Novel Polyimide/Copper-Nickel Ferrite Composites with Tunable Magnetic and Dielectric Properties
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.1.1. Materials
2.1.2. Preparation of Polyimide Composites
2.1.3. Measurements
3. Results and Discussions
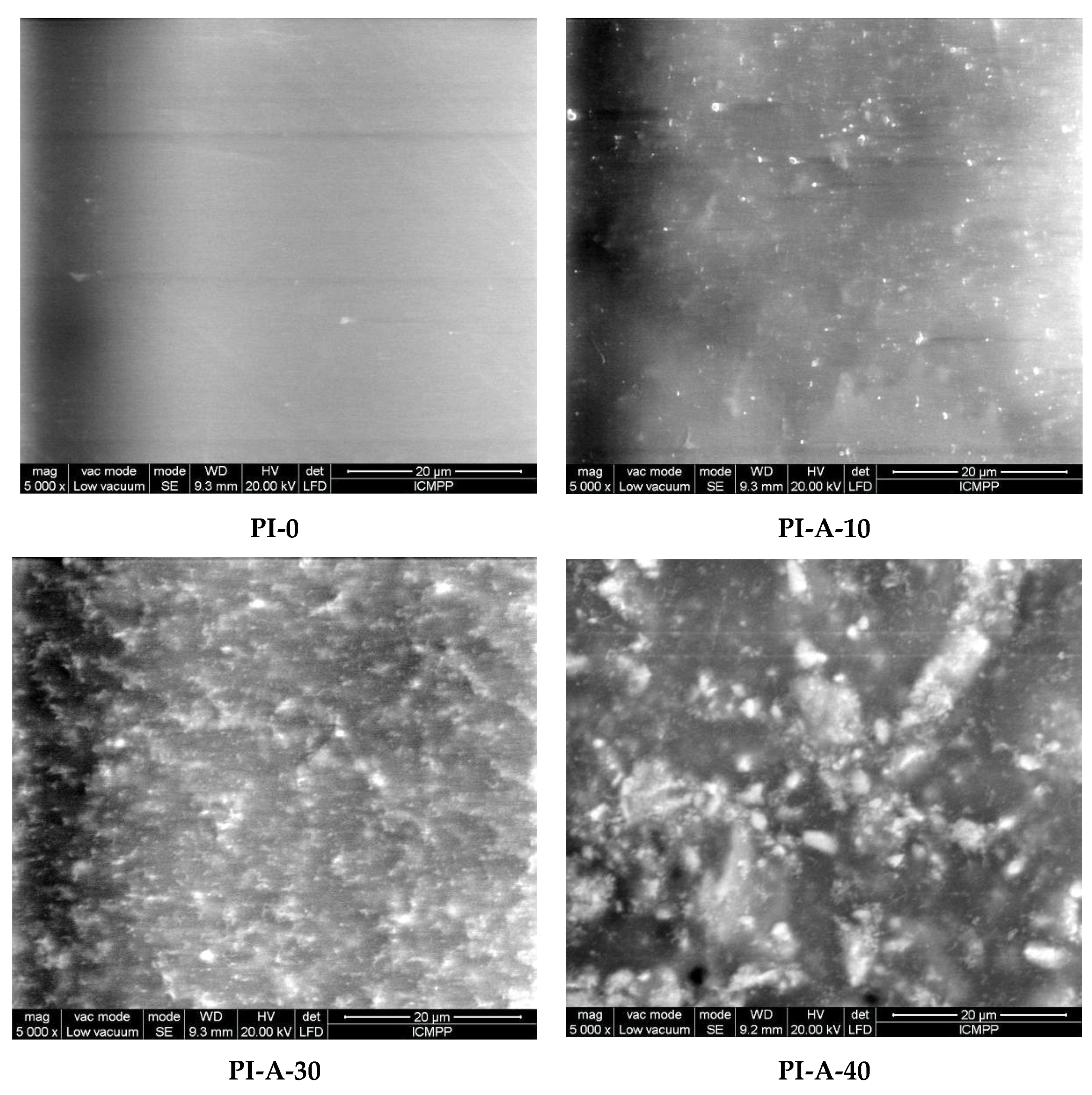
3.1. Structural and Morphological Characterization
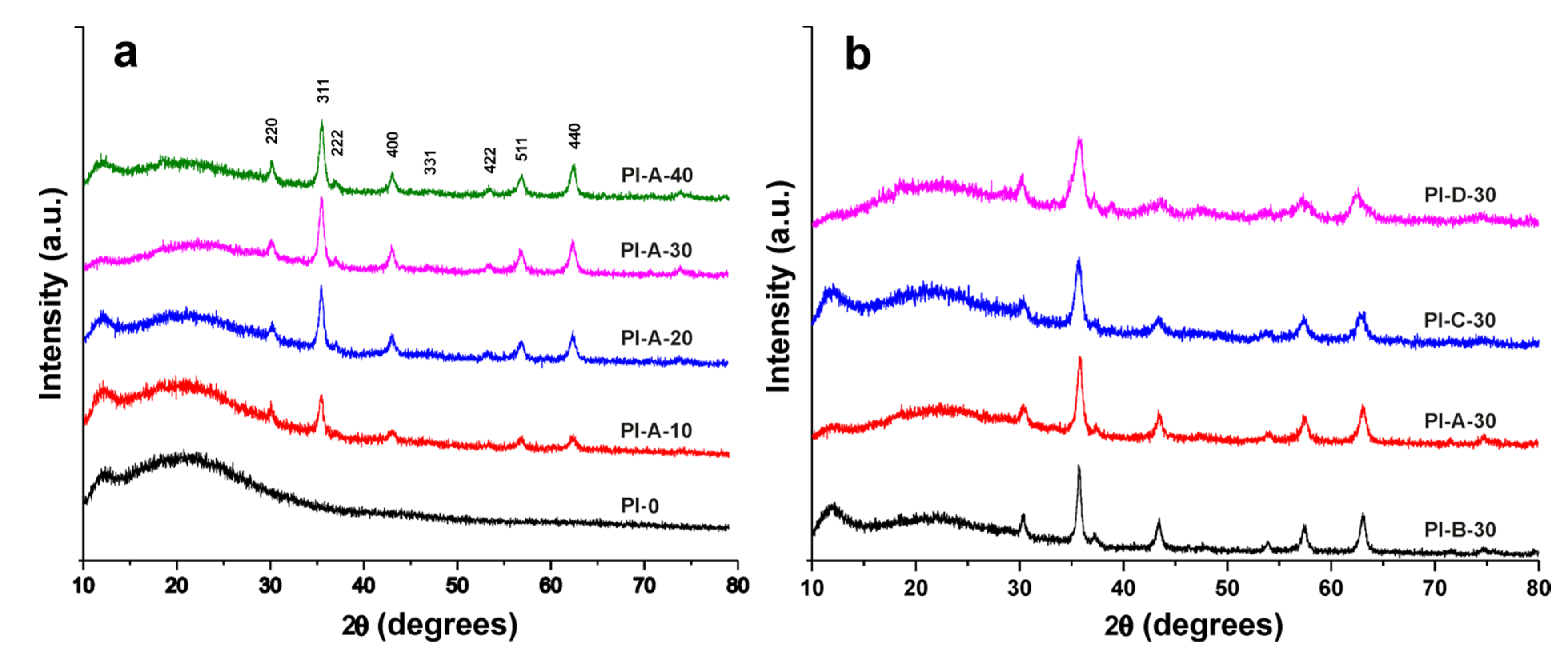
3.2. X-ray Analysis
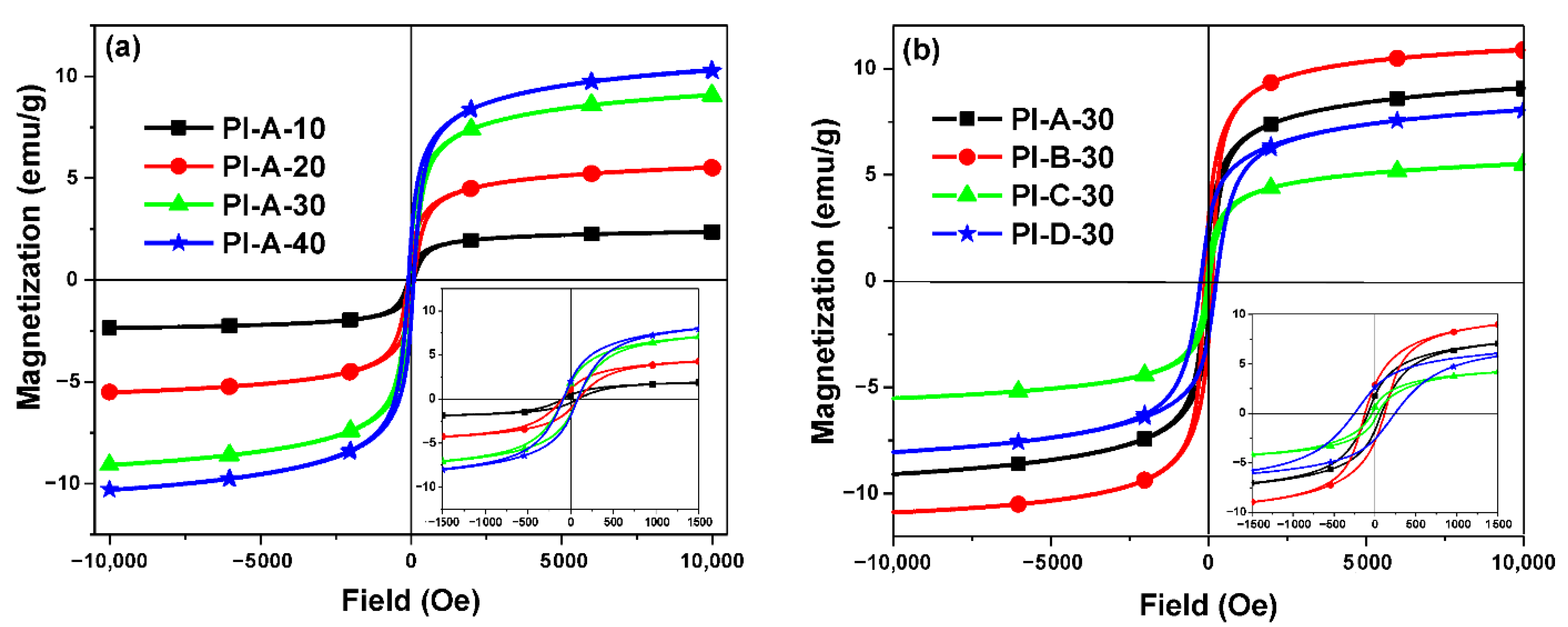
3.3. Thermal and Magnetic Properties
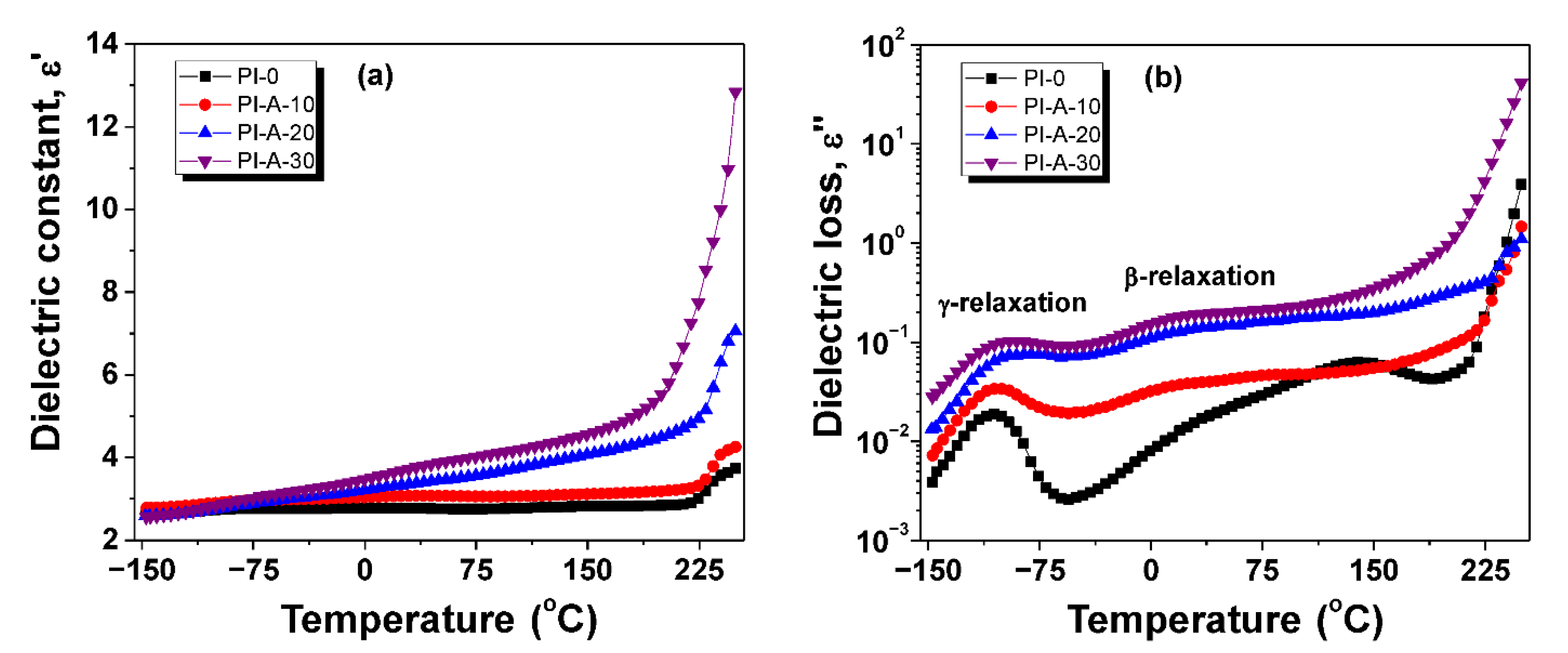
3.4. Dielectric Constant and Dielectric Loss
3.5. Activation Energy
3.6. Conductivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anandhan, S.; Bandyopadhyay, S. Polymer Nanocomposites: From Synthesis to Applications. In Nanocomposites and Polymers with Analytical Methods; Cuppoletti, J., Ed.; InTech Europe: Rijeka, Croatia, 2011. [Google Scholar] [CrossRef] [Green Version]
- Thévenot, J.; Oliveira, H.; Sandre, O.; Lecommandoux, S. Magnetic responsive polymer composite materials. Chem. Soc. Rev. 2013, 42, 7099–7116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalia, S.; Kango, S.; Kumar, A.; Haldorai, Y.; Kumari, B.; Kumar, R. Magnetic polymer nanocomposites for environmental and biomedical applications. Colloid Polym. Sci. 2014, 292, 2025–2052. [Google Scholar] [CrossRef]
- Agami, W.R.; El-Sayed, H.M. Enhancement of the magnetic and dielectric properties of cobalt nanoferrite/polymethyl methacrylate composites. J. Mater. Sci. Mater. Electron. 2015, 26, 3163–3167. [Google Scholar] [CrossRef]
- Antonel, P.; Berhó, F.; Jorge, G.; Molina, F. Magnetic composites of CoFe2O4 nanoparticles in a poly(aniline) matrix: Enhancement of remanence ratio and coercivity. Synth. Met. 2015, 199, 292–302. [Google Scholar] [CrossRef]
