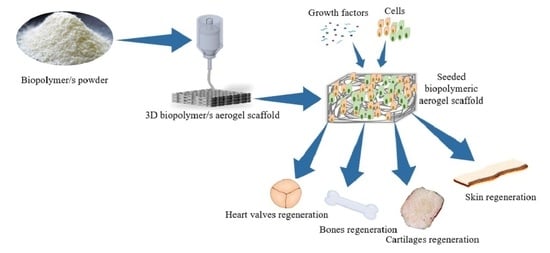
Insights into the Role of Biopolymer Aerogel Scaffolds in Tissue Engineering and Regenerative Medicine
Abstract
:1. Introduction
2. Tissue Scaffolding and Regenerative Medicine
2.1. Chronological Development of Tissue Scaffolding and Regenerative Medicine
2.2. Tissue Regeneration Approaches
3. Biopolymer-Based Aerogels in Tissue Engineering and Regenerative Medicine
3.1. Desirable Characteristics in Biopolymers and Biopolymers Tissue Scaffolds
3.2. Fabrication Techniques of Biopolymeric Scaffold for Tissue Engineering
4. Biopolymers-Based Aerogels in Tissue Engineering for Therapeutic Applications
4.1. Wound Healing and Skin Regeneration
4.2. Cartilage Regeneration
4.3. Bone Regeneration
4.4. Heart Valve Regeneration
5. Challenges and Future Prospects in Tissue Engineering Applications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal stem cells for regenerative medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [Green Version]
- Guidotti, G.; Soccio, M.; Gazzano, M.; Fusaro, L.; Boccafoschi, F.; Munari, A.; Lotti, N. New thermoplastic elastomer triblock copolymer of PLLA for cardiovascular tissue engineering: Annealing as efficient tool to tailor the solid-state properties. Polymer 2021, 213, 123336. [Google Scholar] [CrossRef]
- Palit, S.; Hussain, C.M. Nanodevices applications and recent advancements in nanotechnology and the global pharmaceutical industry. In Nanomaterials in Diagnostic Tools and Devices; Elsevier: Amsterdam, The Netherlands, 2020; pp. 395–415. [Google Scholar]
- Mirtaghavi, A.; Luo, J.; Muthuraj, R. Recent Advances in Porous 3D Cellulose Aerogels for Tissue Engineering Applications: A Review. J. Compos. Sci. 2020, 4, 152. [Google Scholar] [CrossRef]
- Chaudhury, K.; Kumar, V.; Kandasamy, J.; RoyChoudhury, S. Regenerative nanomedicine: Current perspectives and future directions. Int. J. Nanomed. 2014, 9, 4153. [Google Scholar] [CrossRef] [Green Version]
- Rege, A.; Ratke, L.; Külcü, İ.D.; Gurikov, P. Stiffening of biopolymer aerogel networks upon wetting: A model-based study. J. Non Cryst. Solids 2020, 531, 119859. [Google Scholar] [CrossRef]
- Sharma, P.R.; Joshi, R.; Sharma, S.K.; Hsiao, B.S. A simple approach to prepare carboxycellulose nanofibers from untreated biomass. Biomacromolecules 2017, 18, 2333–2342. [Google Scholar] [CrossRef]
- Takeshita, S.; Sadeghpour, A.; Malfait, W.J.; Konishi, A.; Otake, K.; Yoda, S. Formation of nanofibrous structure in biopolymer aerogel during supercritical CO2 processing: The case of chitosan aerogel. Biomacromolecules 2019, 20, 2051–2057. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Lindström, T.; Hsiao, B.S. Nanocellulose-Enabled Membranes for Water Purification: Perspectives. Adv. Sustain. Syst. 2020, 4, 1900114. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Hsiao, B.S. Efficient removal of UO22+ from water using carboxycellulose nanofibers prepared by the nitro-oxidation method. Ind. Eng. Chem. Res. 2017, 56, 13885–13893. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Geng, L.; Amiralian, N.; Martin, D.; Hsiao, B.S. Nanocellulose from spinifex as an effective adsorbent to remove cadmium (II) from water. ACS Sustain. Chem. Eng. 2018, 6, 3279–3290. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Zhan, C.; Sharma, S.K.; Geng, L.; Hsiao, B.S. Lead removal from water using carboxycellulose nanofibers prepared by nitro-oxidation method. Cellulose 2018, 25, 1961–1973. [Google Scholar] [CrossRef]
- Guerrero-Alburquerque, N.; Zhao, S.; Adilien, N.; Koebel, M.M.; Lattuada, M.; Malfait, W.J. Strong, machinable, and insulating chitosan–urea aerogels: Toward ambient pressure drying of biopolymer aerogel monoliths. ACS Appl. Mater. Interfaces 2020, 12, 22037–22049. [Google Scholar] [CrossRef]
- Takeshita, S.; Akasaka, S.; Yoda, S. Structural and acoustic properties of transparent chitosan aerogel. Mater. Lett. 2019, 254, 258–261. [Google Scholar] [CrossRef]
- Alnaief, M.; Obaidat, R.M.; Alsmadi, M.t.M. Preparation of Hybrid Alginate-Chitosan Aerogel as Potential Carriers for Pulmonary Drug Delivery. Polymers 2020, 12, 2223. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Barros, J.; Ardao, I.; Monteiro, F.J.; Alvarez-Lorenzo, C.; Gómez-Amoza, J.L.; García-González, C.A. Vancomycin-loaded chitosan aerogel particles for chronic wound applications. Carbohydr. Polym. 2019, 204, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, M.; Chen, J.; Fan, S.; Liang, J.; Ding, L.; Chen, S. Flexible chitosan/carbon nanotubes aerogel, a robust matrix for in-situ growth and non-enzymatic biosensing applications. Sens. Actuators B Chem. 2016, 232, 750–757. [Google Scholar] [CrossRef]
- Kamel, S.; Khattab, T. Recent advances in cellulose-based biosensors for medical diagnosis. Biosensors 2020, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Deuber, F.; Mousavi, S.; Federer, L.; Adlhart, C. Amphiphilic nanofiber-based aerogels for selective liquid absorption from electrospun biopolymers. Adv. Mater. Interfaces 2017, 4, 1700065. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Adnan, A.; Yahya, E.B.; Olaiya, N.; Safrida, S.; Hossain, M.; Balakrishnan, V.; Gopakumar, D.A.; Abdullah, C.; Oyekanmi, A. A Review on plant cellulose nanofibre-based aerogels for biomedical applications. Polymers 2020, 12, 1759. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.; Su, B.-L.; Xia, H.; Zhao, S.; Gao, C.; Wang, L.; Ogbeide, O.; Feng, J.; Hasan, T. Printed aerogels: Chemistry, processing, and applications. Chem. Soc. Rev. 2021, 50, 3842–3888. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yao, L.; Pan, C.; Tian, J.; Li, L.; Luo, B.; Zhou, C.; Lu, L. 3D printed gellan gum/graphene oxide scaffold for tumor therapy and bone reconstruction. Compos. Sci. Technol. 2021, 208, 108763. [Google Scholar] [CrossRef]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nyström, G. Biopolymer aerogels and foams: Chemistry, properties, and applications. Angew. Chem. Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Jummaat, F.; Yahya, E.B.; Olaiya, N.; Adnan, A.; Abdat, M.; NAM, N.; Halim, A.S.; Kumar, U.; Bairwan, R. A review on micro-to nanocellulose biopolymer scaffold forming for tissue engineering applications. Polymers 2020, 12, 2043. [Google Scholar] [CrossRef]
