Chen, X.; Jiao, C.; Zhang, J. Microencapsulation of ammonium polyphosphate with hydroxyl silicone oil and its flame retardance in thermoplastic polyurethane. J. Therm. Anal. Calorim. 2011, 104, 1037–1043. [Google Scholar] [CrossRef]
- Ruban, L.; Zaikov, G. Importance of Intumescence in Polymers Fire Retardancy. Int. J. Polym. Mater. 2001, 48, 295–310. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Duquesne, S.; Rochery, M. Recent Advances for Intumescent Polymers. Macromol. Mater. Eng. 2004, 289, 499–511. [Google Scholar] [CrossRef]
- Usta, N. Investigation of fire behavior of rigid polyurethane foams containing fly ash and intumescent flame retardant by using a cone calorimeter. J. Appl. Polym. Sci. 2011, 124, 3372–3382. [Google Scholar] [CrossRef]
- Chou, C.-S.; Lin, S.-H.; Wang, C.-I.; Liu, K.-H. A hybrid intumescent fire retardant coating from cake- and eggshell-type IFRC. Powder Technol. 2010, 198, 149–156. [Google Scholar] [CrossRef]
- Réti, C.; Casetta, M.; Duquesne, S.; Bourbigot, S.; Delobel, R. Flammability properties of intumescent PLA including starch and lignin. Polym. Adv. Technol. 2008, 19, 628–635. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-based flame retardants: When nature meets fire protection. Mater. Sci. Eng. R: Rep. 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Ansari, H.; Shabanian, M.; Khonakdar, H.A. Using a β-Cyclodextrin-functional Fe3O4 as a Reinforcement of PLA: Synthesis, Thermal, and Combustion Properties. Polym. Plast. Technol. Eng. 2017, 56, 1366–1373. [Google Scholar] [CrossRef]
- Michałowski, S.; Hebda, E.; Pielichowski, K. Thermal stability and flammability of polyurethane foams chemically reinforced with POSS. J. Therm. Anal. Calorim. 2017, 130, 155–163. [Google Scholar] [CrossRef]
- Morgan, A.B.; Gilman, J.W. An overview of flame retardancy of polymeric materials: Application, technology, and future directions. Fire Mater. 2012, 37, 259–279. [Google Scholar] [CrossRef]
- Bourbigot, S.; Duquesne, S. Fire retardant polymers: Recent developments and opportunities. J. Mater. Chem. 2007, 17, 2283–2300. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Ni, A.; Ding, A.; Han, X.; Sun, Z. The Effects of a Macromolecular Charring Agent with Gas Phase and Condense Phase Synergistic Flame Retardant Capability on the Properties of PP/IFR Composites. Materials 2018, 11, 111. [Google Scholar] [CrossRef]
- Song, Y.; Zong, X.; Wang, N.; Yan, N.; Shan, X.; Li, J. Preparation of γ-Divinyl-3-Aminopropyltriethoxysilane Modified Lignin and Its Application in Flame Retardant Poly(lactic acid). Materials 2018, 11, 1505. [Google Scholar] [CrossRef]