- Lalwani, S.; Marichi, R.B.; Mishra, M.; Gupta, G.; Singh, G.; Sharma, R.K. Edge enriched cobalt ferrite nanorods for symmetric/asymmetric supercapacitive charge storage. Electrochim. Acta 2018, 283, 708–717. [Google Scholar] [CrossRef]
- Routray, K.L.; Saha, S.; Behera, D.; Tripathy, N.; Ghosh, S.P. High dielectric constant, low loss and efficient visible photoluminescence properties of porous rose- flower shaped CoFe2O4 for photovoltaic application. Mater. Lett. 2019, 242, 62–65. [Google Scholar] [CrossRef]
- Stanciu, L.; Won, Y.-H.; Ganesana, M.; Andreescu, S. Magnetic Particle-Based Hybrid Platforms for Bioanalytical Sensors. Sensors 2009, 9, 2976–2999. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Shokrollahi, H. The role of cobalt ferrite magnetic nanoparticles in medical science. Mater. Sci. Eng. C 2013, 33, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, M.W.; Kanwal, F.; Batool, A.; Jamil, T.; Zia-Ul-Haq, M.; Ijaz, B.; Huang, Q.; Ullah, Z. Polymer-coated CoFe2O4 nanoassemblies as biocompatible magnetic nanocarriers for anticancer drug delivery. J. Mater. Sci. 2017, 52, 9282–9293. [Google Scholar] [CrossRef]
- Hashim, M.; Raghasudha, M.; Meena, S.S.; Shah, J.; Shirsath, S.E.; Kumar, S.; Ravinder, D.; Bhatt, P.; Alimuddin; Kumar, R.; et al. Influence of rare earth ion doping (Ce and Dy) on electrical and magnetic properties of cobalt ferrites. J. Magn. Magn. Mater. 2018, 449, 319–327. [Google Scholar] [CrossRef]
- Xiang, J.; Chu, Y.; Shen, X.; Zhou, G.; Guo, Y. Electrospinning preparation, characterization and magnetic properties of cobalt–nickel ferrite (Co1−xNixFe2O4) nanofibers. J. Colloid Interface Sci. 2012, 376, 57–61. [Google Scholar] [CrossRef]
