Interactions in Electrodeposited Poly-3,4-Ethylenedioxythiophene—Tungsten Oxide Composite Films Studied with Spectroelectrochemistry
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Morphology
3.2. Cyclic Voltammetry
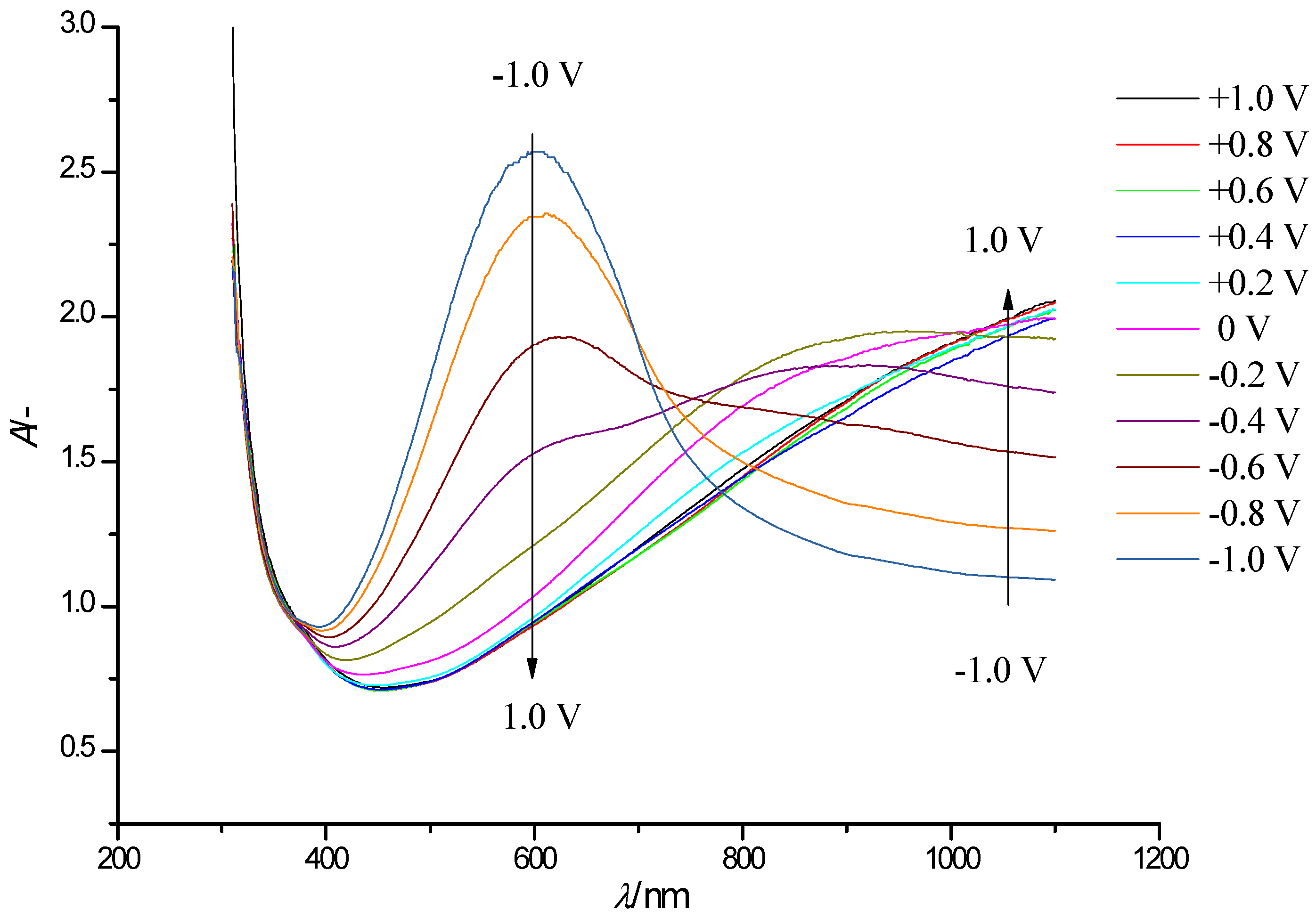
3.3. Spectroelectrochemistry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Deb, S.K. Opportunities and challenges in science and technology of WO3 for electrochromic and related applications. Sol. Energy Mater. Sol. Cells 2008, 92, 245–258. [Google Scholar] [CrossRef]
- Holze, R. Metal oxide/conducting polymers based hybrid materials designed for application in supercapacitors. In Metal Oxides in Supercapacitors; Dubal, D.P., Gomez-Romero, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 219–245. [Google Scholar]
- Meng, Q.; Cai, K.; Chen, Y.; Chen, L. Research progress on conducting polymer based supercapacitor electrode materials. Nano Energy 2017, 36, 268–285. [Google Scholar] [CrossRef]
- Fu, L.; Qu, Q.; Holze, R.; Kondratiev, V.V.; Wu, Y. Composites of metal oxides and intrinsically conducting polymers as supercapacitor electrode materials: The best of both worlds? J. Mater. Chem. A 2019, 7, 14937–14970. [Google Scholar] [CrossRef]
- Sadakane, M.; Sasaki, K.; Kunioku, H.; Ohtani, B.; Ueda, W.; Abe, R. Preparation of nano-structured crystalline tungsten(VI) oxide and enhanced photocatalytic activity for decomposition of organic compounds under visible light irradiation. Chem. Commun. 2008, 6552–6554. [Google Scholar] [CrossRef] [PubMed]
- Granqvist, C.G. Electrochromic tungsten oxide films: Review of progress 1993–1998. Sol. Energy Mater. Sol. Cells 2000, 60, 201–262. [Google Scholar] [CrossRef]
- Granqvist, C.G.; Avendaño, E.; Azens, A. Electrochromic coatings and devices: Survey of some recent advances. Thin Solid Films 2003, 442, 201–211. [Google Scholar] [CrossRef]
- Yang, P.; Sun, P.; Du, L.; Liang, Z.; Xie, W.; Cai, X.; Huang, L.; Tan, S.; Mai, W. Quantitative analysis of charge storage process of tungsten oxide that combines pseudocapacitive and electrochromic properties. J. Phys. Chem. C 2015, 119, 16483–16489. [Google Scholar] [CrossRef]
- Li, W.J.; Fu, Z.W. Nanostructured WO3 thin film as a new anode material for lithium-ion batteries. Appl. Surf. Sci. 2010, 256, 2447–2452. [Google Scholar] [CrossRef]
- Lokhande, V.; Lokhande, A.; Namkoong, G.; Kim, J.H.; Ji, T. Charge storage in WO3 polymorphs and their application as supercapacitor electrode material. Results Phys. 2019, 12, 2012–2020. [Google Scholar] [CrossRef]
