Preparation of Porous Hydroxyapatite Using Cetyl Trimethyl Ammonium Bromide as Surfactant for the Removal of Lead Ions from Aquatic Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Synthesis
2.3. Characterization of HAp-CTAB Composite
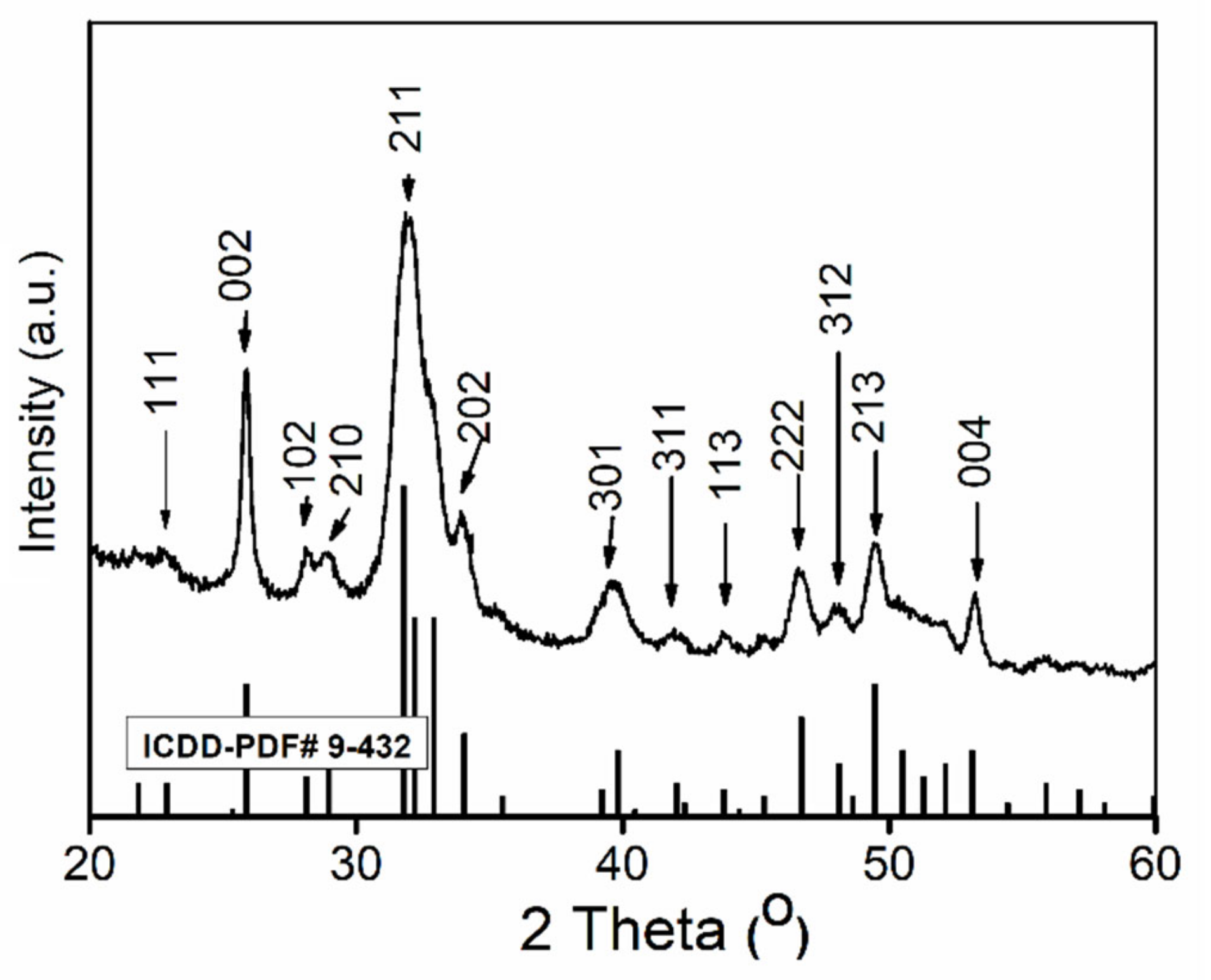
2.3.1. X-ray Diffraction (XRD)
2.3.2. Fourier Transform Infrared Spectroscopy (FT-IR)
2.3.3. Transmission Electron Microscopy (TEM)
2.3.4. Scanning Electron Microscopy (SEM)
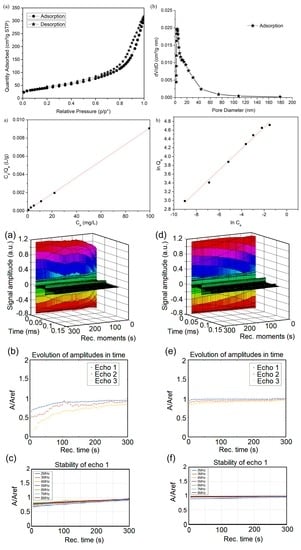
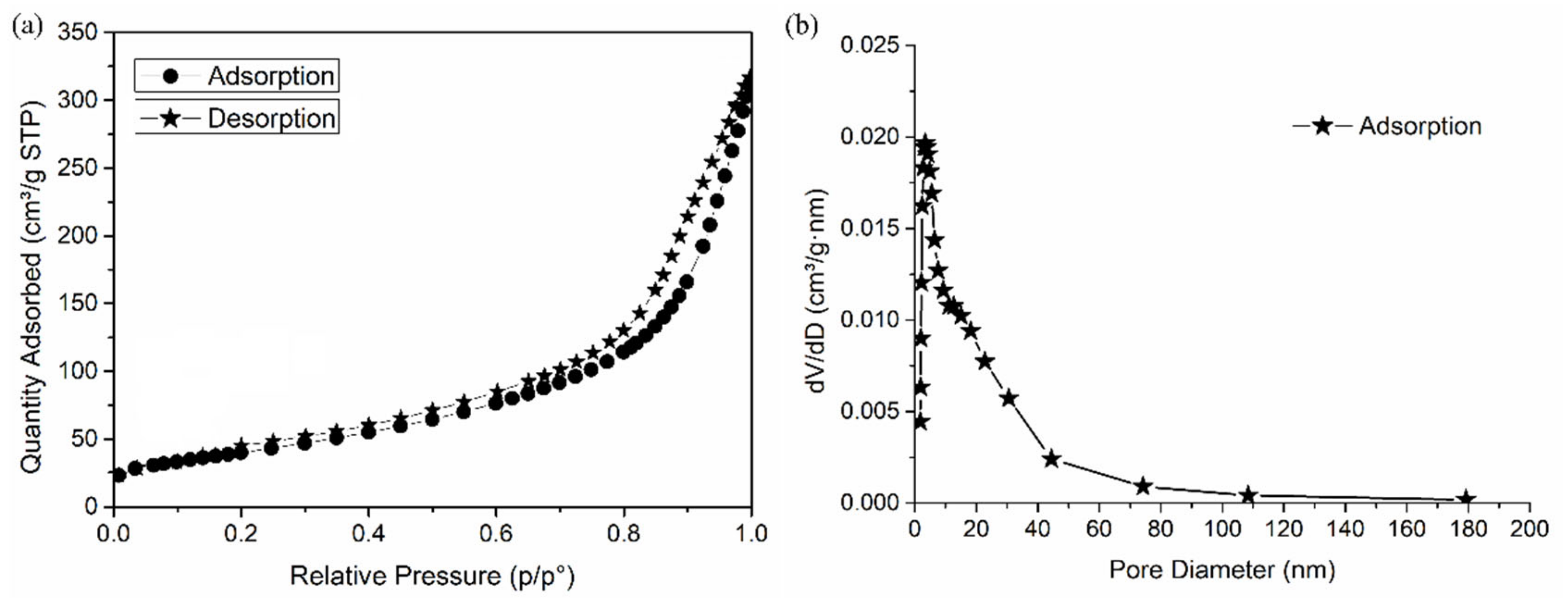
2.3.5. N2 Adsorption/Desorption Analysis
2.4. Evaluation of the Efficiency of HAp-CTAB Composite in Decontamination
2.4.1. Non-Destructive Ultrasound Studies
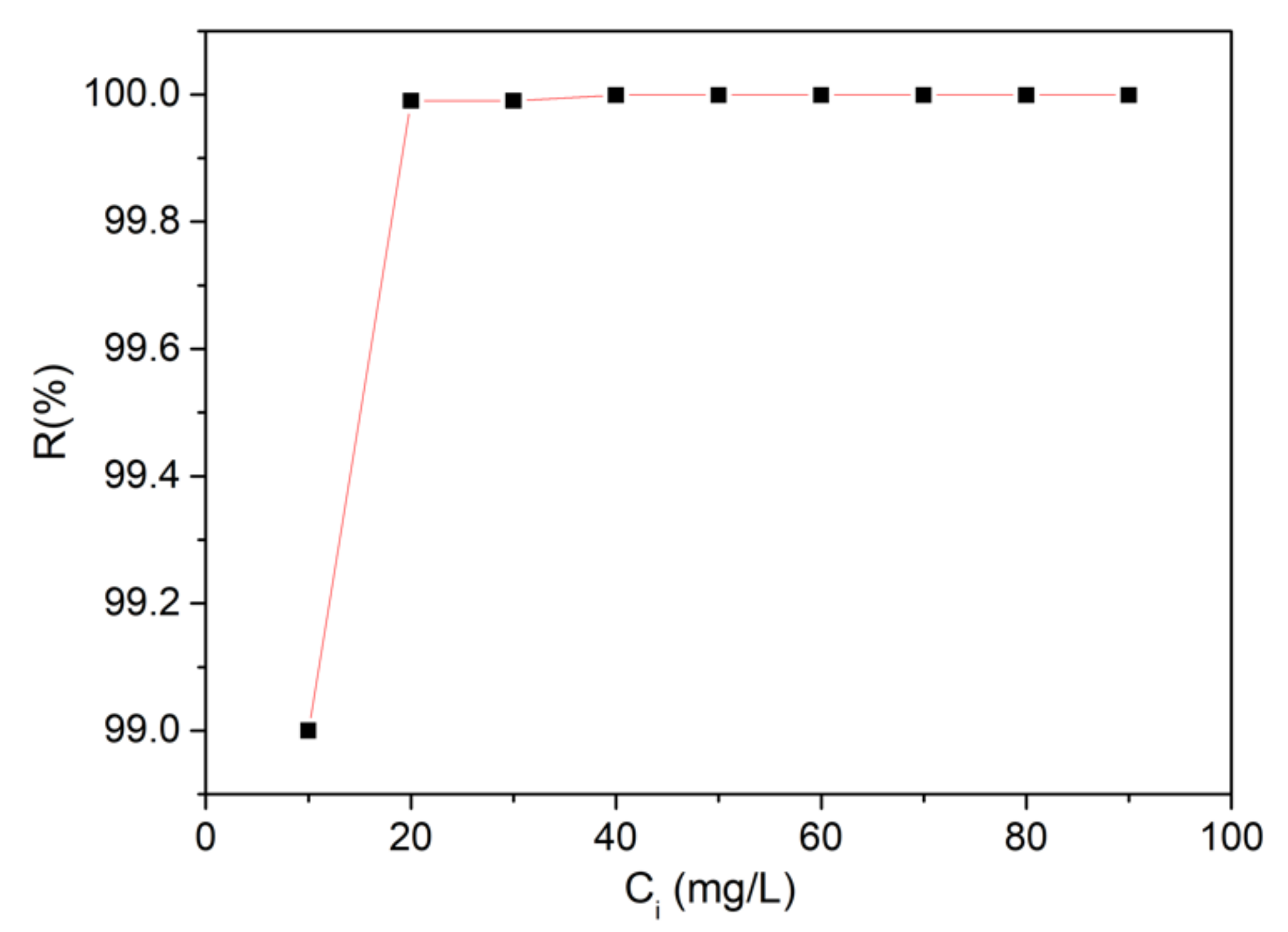
2.4.2. Batch Adsorption Experiments
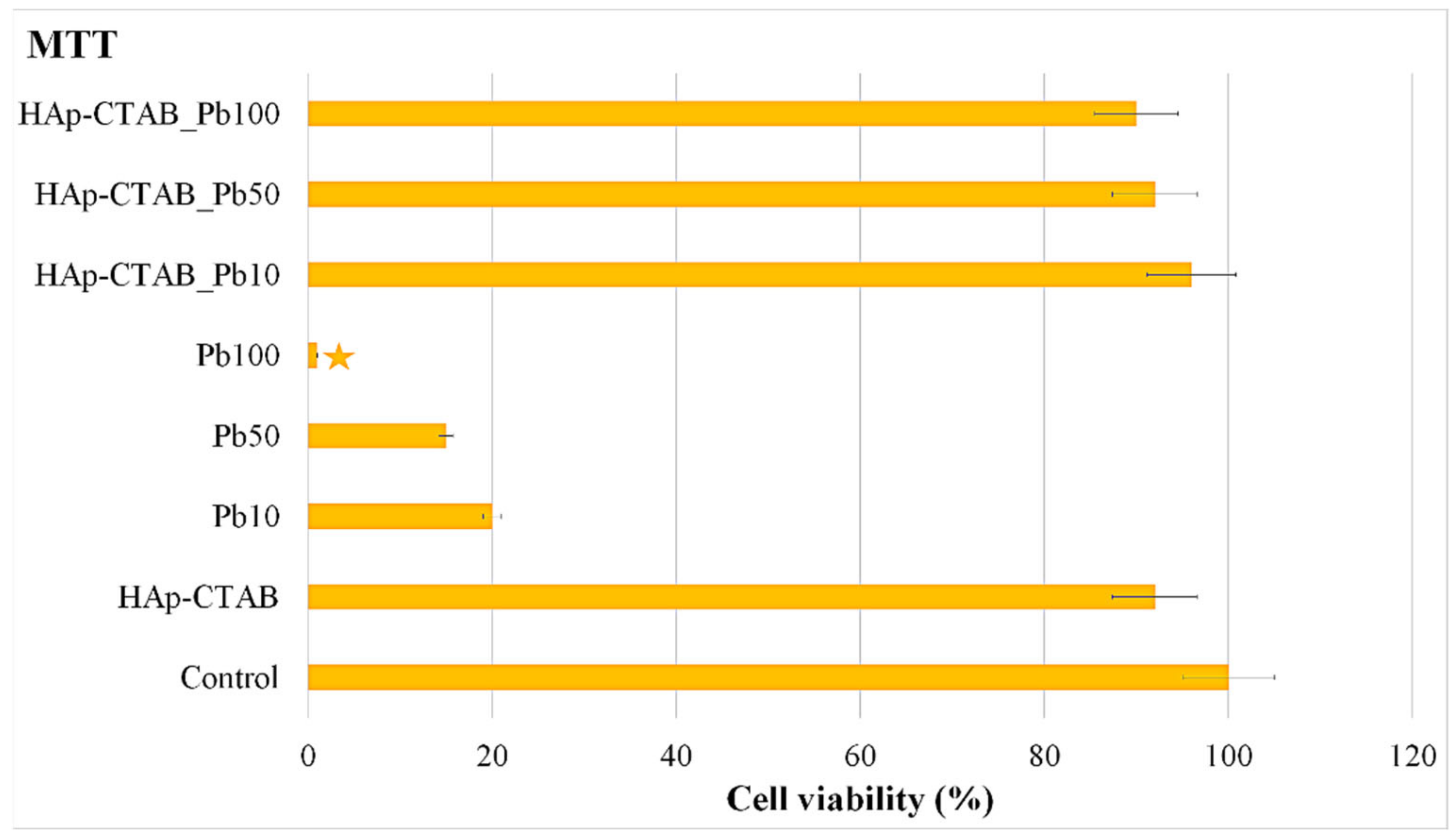
2.5. Biological Investigation
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dexiang, L.; Zheng, W.; Li, X.; Yang, Q.; Yue, X.; Guo, L.; Zeng, G. Removal of lead(II) from aqueous solutions using carbonate hydroxyapatite extracted from eggshell waste. J. Hazard. Mater 2010, 177, 126–130. [Google Scholar]
- Jang, S.H.; Min, B.G.; Jeong, Y.G.; Lyoo, W.S.; Lee, S.C. Removal of lead ions in aqueous solution by hydroxyapatite/polyurethane composite foams. J. Hazard. Mater. 2008, 152, 1285–1292. [Google Scholar] [CrossRef]
- Ghita, R.V.; Iconaru, S.L.; Popa, C.L.; Costescu, A.; Le Coustumer, P.; Motelica-Heino, M.; Ciobanu, C.S.J. Sm: HAp nanopowders present antibacterial activity against enterococcus faecalis. Nanomaterials 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Günay, A.; Arslankaya, A.; Tosun, I. Lead removal from aqueous solution by natural and pretreated clinoptilolite: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2007, 146, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Patrinoiu, G.; Calderón-Moreno, J.M.; Chifiriuc, C.M.; Saviuc, C.; Birjega, R.; Carp, O. Tunable ZnO spheres with high anti-biofilm and antibacterial activity via a simple green hydrothermall. J. Colloid. Interface. Sci. 2016, 462, 64–74. [Google Scholar] [CrossRef]