- Nita, L.E.; Ghilan, A.; Rusu, A.G.; Neamtu, I.; Chiriac, A.P. New trends in bio-based aerogels. Pharmaceutics 2020, 12, 449. [Google Scholar] [CrossRef]
- Park, S.; Oh, Y.; Yun, J.; Yoo, E.; Jung, D.; Park, K.S.; Oh, K.K.; Lee, S.H. Characterization of blended cellulose/biopolymer films prepared using ionic liquid. Cellulose 2020, 27, 5101–5119. [Google Scholar] [CrossRef]
- Sviridov, A.; Osminkina, L.; Kharin, A.Y.; Gongalsky, M.; Kargina, J.; Kudryavtsev, A.; Bezsudnova, Y.I.; Perova, T.; Geloen, A.; Lysenko, V. Cytotoxicity control of silicon nanoparticles by biopolymer coating and ultrasound irradiation for cancer theranostic applications. Nanotechnology 2017, 28, 105102. [Google Scholar] [CrossRef] [PubMed]
- Starbird-Perez, R.; Del Gaudio, P.; García-González, C.A. Biopolymers in Drug Delivery and Regenerative Medicine; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2021. [Google Scholar]
- Baldino, L.; Cardea, S.; Scognamiglio, M.; Reverchon, E. A new tool to produce alginate-based aerogels for medical applications, by supercritical gel drying. J. Supercrit. Fluids 2019, 146, 152–158. [Google Scholar] [CrossRef]
- Reverchon, E.; Baldino, L.; Cardea, S.; De Marco, I. Biodegradable synthetic scaffolds for tendon regeneration. Muscles Ligaments Tendons J. 2012, 2, 181. [Google Scholar]
- Jeong, K.-H.; Park, D.; Lee, Y.-C. Polymer-based hydrogel scaffolds for skin tissue engineering applications: A mini-review. J. Polym. Res. 2017, 24, 1–10. [Google Scholar] [CrossRef]
- Garcia, J.; Dodge, A.; Luepke, P.; Wang, H.L.; Kapila, Y.; Lin, G.H. Effect of membrane exposure on guided bone regeneration: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29, 328–338. [Google Scholar] [CrossRef] [Green Version]
- Kalamegam, G.; Memic, A.; Budd, E.; Abbas, M.; Mobasheri, A. A comprehensive review of stem cells for cartilage regeneration in osteoarthritis. In Cell Biology and Translational Medicine; Springer: New York, NY, USA, 2018; Volume 2, pp. 23–36. [Google Scholar]
- Fioretta, E.S.; Motta, S.E.; Lintas, V.; Loerakker, S.; Parker, K.K.; Baaijens, F.P.; Falk, V.; Hoerstrup, S.P.; Emmert, M.Y. Next-generation tissue-engineered heart valves with repair, remodelling and regeneration capacity. Nat. Rev. Cardiol. 2021, 18, 92–116. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ali, A.; Sheikh, J. A review on chitosan centred scaffolds and their applications in tissue engineering. Int. J. Biol. Macromol. 2018, 116, 849–862. [Google Scholar] [CrossRef]
- Abouzeid, R.E.; Khiari, R.; Beneventi, D.; Dufresne, A. Biomimetic mineralization of three-dimensional printed alginate/TEMPO-oxidized cellulose nanofibril scaffolds for bone tissue engineering. Biomacromolecules 2018, 19, 4442–4452. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef]
- Weinstein-Oppenheimer, C.R.; Brown, D.I.; Coloma, R.; Morales, P.; Reyna-Jeldes, M.; Díaz, M.J.; Sánchez, E.; Acevedo, C.A. Design of a hybrid biomaterial for tissue engineering: Biopolymer-scaffold integrated with an autologous hydrogel carrying mesenchymal stem-cells. Mater. Sci. Eng. C 2017, 79, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Nezhad-Mokhtari, P.; Akrami-Hasan-Kohal, M.; Ghorbani, M. An injectable chitosan-based hydrogel scaffold containing gold nanoparticles for tissue engineering applications. Int. J. Biol. Macromol. 2020, 154, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Opris, H.; Dinu, C.; Baciut, M.; Baciut, G.; Mitre, I.; Crisan, B.; Armencea, G.; Prodan, D.A.; Bran, S. The Influence of Eggshell on Bone Regeneration in Preclinical In Vivo Studies. Biology 2020, 9, 476. [Google Scholar] [CrossRef]
- Almashgab, A.M.; Yahya, E.B.; Banu, A. The Cytotoxicity Effects of Outer Membrane Vesicles Isolated from Hospital and Laboratory Strains of Pseudomonas Aeruginosa on Human Keratinocyte Cell Line. Malays. J. Sci. 2020, 39, 45–53. [Google Scholar] [CrossRef]
- Thorrez, L.; Shansky, J.; Wang, L.; Fast, L.; VandenDriessche, T.; Chuah, M.; Mooney, D.; Vandenburgh, H. Growth, differentiation, transplantation and survival of human skeletal myofibers on biodegradable scaffolds. Biomaterials 2008, 29, 75–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chick, L.R. Brief history and biology of skin grafting. Ann. Plast. Surg. 1988, 21, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Ameer, F.; Singh, A.K.; Kumar, S. Evolution of instruments for harvest of the skin grafts. Indian J. Plast. Surg. Off. Publ. Assoc. Plast. Surg. India 2013, 46, 28. [Google Scholar] [CrossRef] [PubMed]
- Nather, A.; Zheng, S. Evolution of allograft transplantation. In Allograft Procurement, Processing and Transplantation: A Comprehensive Guide for Tissue Bank; World Scientific Publishing Company: Singapore, 2010; Volume 1. [Google Scholar]
- Ziegler, U.; Dietz, U.; Schmidt, K. Skin Grafts. In Surgery in Wounds; Springer: New York, NY, USA, 2004; pp. 179–186. [Google Scholar]