- Hashim, M.; Alimuddin; Shirsath, S.E.; Meena, S.S.; Kotnala, R.; Kumar, S.; Bhatt, P.; Jotania, R.; Kumar, R. Study of structural and magnetic properties of (Co–Cu)Fe2O4/PANI composites. Mater. Chem. Phys. 2013, 141, 406–415. [Google Scholar] [CrossRef]
- Doulabi, F.S.M.; Mohsen-Nia, M. Magnetic cobalt-zinc ferrite/PVAc nanocomposite: Synthesis and characterization. Iran. Polym. J. 2013, 22, 9–14. [Google Scholar] [CrossRef]
- Gherca, D.; Pui, A.; Cornei, N.; Cojocariu, A.; Nica, V.; Caltun, O. Synthesis, characterization and magnetic properties of MFe2O4 (M=Co, Mg, Mn, Ni) nanoparticles using ricin oil as capping agent. J. Magn. Magn. Mater. 2012, 324, 3906–3911. [Google Scholar] [CrossRef]
- Gherca, D.; Pui, A.; Nica, V.; Caltun, O.; Cornei, N. Eco-environmental synthesis and characterization of nanophase powders of Co, Mg, Mn and Ni ferrites. Ceram. Int. 2014, 40, 9599–9607. [Google Scholar] [CrossRef]
- Ciocarlan, R.G.; Pui, A.; Gherca, D.; Virlan, C.; Dobromir, M.; Nica, V.; Craus, M.L.; Gostin, I.N.; Caltun, O.; Hempelman, R.; et al. Quaternary M0.25Cu0.25Mg0.5Fe2O4 (M=Ni, Zn, Co, Mn) ferrite oxides: Synthesis, characterization and magnetic properties. Mater. Res. Bull. 2016, 81, 63–70. [Google Scholar] [CrossRef]
- Mosleh, Z.; Kameli, P.; Poorbaferani, A.; Ranjbar, M.; Salamati, H. Structural, magnetic and microwave absorption properties of Ce-doped barium hexaferrite. J. Magn. Magn. Mater. 2016, 397, 101–107. [Google Scholar] [CrossRef]
- Rahimi-Nasrabadi, M.; Behpour, M.; Sobhani-Nasab, A.; Jeddy, M.R. Nanocrystalline Ce-doped copper ferrite: Synthesis, characterization, and its photocatalyst application. J. Mater. Sci. Mater. Electron. 2016, 27, 11691–11697. [Google Scholar] [CrossRef]