- Chen, X.; Jiao, C.; Li, S.; Hu, Y. Preparation and properties of a single molecule intumescent flame retardant. Fire Saf. J. 2013, 58, 208–212. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, W.; Qiu, Y.; Li, L.; Qian, L.; Xin, F. Terminal group effects of phosphazene-triazine bi-group flame retardant additives in flame retardant polylactic acid composites. Polym. Degrad. Stab. 2017, 140, 166–175. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Aguedo, M.; Richel, A.; Brohez, S.; Delvosalle, C.; Dubois, P. Phosphorus and nitrogen derivatization as efficient route for improvement of lignin flame retardant action in PLA. Eur. Polym. J. 2016, 84, 652–667. [Google Scholar] [CrossRef]
- Sarkanen, K.V.; Lunwing, C.H. Lignins, Occurrence, Formation, Structure and Reactions; Wiley Interscience: New York, NY, USA, 1971. [Google Scholar]
- Zhu, H.; Peng, Z.; Chen, Y.; Li, G.; Wang, L.; Tang, Y.; Pang, R.; Khan, Z.U.H.; Wan, P. Preparation and characterization of flame retardant polyurethane foams containing phosphorus–nitrogen-functionalized lignin. RSC Adv. 2014, 4, 55271–55279. [Google Scholar] [CrossRef]
- Ferry, L.; Dorez, G.; Taguet, A.; Otazaghine, B.; Lopez-Cuesta, J. Chemical modification of lignin by phosphorus molecules to improve the fire behavior of polybutylene succinate. Polym. Degrad. Stab. 2015, 113, 135–143. [Google Scholar] [CrossRef]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Xu, C. Depolymerization of lignins and their applications for the preparation of polyols and rigid polyurethane foams: A review. Renew. Sustain. Energy Rev. 2016, 60, 317–329. [Google Scholar] [CrossRef]
- Bertini, F.; Canetti, M.; Cacciamani, A.; Elegir, G.; Orlandi, M.; Zoia, L. Effect of ligno-derivatives on thermal properties and degradation behavior of poly(3-hydroxybutyrate)-based biocomposites. Polym. Degrad. Stab. 2012, 97, 1979–1987. [Google Scholar] [CrossRef]
- Griffini, G.; Passoni, V.; Suriano, R.; Levi, M.; Turri, S. Polyurethane Coatings Based on Chemically Unmodified Fractionated Lignin. ACS Sustain. Chem. Eng. 2015, 3, 1145–1154. [Google Scholar] [CrossRef]
- Reti, C.; Casetta, M.; Duquesne, S.; Delobel, R.; Soulestin, J.; Bourbigot, S. Intumescent Biobased-Polylactide Films to Flame Retard Nonwovens. J. Eng. Fibers Fabr. 2009, 4, 33–39. [Google Scholar] [CrossRef]
- Cayla, A.; Rault, F.; Giraud, S.; Salaün, F.; Fierro, V.; Celzard, A. PLA with Intumescent System Containing Lignin and Ammonium Polyphosphate for Flame Retardant Textile. Polymers 2016, 8, 331. [Google Scholar] [CrossRef]