- Kaul, H.; Ventikos, Y. On the genealogy of tissue engineering and regenerative medicine. Tissue Eng. Part B Rev. 2015, 21, 203–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blakemore, A.H.; Voorhees Jr, A.B. The use of tubes constructed from vinyon “N” cloth in bridging arterial defects—experimental and clinical. Ann. Surg. 1954, 140, 324. [Google Scholar] [CrossRef] [PubMed]
- Breine, U.; Johansson, B.; Roylance, P.J.; Roeckert, H.; Yoffey, J.M. Regeneration of bone marrow. A clinical and experimental study following removal of bone marrow by curettage. Acta Anat. 1964, 59, 1–46. [Google Scholar] [PubMed]
- Green, W.T., Jr. Articular cartilage repair. Behavior of rabbit chondrocytes during tissue culture and subsequent allografting. Clin. Orthop. Relat. Res. 1977, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Vacanti, J.P.; Morse, M.A.; Saltzman, W.M.; Domb, A.J.; Perez-Atayde, A.; Langer, R. Selective cell transplantation using bioabsorbable artificial polymers as matrices. J. Pediatric Surg. 1988, 23, 3–9. [Google Scholar] [CrossRef]
- Stone, K.R.; Steadman, J.R.; Rodkey, W.G.; Li, S.-T. Regeneration of meniscal cartilage with use of a collagen scaffold. Analysis of preliminary data. JBJS J. Bone Joint Surg. Am. 1997, 79, 1770. [Google Scholar] [CrossRef]
- Zein, I.; Hutmacher, D.W.; Tan, K.C.; Teoh, S.H. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 2002, 23, 1169–1185. [Google Scholar] [CrossRef]
- Svensson, A.; Nicklasson, E.; Harrah, T.; Panilaitis, B.; Kaplan, D.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 2005, 26, 419–431. [Google Scholar] [CrossRef]
- Macchiarini, P.; Jungebluth, P.; Go, T.; Asnaghi, M.A.; Rees, L.E.; Cogan, T.A.; Dodson, A.; Martorell, J.; Bellini, S.; Parnigotto, P.P. Clinical transplantation of a tissue-engineered airway. Lancet 2008, 372, 2023–2030. [Google Scholar] [CrossRef]
- Norotte, C.; Marga, F.S.; Niklason, L.E.; Forgacs, G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 2009, 30, 5910–5917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, S.; Yoon, H.; Kim, G.; Kim, Y.; Lee, S.; Chun, W. Designed three-dimensional collagen scaffolds for skin tissue regeneration. Tissue Eng. Part C Methods 2010, 16, 813–820. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, Q.; Guo, W.; Terrell, L.; Qureshi, A.T.; Hayes, D.J.; Wu, Q. Electrospun bio-nanocomposite scaffolds for bone tissue engineering by cellulose nanocrystals reinforcing maleic anhydride grafted PLA. ACS Appl. Mater. Interfaces 2013, 5, 3847–3854. [Google Scholar] [CrossRef]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef] [Green Version]
- Vikingsson, L.; Gómez-Tejedor, J.A.; Ferrer, G.G.; Ribelles, J.G. An experimental fatigue study of a porous scaffold for the regeneration of articular cartilage. J. Biomech. 2015, 48, 1310–1317. [Google Scholar] [CrossRef] [Green Version]
- Na, S.; Zhang, H.; Huang, F.; Wang, W.; Ding, Y.; Li, D.; Jin, Y. Regeneration of dental pulp/dentine complex with a three-dimensional and scaffold-free stem-cell sheet-derived pellet. J. Tissue Eng. Regen. Med. 2016, 10, 261–270. [Google Scholar] [CrossRef]
- Lastra, M.L.; Molinuevo, M.S.; Blaszczyk-Lezak, I.; Mijangos, C.; Cortizo, M.S. Nanostructured fumarate copolymer-chitosan crosslinked scaffold: An in vitro osteochondrogenesis regeneration study. J. Biomed. Mater. Res. Part A 2018, 106, 570–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, M.; Halperin-Sternfeld, M.; Grinberg, I.; Adler-Abramovich, L. Injectable alginate-peptide composite hydrogel as a scaffold for bone tissue regeneration. Nanomaterials 2019, 9, 497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ElSheshtawy, A.; Nazzal, H.; El Shahawy, O.; El Baz, A.; Ismail, S.; Kang, J.; Ezzat, K. The effect of platelet-rich plasma as a scaffold in regeneration/revitalization endodontics of immature permanent teeth assessed using 2-dimensional radiographs and cone beam computed tomography: A randomized controlled trial. Int. Endod. J. 2020, 53, 905–921. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Lam, P.T.; Robinson, M.L.; Del Rio-Tsonis, K.; Saul, J.M. Design and Characterization of Biomimetic Kerateine Aerogel-Electrospun Polycaprolactone Scaffolds for Retinal Cell Culture. Ann. Biomed. Eng. 2021, 1–12. [Google Scholar] [CrossRef]
- Berthiaume, F.; Maguire, T.J.; Yarmush, M.L. Tissue engineering and regenerative medicine: History, progress, and challenges. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 403–430. [Google Scholar] [CrossRef]
- Liu, N.; Ye, X.; Yao, B.; Zhao, M.; Wu, P.; Liu, G.; Zhuang, D.; Jiang, H.; Chen, X.; He, Y. Advances in 3D bioprinting technology for cardiac tissue engineering and regeneration. Bioact. Mater. 2021, 6, 1388–1401. [Google Scholar] [CrossRef] [PubMed]
- Brehmer, B.; Rohrmann, D.; Becker, C.; Rau, G.; Jakse, G. Different types of scaffolds for reconstruction of the urinary tract by tissue engineering. Urol. Int. 2007, 78, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Atala, A. Bioengineered tissues for urogenital repair in children. Pediatric Res. 2008, 63, 569–575. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Dutt, D.; Kaur, P.; Singh, H.; Mishra, N.C. Microfibrous paper scaffold for tissue engineering application. J. Biomater. Sci. Polym. Ed. 2020, 31, 1091–1106. [Google Scholar] [CrossRef]
- Jahani, B.; Wang, X.; Brooks, A. Additive Manufacturing Techniques for Fabrication of Bone Scaffolds for Tissue Engineering Applications. Recent Prog. Mater. 2020, 2. [Google Scholar] [CrossRef]
- Iqbal, M.O.; Yahya, E.B. In vivo Assessment of Reversing Aminoglycoside Antibiotics Nephrotoxicity Using Jatropha mollissima crude extract. Tissue Cell 2021, 72, 101525. [Google Scholar] [CrossRef]
- Hellman, K. Tissue engineering: Translating science to product. Top. Tissue Eng. 2008, 4, 1–28. [Google Scholar]