- Kalagi, S.S.; Dalavi, D.S.; Mali, S.S.; Inamdar, A.I.; Patil, R.S.; Patil, P.S. Study of novel WO3-PEDOT:PSS bilayered thin film for electrochromic applications. Nanosci. Nanotechnol. Lett. 2012, 4, 1146–1154. [Google Scholar] [CrossRef]
- Zou, B.X.; Liang, Y.; Liu, X.X.; Diamond, D.; Lau, K.T. Electrodeposition and pseudocapacitive properties of tungsten oxide/polyaniline composite. J. Power Sources 2011, 196, 4842–4848. [Google Scholar] [CrossRef] [Green Version]
- Amaechi, I.C.; Nwanya, A.C.; Ekwealor, A.B.C.; Asogwa, P.U.; Osuji, R.U.; Maaza, M.; Ezema, F.I. Electronic thermal conductivity, thermoelectric properties and supercapacitive behaviour of conjugated polymer nanocomposite (polyaniline-WO3) thin film. Eur. Phys. J. Appl. Phys. 2015, 69, 30901. [Google Scholar] [CrossRef]
- Wei, H.; Yan, X.; Wu, S.; Luo, Z.; Wei, S.; Guo, Z. Electropolymerized polyaniline stabilized tungsten oxide nanocomposite films: Electrochromic behavior and electrochemical energy storage. J. Phys. Chem. C 2012, 116, 25052–25064. [Google Scholar] [CrossRef]
- Zou, B.; Gong, S.; Wang, Y.; Liu, X. Tungsten oxide and polyaniline composite fabricated by surfactant-templated electrodeposition and its use in supercapacitors. J. Nanomater. 2014, 2014, 813120. [Google Scholar] [CrossRef]
- Yuksel, R.; Durucan, C.; Unalan, H.E. Ternary nanocomposite SWNT/WO3/PANI thin film electrodes for supercapacitors. J. Alloys Compd. 2016, 658, 183–189. [Google Scholar] [CrossRef]
- Kadam, A.V.; Patil, S.B. Polyaniline globules as a catalyst for WO3 nanoparticles for supercapacitor application. Mater. Res. Expr. 2018, 5, 085036. [Google Scholar] [CrossRef]
- Wang, F.; Zhan, X.; Cheng, Z.; Wang, Z.; Wang, Q.; Xu, K.; Safdar, M.; He, J. Tungsten oxide@polypyrrole core-shell nanowire arrays as novel negative electrodes for asymmetric supercapacitors. Small 2015, 11, 749–755. [Google Scholar] [CrossRef]
- Lokhande, V.C.; Lokhande, A.C.; Lokhande, C.D.; Kim, J.H.; Ji, T. Supercapacitive composite metal oxide electrodes formed with carbon, metal oxides and conducting polymers. J. Alloys Compd. 2016, 685, 381–403. [Google Scholar] [CrossRef]
- Zhuzhelskii, D.V.; Tolstopjatova, E.G.; Eliseeva, S.N.; Ivanov, A.V.; Miao, S.; Kondratiev, V.V. Electrochemical properties of PEDOT/WO3 composite films for high performance supercapacitor application. Electrochim. Acta 2019, 299, 182–190. [Google Scholar] [CrossRef]