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Luo, J.; Yu, D.; Hristovski, K.D.; Fu, K.; Shen, Y.; Westerhoff, P.; Crittenden, J.C. Critical Review of Advances in Engineering Nanomaterial Adsorbents for Metal Removal and Recovery from Water: Mechanism Identification and Engineering Design. Environ. Sci. Technol. 2021, 55, 4287–4304. [Google Scholar] [CrossRef]
- Luo, J.; Fu, K.; Yu, D.; Hristovski, K.D.; Westerhoff, P.; Crittenden, J.C. Review of Advances in Engineering Nanomaterial Adsorbents for Metal Removal and Recovery from Water: Synthesis and Microstructure Impacts. ACS EST Engg. 2021, 1, 623–661. [Google Scholar] [CrossRef]
- Ma, Q.Y.; Traina, S.J.; Logan, T.J.; Ryan, J. In situ lead immobilization by apatite. Environ. Sci. Technol. 1993, 27, 1803–1810. [Google Scholar] [CrossRef]
- Zhang, P.; Ryan, J.A.; Yang, J. In Vitro Soil Pb Solubility in the Presence of Hydroxyapatite. Environ. Sci. Technol. 1998, 32, 2763–2768. [Google Scholar] [CrossRef]
- Mihai, M.M.; Holban, A.M.; Giurcăneanu, C.; Popa, L.G.; Buzea, M.; Filipov, M.; Lazăr, V.; Chifiriuc, M.C.; Popa, M.I. Identification and phenotypic characterization of the most frequent bacterial etiologies in chronic skin ulcers. Rom. J. Morphol. Embryol 2014, 55, 1401–1408. [Google Scholar]
- Choi, S.; Jeong, Y. The Removal of Heavy Metals in Aqueous Solution by Hydroxyapatite/Cellulose Composite. Fibers Polym. 2008, 9, 267–270. [Google Scholar] [CrossRef]
- Yu, D.; Wang, Y.; Wu, M.; Zhang, L.; Wang, L.; Ni, H. Surface functionalization of cellulose with hyperbranched polyamide for efficient adsorption of organic dyes and heavy metals. J. Clean. Prod. 2019, 232, 774–783. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Iconaru, S.L.; Popa, C.L.; Costescu, A.; Motelica-Heino, M.; Predoi, D. Porous Methyltrimethoxysilane Coated Nanoscale-Hydroxyapatite for Removing Lead Ions from Aqueous Solutions. J. Nanomater. 2014, 2014, 361061. [Google Scholar] [CrossRef]
- García, C.; García, C.; Paucar, C. Controlling morphology of hydroxyapatite nanoparticles through hydrothermal microemulsion chemical synthesis. Inorg. Chem. Commun. 2012, 20, 90–92. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, D.; Wang, G. A simple wet chemical synthesis and characterization of hydroxyapatite nanorods. Mater. Chem. Phys. 2004, 86, 69–73. [Google Scholar] [CrossRef]