- Roman, T.; Pui, A.; Lukacs, A.V.; Cimpoesu, N.; Lupescu, S.; Borhan, A.I.; Kordatos, K.; Ntziouni, A.; Postolache, P.; Zaharia, M.; et al. Structural changes of cerium doped copper ferrites during sintering process and magneto-electrical properties assessment. Ceram. Int. 2019, 45, 17243–17251. [Google Scholar] [CrossRef]
- Roman, T.; Ghercă, D.; Borhan, A.-I.; Grigoraș, M.; Stoian, G.; Lupu, N.; Turcan, I.; Cimpoesu, N.; Istrate, B.; Radu, I.; et al. Nanostructured quaternary Ni1-xCuxFe2-yCeyO4 complex system: Cerium content and copper substitution dependence of cation distribution and magnetic-electric properties in spinel ferrites. Ceram. Int. 2021. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Wang, K.-L.; Huang, Y.-C.; Lee, K.-R.; Lai, J.-Y.; Ha, C.-S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Vanherck, K.; Koeckelberghs, G.; Vankelecom, I.F. Crosslinking polyimides for membrane applications: A review. Prog. Polym. Sci. 2013, 38, 874–896. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Q.; You, Q.; Liao, G.; Xia, H.; Wang, D. Porous polyimide framework: A novel versatile adsorbent for highly efficient removals of azo dye and antibiotic. React. Funct. Polym. 2016, 103, 9–16. [Google Scholar] [CrossRef]
- Yi, L.; Huang, W.; Yan, D. Polyimides with side groups: Synthesis and effects of side groups on their properties. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 533–559. [Google Scholar] [CrossRef]
- Mazuera, D.; Perales, Ó.; Suarez, M.; Singh, S.P. Synthesis, characterization and thermal analysis of polyimide–cobalt ferrite nanocomposites. Mater. Sci. Eng. A 2010, 527, 6393–6399. [Google Scholar] [CrossRef]
- Zhan, J.; Wu, D.; Chen, C.; Li, J.; Pan, F.; Chen, J.; Zhan, X. Fabrication of polyimide composite film with both magnetic and surface conductive properties. Desalin. Water Treat. 2011, 34, 344–348. [Google Scholar] [CrossRef]