- Zhang, R.; Xiao, X.; Tai, Q.; Huang, H.; Hu, Y. Modification of lignin and its application as char agent in intumescent flame-retardant poly(lactic acid). Polym. Eng. Sci. 2012, 52, 2620–2626. [Google Scholar] [CrossRef]
- Zhang, R.; Xiao, X.; Tai, Q.; Huang, H.; Yang, J.; Hu, Y. Preparation of lignin–silica hybrids and its application in intumescent flame-retardant poly(lactic acid) system. High Perform. Polym. 2012, 24, 738–746. [Google Scholar] [CrossRef]
- Zhang, R.; Xiao, X.; Tai, Q.; Huang, H.; Yang, J.; Hu, Y. The effect of different organic modified montmorillonites (OMMTs) on the thermal properties and flammability of PLA/MCAPP/lignin systems. J. Appl. Polym. Sci. 2012, 127, 4967–4973. [Google Scholar] [CrossRef]
- Giraud, S.; Rault, F.; Rochery, M.; Specht, C.; Capon, G. Synthesis and characterization of flame retardant polyurethane formulation for textile coating containing lignin as bio-based carbon source. In Proceedings of the 15th European Meeting on Fire Retardancy and Protection of Materials, Berlin, Germany, 22–25 June 2015; pp. 22–25. [Google Scholar]
- Hatakeyama, H.; Hatakeyama, T. Green Polyurethanes and Biocomposites: Molecular Design and Characterization; Nova publishers: New York, NY, USA, 2015. [Google Scholar]
- Zhang, Z.; Li, J.; Zhang, Y.; Jin, Z. Kinetics of partially depolymerized lignin as co-curing agent for epoxy resin. Int. J. Biol. Macromol. 2020, 150, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Le Bras, M.; Bourbigot, S.; Le Tallec, Y.; Laureyns, J. Synergy in intumescence—application to β-cyclodextrin carbonisation agent in intumescent additives for fire retardant polyethylene formulations. Polym. Degrad. Stab. 1997, 56, 11–21. [Google Scholar] [CrossRef]
- Tsai, K.-C.; Kuan, C.-F.; Chen, C.-H.; Kuan, H.-C.; Hsu, S.-W.; Lee, F.-M.; Chiang, C.-L. Study on thermal degradation and flame retardant property of halogen-free polypropylene composites using XPS and cone calorimeter. J. Appl. Polym. Sci. 2013, 127, 1084–1091. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, J.; Yang, S.; Zhang, Q.; Huo, S.; Zhang, Q.; Hu, Y.; Ding, G. Benzimidazolyl-substituted cyclotriphosphazene derivative as latent flame-retardant curing agent for one-component epoxy resin system with excellent comprehensive performance. Compos. Part B Eng. 2019, 177, 107440. [Google Scholar] [CrossRef]