- Shih, W.-J.; Wang, M.-C.; Hon, M.-H. Morphology and crystallinity of the nanosized hydroxyapatite synthesized by hydrolysis using cetyltrimethylammonium bromide (CTAB) as a surfactant. J. Cryst. Growth 2005, 275, e2339–e2344. [Google Scholar] [CrossRef]
- Venditti, F.; Ceglie, A.; Palazzo, G.; Colafemmina, G.; Lopez, F. Removal of chromate from water by a new CTAB–silica gelatin composite. J. Colloid Interface Sci. 2007, 310, 353–361. [Google Scholar] [CrossRef]
- Ercal, N.; Gurer-Orhan, H.; Aykin-Burns, N. Toxic Metals and Oxidative Stress Part I: Mechanisms Involved in Metal induced Oxidative Damage. Curr. Top. Med. Chem. 2001, 1, 529–539. [Google Scholar] [CrossRef]
- Gurer, H.; Ercal, N. Can antioxidants be beneficial in the treatment of lead poisoning. Free Radic. Biol. Med. 2000, 29, 927–945. [Google Scholar] [CrossRef]
- Yiin, S.J.; Lin, T.H. Lead-catalyzed peroxidation ofessential unsaturated fatty acid. Biol. Trace Elem. Res. 1995, 50, 167–172. [Google Scholar] [CrossRef]
- Patterson, A. The Scherrer formula for x-ray particle size determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances 0.1. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V. Dextran-coated zinc-doped hydroxyapatite for biomedical applications. Polymers 2019, 11, 886. [Google Scholar] [CrossRef]
- Ncibi, M.C. Applicability of some statistical tools to predict optimum adsorption isotherm after linear and non-linear regression analysis. J. Hazard. Mater. 2008, 153, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Mckay, G.; Porter, J.F. Adsorption isotherm models for basic dyea dsorption by peat in single and binary component systems. J. Colloid Interface Sci. 2004, 280, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Ho, Y.S.; Huang, C.T.; Huang, H.W. Equilibrium sorption isotherm for metal ions on tree fern. Process. Biochem. 2002, 37, 1421–1430. [Google Scholar] [CrossRef]
- Meroufel, B.; Benali, O.; Benyahia, M.; Benmoussa, Y.; Zenasni, M.A. Adsorptive removal of anionic dye from aqueous solutions by Algerian kaolin: Characteristics, isotherm, kinetic and thermodynamic studies. J. Mater. Environ. Sci. 2013, 44, 482–491. [Google Scholar]