- Jia, H.; Jing, J.; Zhao, X.; Wang, W.; Wang, D.; Liu, C.; Zhou, H. Preparation and characterization of polyimide magnetic hollow nanospheres. Mater. Lett. 2012, 68, 86–89. [Google Scholar] [CrossRef]
- Moghanian, H.; Mobinikhaledi, A.; Baharangiz, Z. Synthesis, characterization and magnetic properties of novel heat resistant polyimide nanocomposites derived from 14H-dibenzo [a,j] xanthene. J. Polym. Res. 2014, 21. [Google Scholar] [CrossRef]
- Ding, D.; Yan, X.; Zhang, X.; He, Q.; Qiu, B.; Jiang, D.; Wei, H.; Guo, J.; Umar, A.; Sun, L.; et al. Preparation and enhanced properties of Fe3O4 nanoparticles reinforced polyimide nanocomposites. Superlattices Microstruct. 2015, 85, 305–320. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Qu, C.; Wang, D.; Feng, H.; Liu, P.; Zhang, Y. Preparation and characterization of magnetic polyimide composite films copolymerized with aminophthalocyanine-coated Fe3O4 nanocrystals. J. Mater. Sci. Mater. Electron. 2015, 26, 4005–4014. [Google Scholar] [CrossRef]
- Luo, C.; Wang, X.; Wang, J.; Pan, K. One-pot preparation of polyimide/Fe3O4 magnetic nanofibers with solvent resistant properties. Compos. Sci. Technol. 2016, 133, 97–103. [Google Scholar] [CrossRef]
- Rybak, A.; Rybak, A.; Kaszuwara, W.; Awietjan, S.; Molak, R.; Sysel, P.; Grzywna, Z.J. The magnetic inorganic-organic hybrid membranes based on polyimide matrices for gas separation. Compos. Part B Eng. 2017, 110, 161–170. [Google Scholar] [CrossRef]
- Faghihi, K.; Moghanian, H.; Mozafari, F.; Shabanian, M. Magnetic and Heat Resistant Poly(imide-ether) Nanocomposites Derived from Methyl Rich 9H-xanthene: Synthesis and Characterization. Chin. J. Polym. Sci. 2018, 36, 822–834. [Google Scholar] [CrossRef]