- Szymanska, D.; Rutkowska, I.A.; Adamczyk, L.; Zoladek, S.; Kulesza, P.J. Effective charge propagation and storage in hybrid films of tungsten oxide and poly(3,4-ethylenedioxythiophene). J. Solid State Electrochem. 2010, 14, 2049–2056. [Google Scholar] [CrossRef]
- Zhu, Y.; Otley, M.T.; Alamer, F.A.; Kumar, A.; Zhang, X.; Mamangun, D.M.D.; Li, M.; Arden, B.G.; Sotzing, G.A. Electrochromic properties as a function of electrolyte on the performance of electrochromic devices consisting of a single-layer polymer. Organ. Electron. 2014, 15, 1378–1386. [Google Scholar] [CrossRef]
- Bredas, J.L.; Scott, J.C.; Yakushi, K.; Street, G.B. Polarons and bipolarons in polypyrrole: Evolution of the band structure and optical spectrum upon doping. Phys. Rev. B Condens. Matter. 1984, 30, 1023–1025. [Google Scholar] [CrossRef]
- Bredas, J.L.; Street, G.B. Polarons, bipolarons and solitons in conducting polymers. Acc. Chem. Res. 1985, 18, 309–315. [Google Scholar] [CrossRef]
- Hoier, S.N.; Park, S.M. Electrochemistry of conductive polymers. 11. Spectroelectrochemical studies of poly(3-methylthiophene) oxidation. J. Phys. Chem. 1992, 96, 5188–5193. [Google Scholar] [CrossRef]
- Trznadel, M.; Zagórska, M.; Lapkowski, M.; Louarn, G.; Lefrant, S.; Pron, A. UV–VIS–NIR and Raman spectroelectrochemistry of regioregular poly(3-octylthiophene): Comparison with its non-regioregular analogue. J. Chem. Soc. Faraday Trans. 1996, 92, 1387–1393. [Google Scholar] [CrossRef]
- Neugebauer, H.; Blomquist, S.; Ahonen, H.J.; Kankare, J.; Ivaska, A. In situ spectroelectrochemical characterization of poly(3,4-ethylenedioxythiophene). Electrochim. Acta 1999, 44, 2739–2750. [Google Scholar] [CrossRef]
- Łapkowski, M.; Proń, A. Electrochemical oxidation of poly(3,4-ethylenedioxythiophene)—“In situ” conductivity and spectroscopic investigations. Synth. Met. 2000, 110, 79–83. [Google Scholar] [CrossRef]
- Tolstopyatova, E.G.; Pogulaichenko, N.A.; Eliseeva, S.N.; Kondratiev, V.V. Spectroelectrochemical study of poly-3,4-ethylenedioxythiophene films in the presence of different supporting electrolytes. Russ. J. Electrochem. 2009, 45, 252–262. [Google Scholar] [CrossRef]
- Chen, X.; Inganaes, O. Three-step redox in polythiophenes: Evidence from electrochemistry at an ultramicroelectrode. J. Phys. Chem. 1996, 100, 15202–15206. [Google Scholar] [CrossRef]
- Carlberg, C.; Chen, X.; Inganaes, O. Ionic transport and electronic structure in poly(3,4-ethylenedioxythiophene). Solid State Ionics 1996, 85, 73–78. [Google Scholar] [CrossRef]