- Mano, J.; Silva, G.; Azevedo, H.S.; Malafaya, P.; Sousa, R.; Silva, S.S.; Boesel, L.; Oliveira, J.M.; Santos, T.; Marques, A. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. R. Soc. Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yahya, E.B.; Jummaat, F.; Amirul, A.; Adnan, A.; Olaiya, N.; Abdullah, C.; Rizal, S.; Mohamad Haafiz, M.; Abdul Khalil, H.P.S. review on revolutionary natural biopolymer-based aerogels for antibacterial delivery. Antibiotics 2020, 9, 648. [Google Scholar] [CrossRef]
- George, A.; Sanjay, M.; Srisuk, R.; Parameswaranpillai, J.; Siengchin, S. A comprehensive review on chemical properties and applications of biopolymers and their composites. Int. J. Biol. Macromol. 2020, 154, 329–338. [Google Scholar] [CrossRef]
- García-González, C.A.; Budtova, T.; Durães, L.; Erkey, C.; Del Gaudio, P.; Gurikov, P.; Koebel, M.; Liebner, F.; Neagu, M.; Smirnova, I. An opinion paper on aerogels for biomedical and environmental applications. Molecules 2019, 24, 1815. [Google Scholar] [CrossRef] [Green Version]
- Rehm, B.H.; Moradali, M.F. Biopolymers for Biomedical and Biotechnological Applications; Wiley Online Library: Hoboken, NJ, USA, 2020. [Google Scholar]
- Jummaat, F.; Yahya, E.B.; Abdul Khalil, H.P.S.; Adnan, A.S.; Alqadhi, A.M.; Abdullah, C.K.; Atty Sofea, A.K.; Olaiya, N.G.; Abdat, M. The Role of Biopolymer-Based Materials in Obstetrics and Gynecology Applications: A Review. Polymers 2021, 13, 633. [Google Scholar] [CrossRef]
- Egorikhina, M.N.; Rubtsova, Y.P.; Charykova, I.N.; Bugrova, M.L.; Bronnikova, I.I.; Mukhina, P.A.; Sosnina, L.N.; Aleynik, D.Y. Biopolymer Hydrogel Scaffold as an Artificial Cell Niche for Mesenchymal Stem Cells. Polymers 2020, 12, 2550. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Ching, Y.C.; Chuah, C.H. Synthesis of chitosan aerogels as promising carriers for drug delivery: A review. Carbohydr. Polym. 2020, 231, 115744. [Google Scholar] [CrossRef]
- Rizal, S.; Lai, T.K.; Muksin, U.; Olaiya, N.; Abdullah, C.; Yahya, E.B.; Chong, E.; Abdul Khalil, H.P.S. Properties of Macroalgae Biopolymer Films Reinforcement with Polysaccharide Microfibre. Polymers 2020, 12, 2554. [Google Scholar] [CrossRef]
- Yahya, E.B.; Alqadhi, A.M. Recent trends in cancer therapy: A review on the current state of gene delivery. Life Sci. 2021, 269, 119087. [Google Scholar] [CrossRef] [PubMed]
- Von Recum, H.A. From Biocompatibility to Immune Engineering. Exp. Biol. Med. 2016, 241, 889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.; Alamry, K.A.; Asiri, A.M. Multifunctional Biopolymers-Based Composite Materials for Biomedical Applications: A Systematic Review. ChemistrySelect 2021, 6, 154–176. [Google Scholar] [CrossRef]
- Palaveniene, A.; Songailiene, K.; Baniukaitiene, O.; Tamburaci, S.; Kimna, C.; Tihminlioğlu, F.; Liesiene, J. The effect of biomimetic coating and cuttlebone microparticle reinforcement on the osteoconductive properties of cellulose-based scaffolds. Int. J. Biol. Macromol. 2020, 152, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Chollakup, R.; Uttayarat, P.; Chworos, A.; Smitthipong, W. Noncovalent Sericin-Chitosan Scaffold: Physical Properties and Low Cytotoxicity Effect. Int. J. Mol. Sci. 2020, 21, 775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Hou, Z.; Wang, J.; Wang, Z.; Li, X.; Liu, J.; Liang, Q.; Zhao, J. Assessment of biological properties of recombinant collagen-hyaluronic acid composite scaffolds. Int. J. Biol. Macromol. 2020, 149, 1275–1284. [Google Scholar] [CrossRef]
- Rodrigues, I.C.; Pereira, K.D.; Woigt, L.F.; Jardini, A.L.; Luchessi, A.D.; Lopes, É.S.; Webster, T.J.; Gabriel, L.P. A novel technique to produce tubular scaffolds based on collagen and elastin. Artif. Organs 2020, 45. [Google Scholar] [CrossRef]
- Lara-Cerón, J.A.; Jiménez-Pérez, V.M.; Molina-Paredes, A.A.; Ochoa, M.E.; Sábio, R.M.; Amaral, A.C.; da Silva, R.R.; Ribeiro, S.J.; Barud, H.d.S.; Muñoz-Flores, B.M. Ultrasound-assisted synthesis of organotin compounds and their application as luminescent dye in silk fibroin scaffolds. Inorg. Chim. Acta 2020, 505, 119490. [Google Scholar] [CrossRef]
- Aranci, K.; Uzun, M.; Su, S.; Cesur, S.; Ulag, S.; Amin, A.; Guncu, M.M.; Aksu, B.; Kolayli, S.; Ustundag, C.B. 3D Propolis-Sodium Alginate Scaffolds: Influence on Structural Parameters, Release Mechanisms, Cell Cytotoxicity and Antibacterial Activity. Molecules 2020, 25, 5082. [Google Scholar] [CrossRef]
- Negrini, N.C.; Celikkin, N.; Tarsini, P.; Farè, S.; Święszkowski, W. Three-dimensional printing of chemically crosslinked gelatin hydrogels for adipose tissue engineering. Biofabrication 2020, 12, 25001. [Google Scholar] [CrossRef]
- Ciarfaglia, N.; Pepe, A.; Piccirillo, G.; Laezza, A.; Daum, R.; Schenke-Layland, K.; Bochicchio, B. Nanocellulose and Elastin Act as Plasticizers of Electrospun Bioinspired Scaffolds. ACS Appl. Polym. Mater. 2020, 2, 4836–4847. [Google Scholar] [CrossRef]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Hyaluronic acid/corn silk extract based injectable nanocomposite: A biomimetic antibacterial scaffold for bone tissue regeneration. Mater. Sci. Eng. C 2020, 107, 110195. [Google Scholar] [CrossRef] [PubMed]
- Watchararot, T.; Prasongchean, W.; Thongnuek, P. Angiogenic property of silk fibroin scaffolds with adipose-derived stem cells on chick chorioallantoic membrane. R. Soc. Open Sci. 2021, 8, 201618. [Google Scholar] [CrossRef]
- Biswal, T. Biopolymers for tissue engineering applications: A review. Mater. Today Proc. 2020, 41. [Google Scholar] [CrossRef]
- Gupta, P.; Lorentz, K.L.; Haskett, D.G.; Cunnane, E.M.; Ramaswamy, A.K.; Weinbaum, J.S.; Vorp, D.A.; Mandal, B.B. Bioresorbable silk grafts for small diameter vascular tissue engineering applications: In vitro and in vivo functional analysis. Acta Biomater. 2020, 105, 146–158. [Google Scholar] [CrossRef]