- Predoi, D.; Vatasescu-Balcan, R.A. Osteoblast interaction with iron oxide nanoparticles coated with dextrin in cell culture. J. Optoelectron. Adv. Mater. 2008, 10, 152–157. [Google Scholar]
- Prodan, A.M.; Beuran, M.; Turculet, C.S.; Popa, M.; Andronescu, E.; Bleotu, C.; Raita, S.M.; Soare, M.; Lupescu, O. In vitro evaluation of glycerol coated iron oxide nanoparticles in solution. Rom. Biotechnol. Lett. 2018, 23, 13901–13908. [Google Scholar]
- Iconaru, S.L.; Turculet, C.; Le Coustumer, P.; Bleotu, C.; Chifiriuc, M.C.; Lazar, V.; Surugiu, A.; Badea, M.; Iordache, F.M.; Soare, M.; et al. Biological studies on dextrin coated iron oxide nanoparticles. Rom. Rep. Phys. 2016, 6868, 1536–1544. [Google Scholar]
- Prodan, A.M.; Iconaru, S.L.; Chifiriuc, C.M.; Bleotu, C.; Ciobanu, S.C.; Motelica-Heino, M.; Sizaret, S.; Predoi, D. Magnetic Properties and Biological Activity Evaluation of Iron Oxide Nanoparticles. J. Nanomater. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Yao, J.; Tjandra, W.; Chen, Y.Z.; Tam, K.C.; Ma, J.; Soh, B. Hydroxyapatite Nanostructure Material Derived Using Cationic Surfactant as a Template. J. Mater. Chem. 2003, 13, 3053–3057. [Google Scholar] [CrossRef]
- Kay, M.I.; Young, R.A.; Posner, A.S. Crystal Structure of Hydroxyapatite. Nature 1964, 204, 1050–1052. [Google Scholar] [CrossRef]
- Fowler, B.O. Infrared Studies of Apatites. I. Vibrational Assignments for Calcium, Strontium, and Barium Hydroxyapatites Utilizing Isotopic Substitution. Inorg. Chem. 1974, 13, 194–207. [Google Scholar] [CrossRef]
- Salimi, E.; Javadpour, J.; Anbia, M. Template-Based Synthesis of Nanoporous Hydroxyapatite. ISRN Ceram. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Costescu, A.; Ciobanu, C.S.; Iconaru, S.L.; Ghita, R.V.; Chifiriuc, M.C.; Marutescu, L.G.; Predoi, D. Fabrication, characterization, and antimicrobial activity, evaluation of low silver concentrations in silver-doped hydroxyapatite nanoparticles. J. Nanomater. 2013, 2013, 5. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Motelica-Heino, M.; Buton, N.; Megier, C. Obtaining and Characterizing Thin Layers of Magnesium Doped Hydroxyapatite by Dip Coating Procedure. Coatings 2020, 10, 510. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V. Fabrication of Silver- and Zinc-Doped Hydroxyapatite Coatings for Enhancing Antimicrobial Effect. Coatings 2020, 10, 905. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption ofgases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Silvestre-Albero, J.; Silvestre-Albero, A.M.; Llewellyn, P.L.; Rodríguez-Reinoso, F. High-resolution N2 adsorption isotherms at77.4 K: Critical effect of the He used during calibration. J. Phys. Chem. C 2013, 117, 16885–16889. [Google Scholar] [CrossRef]
- Sambudi, N.S.; Cho, S.; Cho, K. Porous hollow hydroxyapatite microspheres synthesized by spray pyrolysis using a microalgatemplate: Preparation, drug delivery, and bioactivity. RSC Adv. 2016, 6, 43041–43048. [Google Scholar] [CrossRef]
- Uota, M.; Arakawa, H.; Kitamura, N.; Yoshimura, T.; Tanaka, J.; Kijima, T. Synthesis of high surface area hydroxyapatite nanoparticles by mixed surfactant-mediated approach. Langmuir 2005, 21, 4724–4728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, M.; Chen, W.; Zhu, S.; Liu, N.; Zhu, L. Immobilization of lead and cadmium from aqueous solution and contaminated sediment using nano-hydroxyapatite. Environ. Pollut. 2010, 158, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, M.R.; Meenakshi, G.N.K.S. Removal of copper (II) using chitin/chitosan nano-hydroxyapatite composite. Int. J. Biol. Macromol. 2011, 48, 119–124. [Google Scholar] [CrossRef]
- Vila, M.; Sánchez-Salcedo, S.; Vallet-Regí, M. Hydroxyapatite foams for the immobilization of heavy metals: From waters to the human body. Inorg. Chim. Acta 2012, 393. [Google Scholar] [CrossRef]
- Hafsteinsdóttir, E.G.; Camenzuli, D.; Rocavert, A.L.; Walworth, J.; Gore, D.B. Chemical immobilization of metals and metalloids by phosphates. Appl. Geochem. 2015, 59, 47–62. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Hanafusa, T.; Yamashita, J.; Yamamoto, Y.; Ono, T. Adsorption and removal of strontium in aqueous solution by synthetic hydroxyapatite. J. Radioanal. Nucl. Chem. 2016, 307, 1279–1285. [Google Scholar] [CrossRef]