- Hamciuc, C.; Hamciuc, E.; Asandulesa, M.; Kalvachev, Y.; Dimitrov, L.; Ignat, M. Study on dielectric behavior and harvesting properties of new Ba0.5Sr0.4Ca0.1TiO3/poly(ether imide) composite films. Mater. Res. Bull. 2018, 102, 70–78. [Google Scholar] [CrossRef]
- Patange, S.M.; Shirsath, S.E.; Toksha, B.G.; Jadhav, S.S.; Jadhav, K.M. Electrical and magnetic properties of Cr3+ substituted nanocrystalline nickel ferrite. J. Appl. Phys. 2009, 106, 023914. [Google Scholar] [CrossRef]
- Chern, Y.-T.; Shiue, H.-C. Low Dielectric Constants of Soluble Polyimides Based on Adamantane. Macromolecules 1997, 30, 4646–4651. [Google Scholar] [CrossRef]
- Hamciuc, C.; Hamciuc, E.; Vlad-Bubulac, T.; Vlad, S.; Asandulesa, M.; Wolinska-Grabczyk, A. Silica-containing polyetherimide hybrid films based on methyltriethoxysilane as precursor of inorganic network. Polym. Test. 2016, 52, 94–103. [Google Scholar] [CrossRef]
- Sava, I.; Damaceanu, M.-D.; Constantin, C.-P.; Asandulesa, M.; Wolińska-Grabczyk, A.; Jankowski, A. Structure–promoted high performance properties of triphenylmethane-containing polyimides and copolyimides. Eur. Polym. J. 2018, 108, 554–569. [Google Scholar] [CrossRef]
- Havriliak, S.; Negami, S. A complex plane representation of dielectric and mechanical relaxation processes in some polymers. Polymer 1967, 8, 161–210. [Google Scholar] [CrossRef]


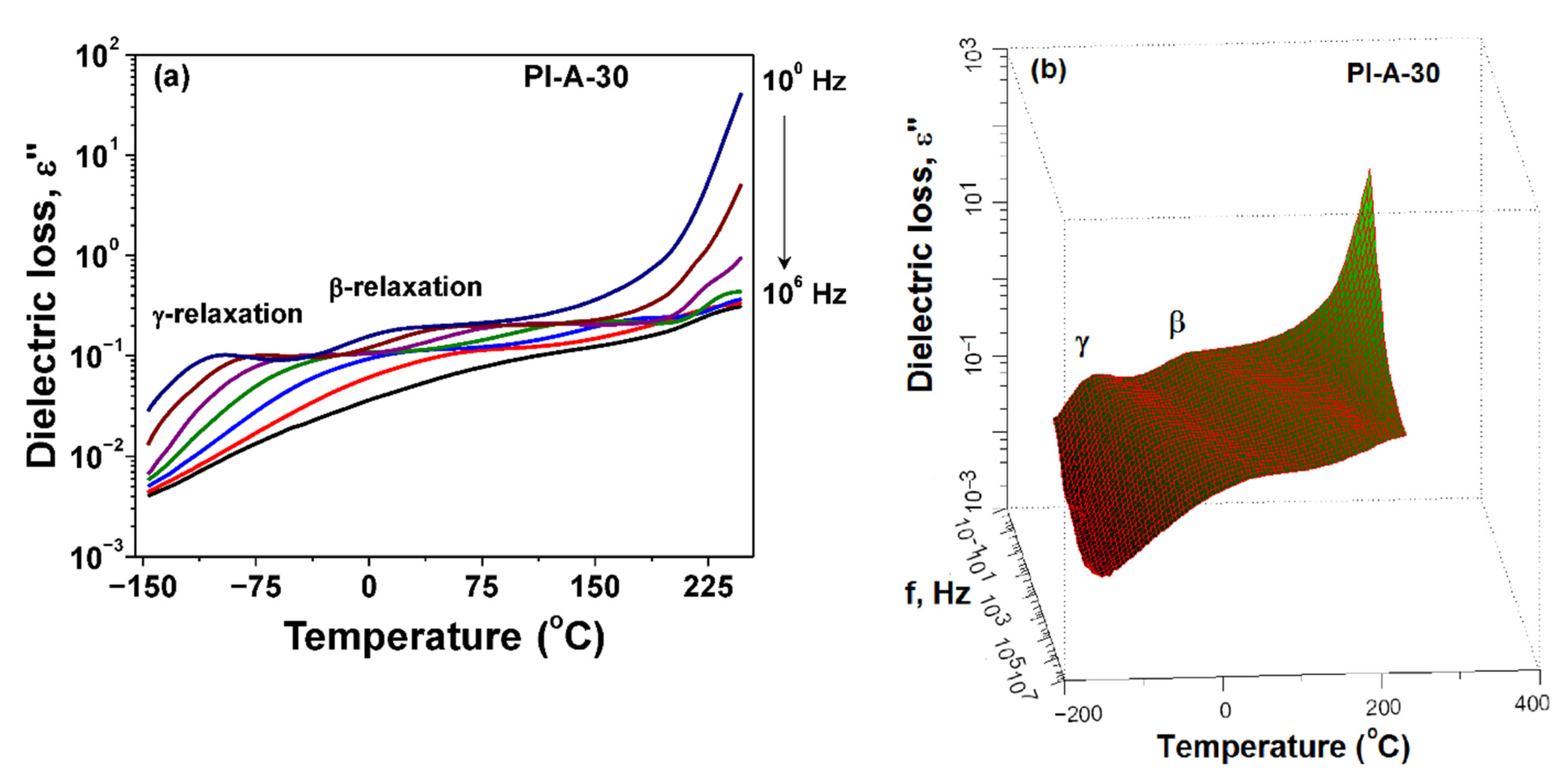
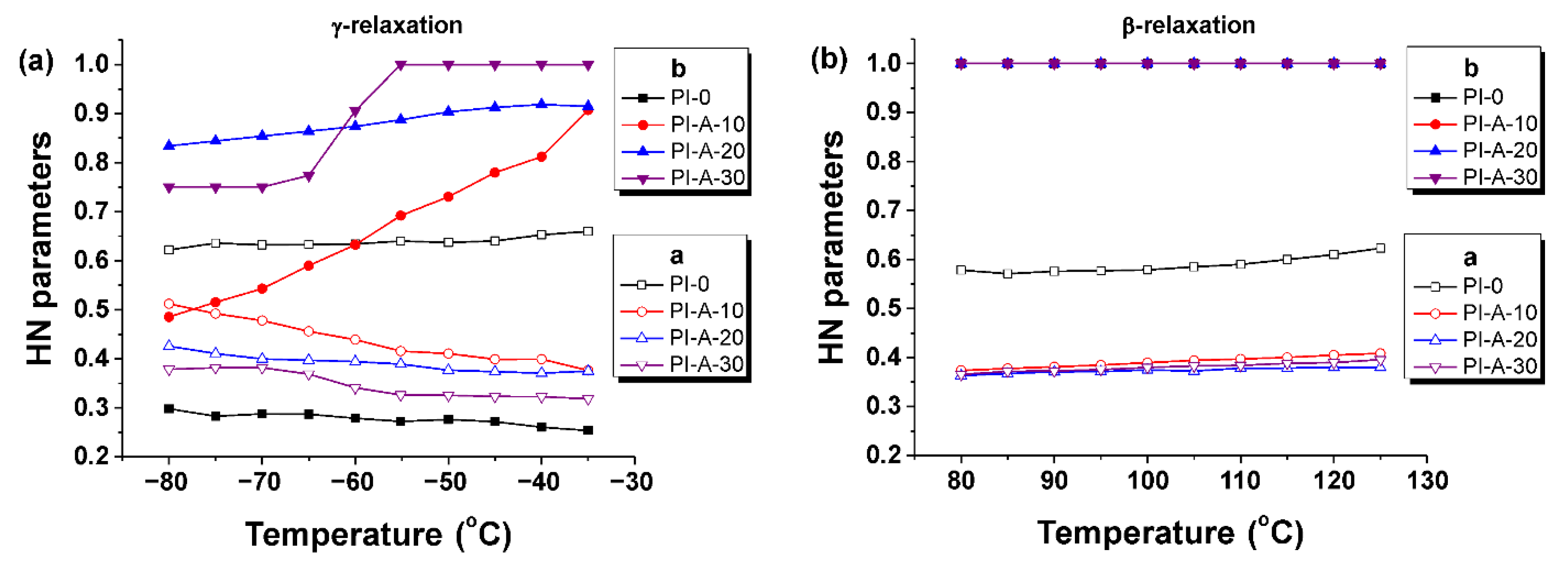
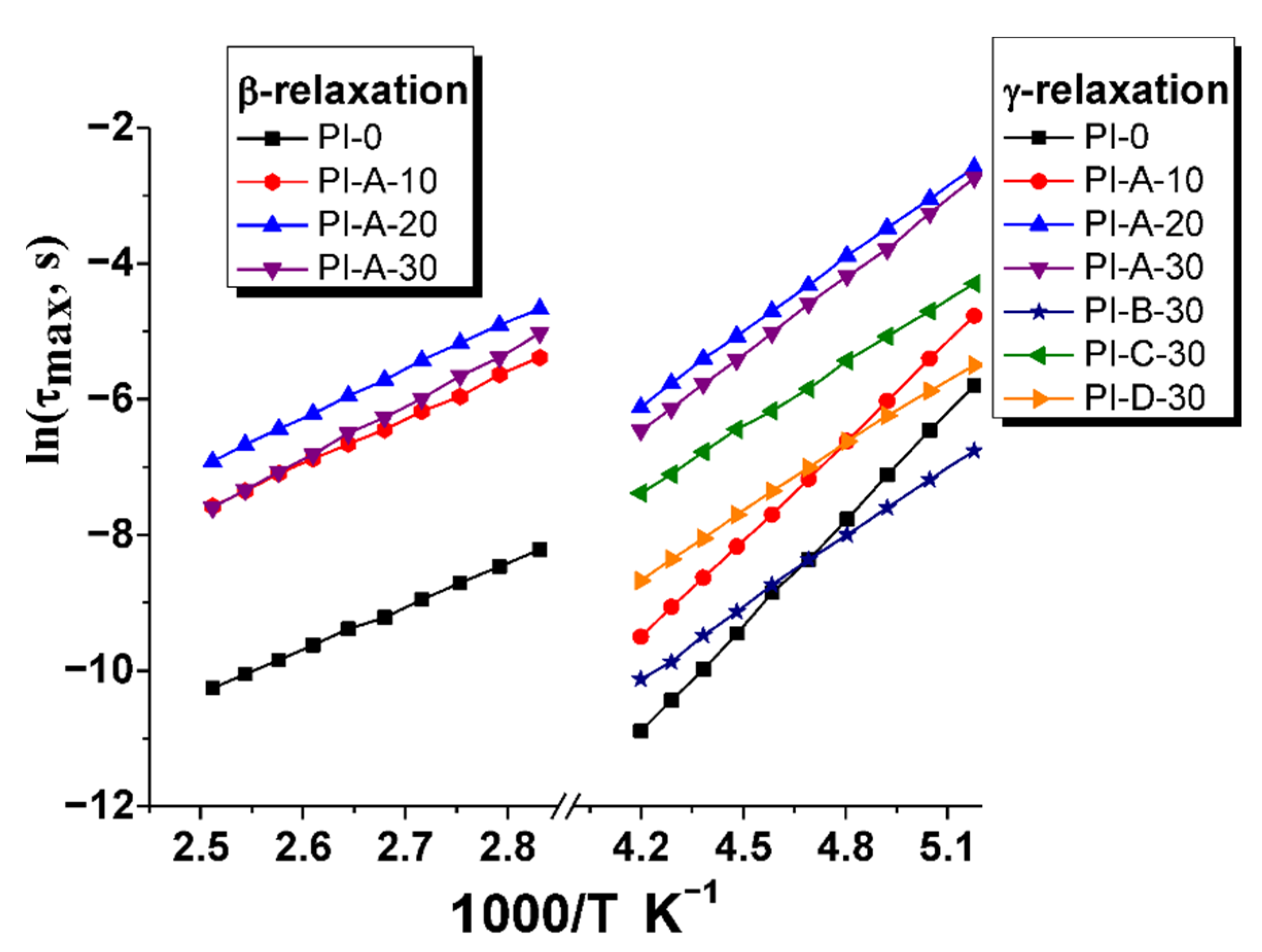
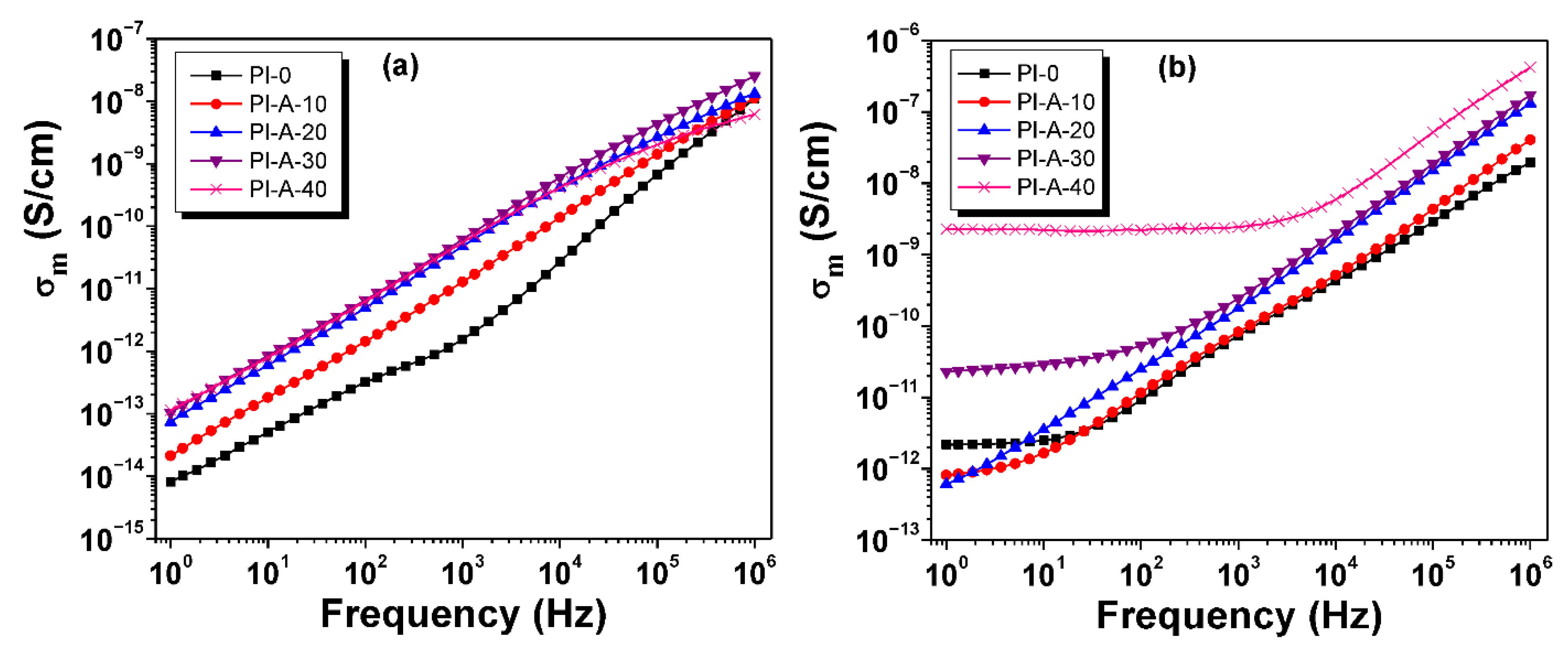
| Ferrite Sample | Chemical Formula | Ms (emu g−1) | Mr (emu g−1) | Hc (Oe) |
|---|---|---|---|---|
| A: [x = 0.3] | Ni0.7 Cu0.3 Fe1.92 Ce0.08 O4 | 25.4 | 3.34 | 53.45 |