- Tuzlakoglu, K.; Bolgen, N.; Salgado, A.; Gomes, M.E.; Piskin, E.; Reis, R. Nano-and micro-fiber combined scaffolds: A new architecture for bone tissue engineering. J. Mater. Sci. Mater. Med. 2005, 16, 1099–1104. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.J.; Kim, J.K. The effect of negative Poisson’s ratio polyurethane scaffolds for articular cartilage tissue engineering applications. Adv. Mater. Sci. Eng. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Ahmed, M.F.; Li, Y.; Zeng, C.; Li, Y. Vascular differentiation from pluripotent stem cells in 3-D auxetic scaffolds. J. Tissue Eng. Regen. Med. 2018, 12, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Ji, J.; Li, J.; Liu, W.; Wang, J.; Sun, Q.; Li, Q. Nanocellulose/PEGDA Aerogels with Tunable Poisson’s Ratio Fabricated by Stereolithography for Mouse Bone Marrow Mesenchymal Stem Cell Culture. Nanomaterials 2021, 11, 603. [Google Scholar] [CrossRef] [PubMed]
- Modo, M. Bioscaffold-induced brain tissue regeneration. Front. Neurosci. 2019, 13, 1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizal, S.; Saharudin, N.; Olaiya, N.; Abdul Khalil, H.P.S.; Haafiz, M.; Ikramullah, I.; Muksin, U.; Olaiya, F.G.; Abdullah, C.; Yahya, E.B. Functional Properties and Molecular Degradation of Schizostachyum Brachycladum Bamboo Cellulose Nanofibre in PLA-Chitosan Bionanocomposites. Molecules 2021, 26, 2008. [Google Scholar] [CrossRef]
- Mahumane, G.D.; Kumar, P.; du Toit, L.C.; Choonara, Y.E.; Pillay, V. 3D scaffolds for brain tissue regeneration: Architectural challenges. Biomater. Sci. 2018, 6, 2812–2837. [Google Scholar] [CrossRef] [PubMed]
- Ghafari, R.; Jonoobi, M.; Amirabad, L.M.; Oksman, K.; Taheri, A.R. Fabrication and characterization of novel bilayer scaffold from nanocellulose based aerogel for skin tissue engineering applications. Int. J. Biol. Macromol. 2019, 136, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Asim, N.; Badiei, M.; Alghoul, M.A.; Mohammad, M.; Fudholi, A.; Akhtaruzzaman, M.; Amin, N.; Sopian, K. Biomass and industrial wastes as resource materials for aerogel preparation: Opportunities, challenges, and research directions. Ind. Eng. Chem. Res. 2019, 58, 17621–17645. [Google Scholar] [CrossRef]
- Osorio, D.A.; Lee, B.E.; Kwiecien, J.M.; Wang, X.; Shahid, I.; Hurley, A.L.; Cranston, E.D.; Grandfield, K. Cross-linked cellulose nanocrystal aerogels as viable bone tissue scaffolds. Acta Biomater. 2019, 87, 152–165. [Google Scholar] [CrossRef]
- Goimil, L.; Santos-Rosales, V.; Delgado, A.; Evora, C.; Reyes, R.; Lozano-Perez, A.A.; Aznar-Cervantes, S.D.; Cenis, J.L.; Gómez-Amoza, J.L.; Concheiro, A. scCO2-foamed silk fibroin aerogel/poly (ε-caprolactone) scaffolds containing dexamethasone for bone regeneration. J. CO2 Util. 2019, 31, 51–64. [Google Scholar] [CrossRef]
- Gopinath, A.; Waclawczyk, T.; Bedi, R.; Babu, A.; Thomas, S.; Tom, P. Rapid Prototyping Methods in Manufacturing of Biomedical Implants: A Review. In 3D Printing in Biomedical Engineering; Springer: Singapore, 2020; pp. 187–208. [Google Scholar]
- Nguyen, T.T.T.; Chung, O.H.; Park, J.S. Coaxial electrospun poly (lactic acid)/chitosan (core/shell) composite nanofibers and their antibacterial activity. Carbohydr. Polym. 2011, 86, 1799–1806. [Google Scholar] [CrossRef]
- Movahedi, M.; Asefnejad, A.; Rafienia, M.; Khorasani, M.T. Potential of novel electrospun core-shell structured polyurethane/starch (hyaluronic acid) nanofibers for skin tissue engineering: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2020, 146, 627–637. [Google Scholar] [CrossRef]
- Sivashankari, P.; Prabaharan, M. Three-dimensional porous scaffolds based on agarose/chitosan/graphene oxide composite for tissue engineering. Int. J. Biol. Macromol. 2020, 146, 222–231. [Google Scholar] [CrossRef]
- Joshi, V.; Srivastava, C.M.; Gupta, A.P.; Vats, M. Electrospun Nano-architectures for Tissue Engineering and Regenerative Medicine. Nanoscience in Medicine; Springer: Berlin, Germany, 2020; Volume 1, pp. 213–248. [Google Scholar]
- Gheysari, H.; Mohandes, F.; Mazaheri, M.; Dolatyar, B.; Askari, M.; Simchi, A. Extraction of Hydroxyapatite Nanostructures from Marine Wastes for the Fabrication of Biopolymer-Based Porous Scaffolds. Mar. Drugs 2020, 18, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campodoni, E.; Heggset, E.B.; Rashad, A.; Ramírez-Rodríguez, G.B.; Mustafa, K.; Syverud, K.; Tampieri, A.; Sandri, M. Polymeric 3D scaffolds for tissue regeneration: Evaluation of biopolymer nanocomposite reinforced with cellulose nanofibrils. Mater. Sci. Eng. C 2019, 94, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Elomaa, L.; Keshi, E.; Sauer, I.M.; Weinhart, M. Development of GelMA/PCL and dECM/PCL resins for 3D printing of acellular in vitro tissue scaffolds by stereolithography. Mater. Sci. Eng. C 2020, 112, 110958. [Google Scholar] [CrossRef]
- Alonzo, M.; Primo, F.A.; Kumar, S.A.; Mudloff, J.A.; Dominguez, E.; Fregoso, G.; Ortiz, N.; Weiss, W.M.; Joddar, B. Bone tissue engineering techniques, advances and scaffolds for treatment of bone defects. Curr. Opin. Biomed. Eng. 2020, 17, 100248. [Google Scholar] [CrossRef]
- Annabi, N.; Fathi, A.; Mithieux, S.M.; Martens, P.; Weiss, A.S.; Dehghani, F. The effect of elastin on chondrocyte adhesion and proliferation on poly (ɛ-caprolactone)/elastin composites. Biomaterials 2011, 32, 1517–1525. [Google Scholar] [CrossRef]
- Salmoria, G.V.; Klauss, P.; Paggi, R.A.; Kanis, L.A.; Lago, A. Structure and mechanical properties of cellulose based scaffolds fabricated by selective laser sintering. Polym. Test. 2009, 28, 648–652. [Google Scholar] [CrossRef]