- Malek, A.; Farooq, S. Comparison of isotherm models for hydrocarbon adsorption on activated carbon. AIChE J. 1996, 42, 3191–3201. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Bulut, E.; Ozacar, M.; Sengil, I.A. Adsorption of malachite green onto bentonite: Equilibrium and kinetic studies and process design. Micropor. Mesopor. Mater. 2008, 115, 234–246. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Padmesh, T.V.N.; Palanivelu, K.; Velan, M. Biosorption of nickel (II) ions onto Sargassum wightii: Application of two-parameterand three parameter isotherm models. J. Hazard. Mater. 2006, B133, 304–308. [Google Scholar] [CrossRef]
- Kundu, S.; Gupta, A.K. Arsenic adsorption onto iron oxide-coated cement (IOCC): Regression analysis of equilibrium data with several isotherm models and their optimization. Chem. Eng. J. 2006, 122, 93–106. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Motelica-Heino, M.; Guegan, R.; Beuran, M.; Costescu, A.; Predoi, D. Adsorption of Pb (II) Ions onto Hydroxyapatite Nanopowders in Aqueous Solutions. Materials 2018, 11, 2204. [Google Scholar] [CrossRef]
- Predoi, D.; Predoi, M.V.; Iconaru, S.L.; Ech Cherif El Kettani, M.; Leduc, D.; Prodan, A.M. Ultrasonic Measurements on β Cyclodextrin/Hydroxyapatite Composites for Potential Water Depollution. Materials 2017, 10, 681. [Google Scholar] [CrossRef]
- Lee, C.-K.; Kim, H.-S.; Kwon, J.-H. The removal of heavy metals using hydroxyapatite. Environ Eng. Res. 2005, 10, 205–212. [Google Scholar] [CrossRef]
- Ghita, R.V.; Iconaru, S.L.; Popa, C.L.; Costescu, A.; Le Coustumer, P.; Motelica-Heino, M.; Ciobanu, C.S. Tetraethyl Orthosilicate Coated Hydroxyapatite Powders for Lead Ions Removal from Aqueous Solutions. J. Nanomater. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Ho, Y.S. Selection of optimum sorption isotherm. Carbon 2004, 42, 2115–2116. [Google Scholar] [CrossRef]
- He, J.; Hong, S.; Zhang, L.; Gan, F.; Ho, Y.-S. Equilibrium and Thermodynamic parameters of adsorption of merhylene blue onto rectorite. Fresen. Environ. Bull. 2010, 19, 2651–2656. [Google Scholar]