- Tourillon, G. Polythiophene and its derivatives. In Handbook of Conducting Polymers; Skotheim, T.A., Ed.; Marcel Dekker: New York, NY, USA, 1986; pp. 293–350. [Google Scholar]
- Darmawi, S.; Burkhardt, S.; Leichtweiss, T.; Weber, D.A.; Wenzel, S.; Janek, J.; Elm, M.T.; Klar, P.J. Correlation of electrochromic properties and oxidation states in nanocrystalline tungsten trioxide. Phys. Chem. Chem. Phys. 2015, 17, 15903–15911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deepa, M.; Joshi, A.G.; Srivastava, A.K.; Shivaprasad, S.M.; Agnihotry, S.A. Electrochromic nanostructured tungsten oxide films by sol-gel: Structure and intercalation properties. J. Electrochem. Soc. 2006, 153, C365–C376. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Tang, K.; Cui, J.; Shu, X.; Wang, Y.; Liu, J.; Jiang, Y.; Tan, H.H.; Wu, Y. Designed growth of WO3/PEDOT core/shell hybrid nanorod arrays with modulated electrochromic properties. Chem. Eng. J. 2019, 355, 942–951. [Google Scholar] [CrossRef]
- Zhuzhelskii, D.V.; Tolstopjatova, E.G.; Volkov, A.I.; Eliseeva, S.N.; Láng, G.G.; Kondratiev, V.V. Insights on the electrodeposition mechanism of tungsten oxide into conducting polymers: Potentiostatic vs. potentiodynamic deposition. Synth. Met. 2020, 267, 116469. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efremova, A.O.; Tolstopjatova, E.G.; Holze, R.; Kondratiev, V.V. Interactions in Electrodeposited Poly-3,4-Ethylenedioxythiophene—Tungsten Oxide Composite Films Studied with Spectroelectrochemistry. Polymers 2021, 13, 1630. https://doi.org/10.3390/polym13101630
Efremova AO, Tolstopjatova EG, Holze R, Kondratiev VV. Interactions in Electrodeposited Poly-3,4-Ethylenedioxythiophene—Tungsten Oxide Composite Films Studied with Spectroelectrochemistry. Polymers. 2021; 13(10):1630. https://doi.org/10.3390/polym13101630
Chicago/Turabian StyleEfremova, Aleksandra O., Elena G. Tolstopjatova, Rudolf Holze, and Veniamin V. Kondratiev. 2021. "Interactions in Electrodeposited Poly-3,4-Ethylenedioxythiophene—Tungsten Oxide Composite Films Studied with Spectroelectrochemistry" Polymers 13, no. 10: 1630. https://doi.org/10.3390/polym13101630
APA StyleEfremova, A. O., Tolstopjatova, E. G., Holze, R., & Kondratiev, V. V. (2021). Interactions in Electrodeposited Poly-3,4-Ethylenedioxythiophene—Tungsten Oxide Composite Films Studied with Spectroelectrochemistry. Polymers, 13(10), 1630. https://doi.org/10.3390/polym13101630