- Alemán-Domínguez, M.E.; Giusto, E.; Ortega, Z.; Tamaddon, M.; Benítez, A.N.; Liu, C. Three-dimensional printed polycaprolactone-microcrystalline cellulose scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 521–528. [Google Scholar] [CrossRef]
- Li, V.C.-F.; Dunn, C.K.; Zhang, Z.; Deng, Y.; Qi, H.J. Direct ink write (DIW) 3D printed cellulose nanocrystal aerogel structures. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Batista, M.; Gonçalves, V.S.; Gaspar, F.; Nogueira, I.; Matias, A.A.; Gurikov, P. Novel alginate-chitosan aerogel fibres for potential wound healing applications. Int. J. Biol. Macromol. 2020, 156, 773–782. [Google Scholar] [CrossRef] [Green Version]
- Abdul Khalili, H.P.S.; Khorasani, S.N.; Razavi, S.M.; Hashemibeni, B.; Tamayol, A. Nanofibrous scaffolds with biomimetic composition for skin regeneration. Appl. Biochem. Biotechnol. 2019, 187, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ul-Islam, M.; Ikram, M.; Islam, S.U.; Ullah, M.W.; Israr, M.; Jang, J.H.; Yoon, S.; Park, J.K. Preparation and structural characterization of surface modified microporous bacterial cellulose scaffolds: A potential material for skin regeneration applications in vitro and in vivo. Int. J. Biol. Macromol. 2018, 117, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Srivas, P.K.; Dadhich, P.; Das, B.; Maulik, D.; Dhara, S. Nano-/microfibrous cotton-wool-like 3D scaffold with core–shell architecture by emulsion electrospinning for skin tissue regeneration. ACS Biomater. Sci. Eng. 2017, 3, 3563–3575. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.S. Nanotechnologies for the Life Sciences; Wiley-VCH: Weinheim, Germany, 2005; Volume 1. [Google Scholar]
- Ghaee, A.; Bagheri-Khoulenjani, S.; Afshar, H.A.; Bogheiri, H. Biomimetic nanocomposite scaffolds based on surface modified PCL-nanofibers containing curcumin embedded in chitosan/gelatin for skin regeneration. Compos. Part B Eng. 2019, 177, 107339. [Google Scholar] [CrossRef]
- Sun, L.; Li, J.; Gao, W.; Shi, M.; Tang, F.; Fu, X.; Chen, X. Coaxial nanofibrous scaffolds mimicking the extracellular matrix transition in the wound healing process promoting skin regeneration through enhancing immunomodulation. J. Mater. Chem. B 2021, 9, 1395–1405. [Google Scholar] [CrossRef]
- Yang, W.; Xu, H.; Lan, Y.; Zhu, Q.; Liu, Y.; Huang, S.; Shi, S.; Hancharou, A.; Tang, B.; Guo, R. Preparation and characterisation of a novel silk fibroin/hyaluronic acid/sodium alginate scaffold for skin repair. Int. J. Biol. Macromol. 2019, 130, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. C 2018, 83, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Lien, S.-M.; Ko, L.-Y.; Huang, T.-J. Effect of pore size on ECM secretion and cell growth in gelatin scaffold for articular cartilage tissue engineering. Acta Biomater. 2009, 5, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Sun, M.; Hu, X.; Ren, B.; Cheng, J.; Li, C.; Duan, X.; Fu, X.; Zhang, J.; Chen, H. Structurally and functionally optimized silk-fibroin–gelatin scaffold using 3D printing to repair cartilage injury in vitro and in vivo. Adv. Mater. 2017, 29, 1701089. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Hägg, D.A.; Forsman, A.; Ekholm, J.; Nimkingratana, P.; Brantsing, C.; Kalogeropoulos, T.; Zaunz, S.; Concaro, S.; Brittberg, M. Cartilage tissue engineering by the 3D bioprinting of iPS cells in a nanocellulose/alginate bioink. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Saatchi, A.; Arani, A.R.; Moghanian, A.; Mozafari, M. Synthesis and characterization of electrospun cerium-doped bioactive glass/chitosan/polyethylene oxide composite scaffolds for tissue engineering applications. Ceram. Int. 2021, 47, 260–271. [Google Scholar] [CrossRef]
- Shokraei, S.; Mirzaei, E.; Shokraei, N.; Derakhshan, M.A.; Ghanbari, H.; Faridi-Majidi, R. Fabrication and characterization of chitosan/kefiran electrospun nanofibers for tissue engineering applications. J. Appl. Polym. Sci. 2021, 50547. [Google Scholar] [CrossRef]
- Chen, W.; Xu, Y.; Liu, Y.; Wang, Z.; Li, Y.; Jiang, G.; Mo, X.; Zhou, G. Three-dimensional printed electrospun fiber-based scaffold for cartilage regeneration. Mater. Des. 2019, 179, 107886. [Google Scholar] [CrossRef]
- Jordahl, J.H.; Solorio, L.; Sun, H.; Ramcharan, S.; Teeple, C.B.; Haley, H.R.; Lee, K.J.; Eyster, T.W.; Luker, G.D.; Krebsbach, P.H. 3D jet writing: Functional microtissues based on tessellated scaffold architectures. Adv. Mater. 2018, 30, 1707196. [Google Scholar] [CrossRef]
- Chen, W.; Chen, S.; Morsi, Y.; El-Hamshary, H.; El-Newhy, M.; Fan, C.; Mo, X. Superabsorbent 3D scaffold based on electrospun nanofibers for cartilage tissue engineering. ACS Appl. Mater. Interfaces 2016, 8, 24415–24425. [Google Scholar] [CrossRef]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.-h.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Muschler, G.F.; Nakamoto, C.; Griffith, L.G. Engineering principles of clinical cell-based tissue engineering. JBJS J. Bone Jt. Surg. Am. 2004, 86, 1541–1558. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Hao, N.; Bhagia, S.; Li, M.; Meng, X.; Pu, Y.; Yong, Q.; Ragauskas, A.J. Porous artificial bone scaffold synthesized from a facile in situ hydroxyapatite coating and crosslinking reaction of crystalline nanocellulose. Materialia 2018, 4, 237–246. [Google Scholar] [CrossRef]
- Wei, L.; Wu, S.; Kuss, M.; Jiang, X.; Sun, R.; Reid, P.; Qin, X.; Duan, B. 3D printing of silk fibroin-based hybrid scaffold treated with platelet rich plasma for bone tissue engineering. Bioactive Mater. 2019, 4, 256–260. [Google Scholar] [CrossRef]