- Minh, D.P.; Tran, N.D.; Nzihou, A.; Sharrock, P. Hydroxyapatite gel for the improved removal of Pb2+ ions from aqueous. Solution Chem. Eng. J. 2013, 232, 128–138. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces, 6th ed.; Wiley-Interscience: New York, NY, USA, 1997. [Google Scholar]
- Voudrias, E.; Ytianos, F.; Bozani, E. Sorption Description isotherms of Dyes from aqueous solutions and Waste Waters with Different Sorbent materials. Global Nest. Int. J. 2002, 4, 75–83. [Google Scholar]
- Mohan, S.; Karthikeyan, J. Removal of lignin and tannin color from aqueous solution by adsorption on to activated carbon solution by adsorption on to activated charcoal. Environ. Pollut. 1997, 97, 183–187. [Google Scholar] [CrossRef]
- Khayyun, T.S.; Mseer, A.S. Comparison of the experimental results with the Langmuir and Freundlich models for copper removal on limestone adsorbent. Appl. Water Sci. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Urick, R.J. The absorption of sound in suspensions of irregular particles. J. Acoust. Soc. Am. 1948, 20, 283–289. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Motelica-Heino, M.; Guegan, R.; Predoi, M.V.; Prodan, A.M.; Predoi, D. Removal of Zinc Ions Using Hydroxyapatite and Study of Ultrasound Behavior of Aqueous Media. Materials 2018, 11, 1350. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Motelica-Heino, M. Removal and Oxidation of As(III) from Water Using Iron Oxide Coated CTAB as Adsorbent. Polymers 2020, 12, 1687. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Foxx, D.N.; Ishaque, A.B.; Shen, E. Lead-induced cytotoxicity and transcriptional activation of stress genes in human liver carcinoma (HepG2) cells. Mol. Cell. Biochem. 2004, 255, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Lead; Public Health Service, U.S. Department of Health and Human Services: Atlanta, GA, USA, 1999. [Google Scholar]
- Pirkle, J.L.; Kaufmann, R.B.; Brody, D.J.; Hickman, T.; Gunter, E.W.; Paschal, D.C. Exposure of the U.S. population to lead, 1991–1994. Environ. Health Perspect. 1998, 106, 745–750. [Google Scholar] [CrossRef]