- Huo, S.; Yang, S.; Wang, J.; Cheng, J.; Zhang, Q.; Hu, Y.; Ding, G.; Zhang, Q.; Song, P. A liquid phosphorus-containing imidazole derivative as flame-retardant curing agent for epoxy resin with enhanced thermal latency, mechanical, and flame-retardant performances. J. Hazard. Mater. 2020, 386, 121984. [Google Scholar] [CrossRef] [PubMed]
- Li, B. An investigation of the smoke suppression and the thermal degradation in the smouldering mode of poly (vinyl chloride) containing a combination of cuprous oxide and molybdenum trioxide. Polym. Degrad. Stab. 2001, 74, 195–199. [Google Scholar] [CrossRef]
- Bras, M.; Bourbigot, S.; Delporte, C.; Siat, C.; Tallec, Y. New intumescent formulations of fire-retardant polypropylene-discussion of the free radical mechanism of the formation of carbonaceous protective material during the thermo-oxidative treatment of the additives. Fire Mater. 1996, 20, 191–203. [Google Scholar] [CrossRef]
- Ke, C.-H.; Li, J.; Fang, K.-Y.; Zhu, Q.-L.; Zhu, J.; Yan, Q.; Wang, Y.-Z. Synergistic effect between a novel hyperbranched charring agent and ammonium polyphosphate on the flame retardant and anti-dripping properties of polylactide. Polym. Degrad. Stab. 2010, 95, 763–770. [Google Scholar] [CrossRef]
- Zhong, Y.; Wu, W.; Wu, R.; Luo, Q.; Wang, Z. The flame retarding mechanism of the novolac as char agent with the fire retardant containing phosphorous–nitrogen in thermoplastic poly(ether ester) elastomer system. Polym. Degrad. Stab. 2014, 105, 166–177. [Google Scholar] [CrossRef]
- Su, X.; Yi, Y.; Tao, J.; Qi, H.; Li, D. Synergistic effect between a novel triazine charring agent and ammonium polyphosphate on flame retardancy and thermal behavior of polypropylene. Polym. Degrad. Stab. 2014, 105, 12–20. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials—Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
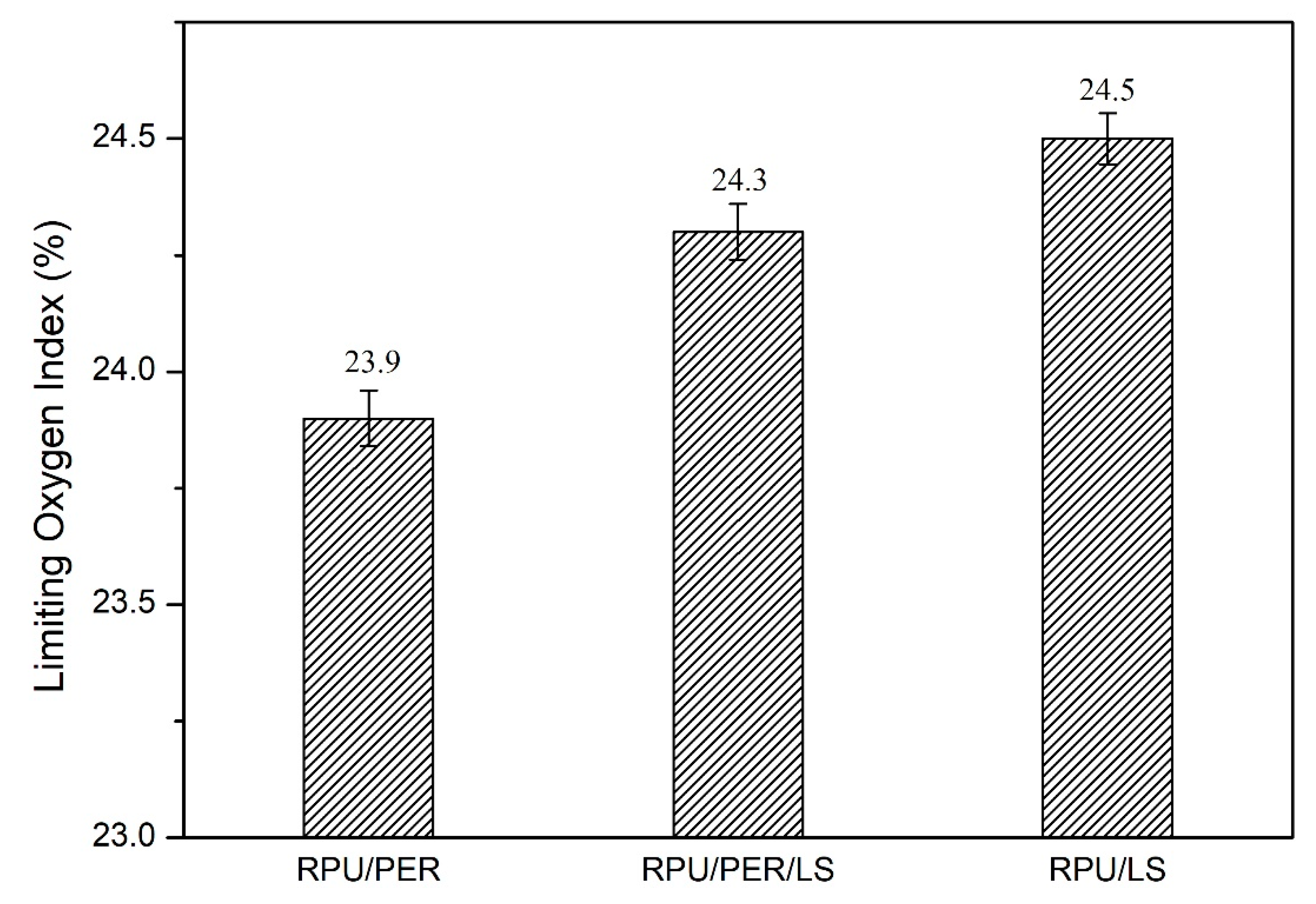





| Sample | APP (g) | MEL (g) | PER (g) | LS (g) | Density (kg/m3) |
|---|---|---|---|---|---|
| RPU/PER | 10.2 | 3.4 | 3.4 | - | 58.0 |
| RPU/PER/LS | 10.2 | 3.4 | 1.7 | 1.7 | 57.6 |
| RPU/LS | 10.2 | 3.4 | - | 3.4 | 57.5 |
| Sample | APP (g) | MEL (g) | LS (g) | APP:MEL:LS Ratio | Density (kg/m3) |
|---|---|---|---|---|---|
| RPU/LS0.5 | 10.2 | 3.4 | 1.7 | 3:1:0.5 | 57.3 |
| RPU/LS1 | 10.2 | 3.4 | 3.4 | 3:1:1 | 57.5 |
| RPU/LS1.5 | 10.2 | 3.4 | 5.1 | 3:1:1.5 | 57.6 |
| RPU/LS2 | 10.2 | 3.4 | 6.8 | 3:1:2 | 57.8 |
| RPU/LS3 | 10.2 | 3.4 | 10.2 | 3:1:3 | 58.0 |
| RPU/LS4 | 10.2 | 3.4 | 13.6 | 3:1:4 | 58.2 |
| Sample | HRR (kW/m2) | pHRR (kW/m2) | tpHRR (s) | THR (MJ/m2) | FGRI (kW/m2·s) |
|---|---|---|---|---|---|
| RPU/PER | 46.1 | 215.1 | 104 | 28.4 | 2.1 |
| RPU/PER/LS | 45.7 | 202.2 | 110 | 27.8 | 1.8 |