| B: [x = 0.0] | NiFe1.92 Ce0.08 O4 | 33.86 | 5.23 | 65.75 |
| C: [x = 0.6] | Ni0.4Cu0.6 Fe1.92 Ce0.08 O4 | 20.77 | 1.73 | 32.32 |
| D: [x = 1.0] | CuFe1.92 Ce0.08 O4 | 19.44 | 6.47 | 246.2 |
| Polyimide Composite | Type of Ferrite Particles | Concentration of Particles in Polyimide Composite (wt%) |
|---|---|---|
| PI-0 | – | – |
| PI-A-10 | Ni0.7 Cu0.3 Fe1.92 Ce0.08 O4 | 10 |
| PI-A-20 | Ni0.7 Cu0.3 Fe1.92 Ce0.08 O4 | 20 |
| PI-A-30 | Ni0.7 Cu0.3 Fe1.92 Ce0.08 O4 | 30 |
| PI-A-40 | Ni0.7 Cu0.3 Fe1.92 Ce0.08 O4 | 40 |
| PI-B-30 | NiFe1.92 Ce0.08 O4 | 30 |
| PI-C-30 | Ni0.4Cu0.6 Fe1.92 Ce0.08 O4 | 30 |
| PI-D-30 | CuFe1.92 Ce0.08 O4 | 30 |
| Sample | T5a (°C) | T10b (°C) | Tmax c (°C) | Char Yield at 900 °C (%) | Tgd (°C) | Ms (emu g−1) | Mr (emu g−1) | Hc (Oe) |
|---|---|---|---|---|---|---|---|---|
| PI-0 | 509 | 522 | 527 | 45 | 241 | - | - | - |
| PI-A-10 | 507 | 523 | 522 | 26 | 234 | 2.37 | 0.45 | 84 |
| PI-A-20 | 506 | 520 | 515 | 43 | 235 | 5.52 | 1.11 | 90 |
| PI-A-30 | 501 | 513 | 509 | 53 | 234 | 9.05 | 1.76 | 79 |
| PI-A-40 | 498 | 511 | 511 | 49 | 233 | 10.28 | 1.98 | 83 |
| PI-B-30 | 500 | 512 | 512 | 42 | 235 | 10.90 | 2.84 | 119 |
| PI-C-30 | 502 | 515 | 513 | 47 | 234 | 5.53 | 0.63 | 32 |
| PI-D-30 | 495 | 508 | 510 | 48 | 233 | 8.02 | 2.61 | 244 |
| Sample | Dielectric Constant | Dielectric Loss | Conductivity (S cm−1) | |||
|---|---|---|---|---|---|---|
| 1 Hz | 104 Hz | 1 Hz | 104 Hz | 1 Hz | 104 Hz | |
| PI-0 | 3.0 | 3.0 | 1.6 × 10−2 | 0.5 × 10−2 | 8.8 × 10−15 | 2.9 × 10−11 |
| PI-A-10 | 3.3 | 3.1 | 4.1 × 10−2 | 2.7 × 10−2 | 2.3 × 10−14 | 1.5 × 10−10 |
| PI-A-20 | 3.5 | 2.9 | 13.9 × 10−2 | 7.9 × 10−2 | 7.8 × 10−14 | 4.4 × 10−10 |
| PI-A-30 | 3.9 | 3.1 | 19.6 × 10−2 | 11.5 × 10−2 | 1.1 × 10−13 | 6.4 × 10−10 |