- Jiang, T.; Nukavarapu, S.P.; Deng, M.; Jabbarzadeh, E.; Kofron, M.D.; Doty, S.B.; Abdel-Fattah, W.I.; Laurencin, C.T. Chitosan–poly (lactide-co-glycolide) microsphere-based scaffolds for bone tissue engineering: In vitro degradation and in vivo bone regeneration studies. Acta Biomater. 2010, 6, 3457–3470. [Google Scholar] [CrossRef] [PubMed]
- Dijkman, P.E.; Fioretta, E.S.; Frese, L.; Pasqualini, F.S.; Hoerstrup, S.P. Heart valve replacements with regenerative capacity. Transfus. Med. Hemotherapy 2016, 43, 282–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, D.; Waldman, S.D.; Li, S. From in vitro to in situ tissue engineering. Ann. Biomed. Eng. 2014, 42, 1537–1545. [Google Scholar] [CrossRef]
- Wang, X.; Ali, M.S.; Lacerda, C.M. A three-dimensional collagen-elastin scaffold for heart valve tissue engineering. Bioengineering 2018, 5, 69. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.-H.; Zhao, M.; Lin, Y.-R.; Tian, X.-D.; Wang, Y.-D.; Wang, Z.-X.; Wang, L.-X. Degradable chitosan-collagen composites seeded with cells as tissue engineered heart valves. Heart Lung Circ. 2017, 26, 94–100. [Google Scholar] [CrossRef]
- Jahnavi, S.; Saravanan, U.; Arthi, N.; Bhuvaneshwar, G.; Kumary, T.; Rajan, S.; Verma, R. Biological and mechanical evaluation of a Bio-Hybrid scaffold for autologous valve tissue engineering. Mater. Sci. Eng. C 2017, 73, 59–71. [Google Scholar] [CrossRef]
- Du, J.; Zhu, T.; Yu, H.; Zhu, J.; Sun, C.; Wang, J.; Chen, S.; Wang, J.; Guo, X. Potential applications of three-dimensional structure of silk fibroin/poly (ester-urethane) urea nanofibrous scaffold in heart valve tissue engineering. Appl. Surf. Sci. 2018, 447, 269–278. [Google Scholar] [CrossRef]
- Driessen-Mol, A.; Emmert, M.Y.; Dijkman, P.E.; Frese, L.; Sanders, B.; Weber, B.; Cesarovic, N.; Sidler, M.; Leenders, J.; Jenni, R. Transcatheter implantation of homologous “off-the-shelf” tissue-engineered heart valves with self-repair capacity: Long-term functionality and rapid in vivo remodeling in sheep. J. Am. Coll. Cardiol. 2014, 63, 1320–1329. [Google Scholar] [CrossRef]
- Weber, B.; Dijkman, P.E.; Scherman, J.; Sanders, B.; Emmert, M.Y.; Grünenfelder, J.; Verbeek, R.; Bracher, M.; Black, M.; Franz, T. Off-the-shelf human decellularized tissue-engineered heart valves in a non-human primate model. Biomaterials 2013, 34, 7269–7280. [Google Scholar] [CrossRef]
- Pillai, M.M.; Kumar, G.S.; Houshyar, S.; Padhye, R.; Bhattacharyya, A. Effect of nanocomposite coating and biomolecule functionalization on silk fibroin based conducting 3D braided scaffolds for peripheral nerve tissue engineering. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102131. [Google Scholar] [CrossRef] [PubMed]
- Arslantunali, D.; Dursun, T.; Yucel, D.; Hasirci, N.; Hasirci, V. Peripheral nerve conduits: Technology update. Med Devices 2014, 7, 405. [Google Scholar]
- Sun, W.; Gregory, D.A.; Tomeh, M.A.; Zhao, X. Silk fibroin as a functional biomaterial for tissue engineering. Int. J. Mol. Sci. 2021, 22, 1499. [Google Scholar] [CrossRef]
- Nishikawa, T.; Masuno, K.; Tominaga, K.; Koyama, Y.; Yamada, T.; Takakuda, K.; Kikuchi, M.; Tanaka, J.; Tanaka, A. Bone repair analysis in a novel biodegradable hydroxyapatite/collagen composite implanted in bone. Implant Dent. 2005, 14, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Morouço, P.; Lattanzi, W.; Alves, N. Four-dimensional bioprinting as a new era for tissue engineering and regenerative medicine. Front. Bioeng. Biotechnol. 2017, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, A.; Maxson, R.; Stoychev, G.; Gomillion, C.T.; Ionov, L. 4D biofabrication using shape-morphing hydrogels. Adv. Mater. 2017, 29, 1703443. [Google Scholar] [CrossRef] [PubMed]








| Scientist/s and Year | Type of Tissue | Scaffold Material | Remark | Ref |
|---|---|---|---|---|
| Indians in 600 BC. | Skin and cartilage. | Free gluteal fat. | Using secret cement for adhesion. | [45] |
| Brancas in 1442. | Nose cartilage. | Isograft. | The nose of slave to his master. | [46] |
| Boronio in 1804. | Skin substitute. | Autograft. | Auto-graft of full-thickness skin grafts on a sheep. | [47] |
| Bunger in 1823. | Skin tissues. | Autograft. | Skin is taken from the thigh for the repair of nasal defects. | [45] |
| Alexis Carrel in 1911. | Endothermal animal cells. | Thin layer of clotted plasma. | Recipient of Nobel Prize in Medicine for tissue culture. | [48] |
| Blakemore et al. in 1954. | Vascular graft. | Silk handkerchief and Vinyon. | The first prosthetic vascular graft implanted in a human patient. | [49] |
| Per Ingvar Brånemark in 1960s. | Bone tissue. | Titanium cylinder. | The establishment of the osseointegration concept. | [50] |
| W. T. Green in the 1970s. | Cartilage tissue. | Spicules of bone. | Seeding cells onto spicules of bone and implanting them in nude mice. | [51] |
| Vacanti et al. in 1988. | Different fetal and adult rat and mouse cells. | Polyanhydrides, polyglactin 910, and polyorthoester. | Successful transplantation of cells in synthetic biodegradable polymers. | [52] |
| Stone et al. in 1997. | Meniscal cartilage. | Collagen-based scaffold. | No adverse immunological reactions were reported. | [53] |
| Zein et al. in 2002. | Different human tissues. | Bioresorbable polymer. | Fused deposition modelling used for aerogel scaffold fabrication. | [54] |
| Svensson et al. in 2005. | Cartilage tissue. | Bacterial cellulose scaffold. | Concluded a high potential for this biopolymer in tissue regeneration. | [55] |
| Macchiarini et al. in 2008. | Engineered trachea. | Decellularized matrix of human donor trachea. | Removing all the antigens from donor trachea and seeding it with human stem cells. | [56] |