- Silbergeld, E.K. Facilitative mechanisms of lead as a carcinogen. Mutat. Res. 2003, 533, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, A.; Schlepegrell, R.; Beyersmann, D. Indirect mechanism of lead-induced genotoxicity in cultured mammalian cells. Mutat. Res. 1990, 241, 75–82. [Google Scholar] [CrossRef]
- Frenkel, G.D.; Middleton, C. Effects of lead acetate on DNA and RNA synthesis by intact HeLa cells, isolated nuclei and purified polymerases. Biochem. Pharmacol. 1987, 36, 265–268. [Google Scholar] [CrossRef]
- Pounds, J.G.; Long, G.J.; Rosent, J.F. Cellular and Molecular Toxicity of Lead in Bone. Environ. Health. Perspect. 1991, 91, 17–32. [Google Scholar] [CrossRef] [PubMed]



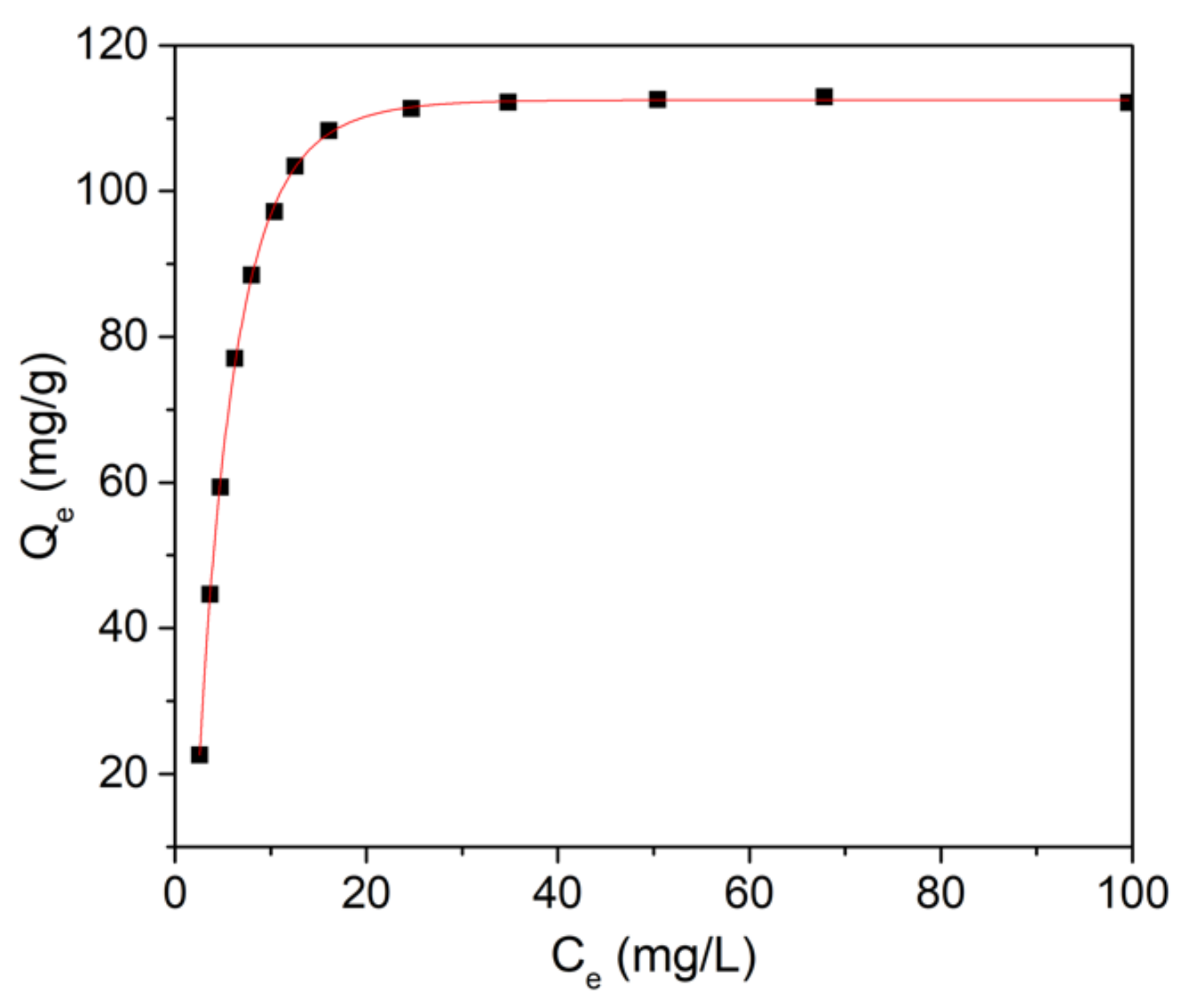




| Sample | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| HAp-CTAB | R2 | qm (mg/g) | KL (L/mg) | R2 | n | kf |
| 0.999 | 110.5 | 166.49 | 0.993 | 4.11 | 172.25 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Predoi, S.-A.; Ciobanu, C.S.; Motelica-Heino, M.; Chifiriuc, M.C.; Badea, M.L.; Iconaru, S.L. Preparation of Porous Hydroxyapatite Using Cetyl Trimethyl Ammonium Bromide as Surfactant for the Removal of Lead Ions from Aquatic Solutions. Polymers 2021, 13, 1617. https://doi.org/10.3390/polym13101617
Predoi S-A, Ciobanu CS, Motelica-Heino M, Chifiriuc MC, Badea ML, Iconaru SL. Preparation of Porous Hydroxyapatite Using Cetyl Trimethyl Ammonium Bromide as Surfactant for the Removal of Lead Ions from Aquatic Solutions. Polymers. 2021; 13(10):1617. https://doi.org/10.3390/polym13101617
Chicago/Turabian StylePredoi, Silviu-Adrian, Carmen Steluta Ciobanu, Mikael Motelica-Heino, Mariana Carmen Chifiriuc, Monica Luminita Badea, and Simona Liliana Iconaru. 2021. "Preparation of Porous Hydroxyapatite Using Cetyl Trimethyl Ammonium Bromide as Surfactant for the Removal of Lead Ions from Aquatic Solutions" Polymers 13, no. 10: 1617. https://doi.org/10.3390/polym13101617
APA StylePredoi, S.-A., Ciobanu, C. S., Motelica-Heino, M., Chifiriuc, M. C., Badea, M. L., & Iconaru, S. L. (2021). Preparation of Porous Hydroxyapatite Using Cetyl Trimethyl Ammonium Bromide as Surfactant for the Removal of Lead Ions from Aquatic Solutions. Polymers, 13(10), 1617. https://doi.org/10.3390/polym13101617