| PI-B-30 | 4.3 | 3.6 | 19.7 × 10−2 | 13.3 × 10−2 | 1.1 × 10−13 | 7.4 × 10−10 |
| PI-C-30 | 3.4 | 3.1 | 2.7 × 10−2 | 10.8 × 10−2 | 1.5 × 10−14 | 6.0 × 10−10 |
| PI-D-30 | 3.7 | 3.1 | 18.2 × 10−2 | 14.8 × 10−2 | 1.0 × 10−13 | 8.2 × 10−10 |
| Sample | γ-Relaxation | β-Relaxation | ||
|---|---|---|---|---|
| τ0 (s) | Eγ (kJ mol−1) | τ0 (s) | Eβ (kJ mol−1) | |
| PI-0 | 6.0 × 10−15 | 43.3 | 3.9 × 10−12 | 53.1 |
| PI-A-10 | 1.1 × 10−13 | 40.3 | 1.4 × 10−11 | 57.7 |
| PI-A-20 | 6.1 × 10−10 | 30.0 | 1.7 × 10−11 | 59.2 |
| PI-A-30 | 1.9 × 10−10 | 31.6 | 1.1 × 10−12 | 66.3 |
| PI-B-30 | 1.8 × 10−11 | 29.0 | – | – |
| PI-C-30 | 8.7 × 10−10 | 27.0 | – | – |
| PI-D-30 | 1.9 × 10−10 | 27.3 | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamciuc, C.; Asandulesa, M.; Hamciuc, E.; Roman, T.; Olariu, M.A.; Pui, A. Novel Polyimide/Copper-Nickel Ferrite Composites with Tunable Magnetic and Dielectric Properties. Polymers 2021, 13, 1646. https://doi.org/10.3390/polym13101646
Hamciuc C, Asandulesa M, Hamciuc E, Roman T, Olariu MA, Pui A. Novel Polyimide/Copper-Nickel Ferrite Composites with Tunable Magnetic and Dielectric Properties. Polymers. 2021; 13(10):1646. https://doi.org/10.3390/polym13101646
Chicago/Turabian StyleHamciuc, Corneliu, Mihai Asandulesa, Elena Hamciuc, Tiberiu Roman, Marius Andrei Olariu, and Aurel Pui. 2021. "Novel Polyimide/Copper-Nickel Ferrite Composites with Tunable Magnetic and Dielectric Properties" Polymers 13, no. 10: 1646. https://doi.org/10.3390/polym13101646
APA StyleHamciuc, C., Asandulesa, M., Hamciuc, E., Roman, T., Olariu, M. A., & Pui, A. (2021). Novel Polyimide/Copper-Nickel Ferrite Composites with Tunable Magnetic and Dielectric Properties. Polymers, 13(10), 1646. https://doi.org/10.3390/polym13101646