| RPU/LS | 45.0 | 191.4 | 110 | 27.4 | 1.7 |
| Sample | TSR (m2/m2) | SEA (m2/kg) | EHC (MJ/kg) | pCO (kg/kg) | mCOY (g/kg) | mCO2Y (kg/kg) |
|---|---|---|---|---|---|---|
| RPU/PER | 717.4 | 430.2 | 18.1 | 0.14 | 0.55 | 2.05 |
| RPU/PER/LS | 765.9 | 444.3 | 16.7 | 0.18 | 0.49 | 1.95 |
| RPU/LS | 700.4 | 343.8 | 17.0 | 0.03 | 0.68 | 2.03 |
| Sample | CCT Results | TG Results (Second Step of Mass Loss) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PMLR (g/s) | SMLR (g/s.m2) | tPMLR (s) | Char600 s (g) | Tonset2 (°C) | Tmax2 (°C) | Tfinal2 (°C) | Mass Loss (wt%) | Char800 °C (wt%) | |
| RPU/PER | 0.48 | 11.1 | 9 | 2.1 | 273 | 311 | 361 | 61.9 | 29.9 |
| RPU/PER/LS | 0.30 | 10.3 | 6 | 2.1 | 274 | 309 | 364 | 63.0 | 29.5 |
| RPU/LS | 0.26 | 9.6 | 35 | 2.6 | 277 | 311 | 356 | 50.7 | 34.3 |
| Sample | THR (MJ/m2) | HRR (kW m2) | EHC (MJ/kg) | PMLR (g/s) | tPCO2 (s) |
|---|---|---|---|---|---|
| RPU/LS0.5 | 39.3 | 65.9 | 17.4 | 0.35 | 118 |
| RPU/LS1 | 27.4 | 46.0 | 17.0 | 0.26 | 596 |
| RPU/LS1.5 | 32.6 | 54.6 | 16.9 | 0.44 | 563 |
| RPU/LS2 | 32.5 | 54.5 | 19.3 | 0.61 | 205 |
| RPU/LS3 | 28.2 | 47.4 | 18.8 | 0.29 | 366 |
| RPU/LS4 | 39.6 | 66.5 | 18.5 | 0.29 | 565 |
| Sample | Tmax1 (°C) | Mass Loss1 (wt%) | Tmax2 (°C) | Mass Loss2 (wt%) | Char800 °C (wt%) |
|---|---|---|---|---|---|
| RPU/LS0.5 | 181 | 2.4 | 302 | 59.3 | 29.6 |
| RPU/LS1 | 174 | 2.4 | 311 | 50.6 | 34.3 |
| RPU/LS1.5 | 169 | 2.5 | 307 | 58.3 | 31.2 |
| RPU/LS2 | 173 | 2.7 | 307 | 59.8 | 32.3 |
| RPU/LS3 | 173 | 2.9 | 304 | 57.5 | 31.4 |
| RPU/LS4 | 183 | 2.4 | 308 | 55.4 | 32.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, W.; Ye, J.; Zhu, L.; Jin, Z.; Matsumoto, Y. Intumescent Flame Retardant Mechanism of Lignosulfonate as a Char Forming Agent in Rigid Polyurethane Foam. Polymers 2021, 13, 1585. https://doi.org/10.3390/polym13101585
Lu W, Ye J, Zhu L, Jin Z, Matsumoto Y. Intumescent Flame Retardant Mechanism of Lignosulfonate as a Char Forming Agent in Rigid Polyurethane Foam. Polymers. 2021; 13(10):1585. https://doi.org/10.3390/polym13101585
Chicago/Turabian StyleLu, Weimiao, Jiewang Ye, Lianghai Zhu, Zhenfu Jin, and Yuji Matsumoto. 2021. "Intumescent Flame Retardant Mechanism of Lignosulfonate as a Char Forming Agent in Rigid Polyurethane Foam" Polymers 13, no. 10: 1585. https://doi.org/10.3390/polym13101585
APA StyleLu, W., Ye, J., Zhu, L., Jin, Z., & Matsumoto, Y. (2021). Intumescent Flame Retardant Mechanism of Lignosulfonate as a Char Forming Agent in Rigid Polyurethane Foam. Polymers, 13(10), 1585. https://doi.org/10.3390/polym13101585