| Norotte et al. in 2009. | Various vascular cell types. | Direct bioprinting. | Fully biological self-assembly approach for tissue engineering. | [57] |
| Ahn et al. in 2010. | Skin tissue regeneration. | 3D collagen scaffolds. | The scaffold supported the migration and infiltration of cells. | [58] |
| Zhou et al. in 2013. | Bone tissue. | Bio-nanocomposite scaffolds. | Using the electrospun technique. | [59] |
| Inzana et al. in 2014. | Bone regeneration. | Calcium phosphate and collagen scaffolds | Using 3D printing technique to control the shape of scaffold. | [60] |
| Vikingsson et al. in 2015. | Articular cartilage regeneration. | Polycaprolactone-polyvinyl alcohol. | The composite scaffold possesses great potential for articular cartilage. | [61] |
| Na et al. in 2016. | Dental pulp regeneration. | 3D stem cell sheet-derived pellet. | Odontogenic stem cells used for designing 3D stem cell sheet-derived pellet. | [62] |
| Lastra et al. in 2018. | Osteochondrogenesis regeneration. | Copolymer chitosan crosslinked scaffold | The nanostructured scaffold was highly biocompatible and non-cytotoxic. | [63] |
| Ghosh et al. in 2019. | For bone repair and regeneration. | Injectable alginate–peptide scaffolds | The scaffold served as a biomaterial for bone regeneration. | [64] |
| ElSheshtawy et al. in 2020. | Endodontics regeneration. | Plateletrich plasma-based scaffold | Using 2D radiographs and cone-beam computed tomography. | [65] |
| Zeng et al. in 2021. | Retinal cell culture. | Polycaprolactone scaffolds. | Biomimetic kerateine aerogel electrospun scaffolds. | [66] |
| Biopolymeric Scaffold | Cell Type | Conclusion | Ref |
|---|---|---|---|
| 3D porous cellulose scaffolds. | Osteoblast-like MG-63 cells. | The scaffold did not show any cytotoxic effect. | [87] |
| Non-covalent sericin–chitosan scaffold. | Human dermal fibroblasts. | No cytotoxic effect for the scaffold was observed against the human skin cells. | [88] |
| Recombinant collagen/hyaluronic acid composite scaffolds. | Mouse fibroblasts cells (L929 cells). | No cytotoxicity and good biodegradability was observed. | [89] |
| Collagen- and elastin-based scaffolds. | Human umbilical vein endothelial cells. | The scaffolds were highly compatible and non-cytotoxic. | [90] |
| Silk fibroin-based scaffolds. | Human fibroblast cells (GM07492). | High cellular viability and seemed to be non-cytotoxic. | [91] |
| Propolis/sodium alginate scaffolds. | Human dermal fibroblasts (HFFF2). | The scaffolds were non-toxic at low concentrations. | [92] |
| Gelatin hydrogels tissue scaffold. | Human pre-adipocytes (3T3-L1). | The scaffolds showed no cytotoxic effects on the cells. | [93] |
| Nanocellulose- and elastin-based scaffolds. | Human fibroblast cells. | All the prepared scaffolds seemed to be non-cytotoxic and biocompatible. | [94] |
| Hyaluronic acid/corn silk extract scaffold. | Mesenchymal stem cells. | High cellular differentiation without any cytotoxic effect. | [95] |
| Salt leached silk fibroin-based scaffolds. | Human adipose stem cells. | The scaffolds were highly biocompatible and non-cytotoxic. | [96] |
| Technique | Principal | Ref |
|---|---|---|
| Electrospinning technique | Charged threads of biopolymeric solution or biopolymer melt are drawn using a special machine by high voltage electricity. | [114] |
| Solvent casting and practical leaching technique | Dissolving the polymeric powder in suitable solvents containing salt particles, which are then evaporated with the salts leaching out. | [115] |
| Freeze-drying technique | Freezing the dissolved polymer hydrogel and drying it under the vacuum to maintain the structural integrity of the hydrogel. | [116] |
| Stereolithography technique | Computer-aided technique prints photosensitive liquid of biopolymer layer-by-layer using an ultraviolet laser. | [117] |
| Injection molding technique | Melting and injecting the biopolymeric material into a mold, after which it cools and solidifies. | [118] |
| Gas foaming technique | Dissolving the biopolymer in organic solvents and then inserting gases used to pressurize the modelled until it is full of gas bubbles. | [119] |
| Selective laser sintering technique | The biopolymeric solution is printed by selective laser, which sinters the material in thin layers leading to 3D scaffold printing. | [120] |
| Fused deposition modelling technique | Deposition of biopolymeric materials extruded layer-by-layer through a special nozzle to form 3D multiple layers scaffolds. | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yahya, E.B.; Amirul, A.A.; H.P.S., A.K.; Olaiya, N.G.; Iqbal, M.O.; Jummaat, F.; A.K., A.S.; Adnan, A.S. Insights into the Role of Biopolymer Aerogel Scaffolds in Tissue Engineering and Regenerative Medicine. Polymers 2021, 13, 1612. https://doi.org/10.3390/polym13101612
Yahya EB, Amirul AA, H.P.S. AK, Olaiya NG, Iqbal MO, Jummaat F, A.K. AS, Adnan AS. Insights into the Role of Biopolymer Aerogel Scaffolds in Tissue Engineering and Regenerative Medicine. Polymers. 2021; 13(10):1612. https://doi.org/10.3390/polym13101612
Chicago/Turabian StyleYahya, Esam Bashir, A. A. Amirul, Abdul Khalil H.P.S., Niyi Gideon Olaiya, Muhammad Omer Iqbal, Fauziah Jummaat, Atty Sofea A.K., and A. S. Adnan. 2021. "Insights into the Role of Biopolymer Aerogel Scaffolds in Tissue Engineering and Regenerative Medicine" Polymers 13, no. 10: 1612. https://doi.org/10.3390/polym13101612
APA StyleYahya, E. B., Amirul, A. A., H.P.S., A. K., Olaiya, N. G., Iqbal, M. O., Jummaat, F., A.K., A. S., & Adnan, A. S. (2021). Insights into the Role of Biopolymer Aerogel Scaffolds in Tissue Engineering and Regenerative Medicine. Polymers, 13(10), 1612. https://doi.org/10.3390/polym13101612