Ring-Opening Copolymerization of Cyclohexene Oxide and Cyclic Anhydrides Catalyzed by Bimetallic Scorpionate Zinc Catalysts
Abstract
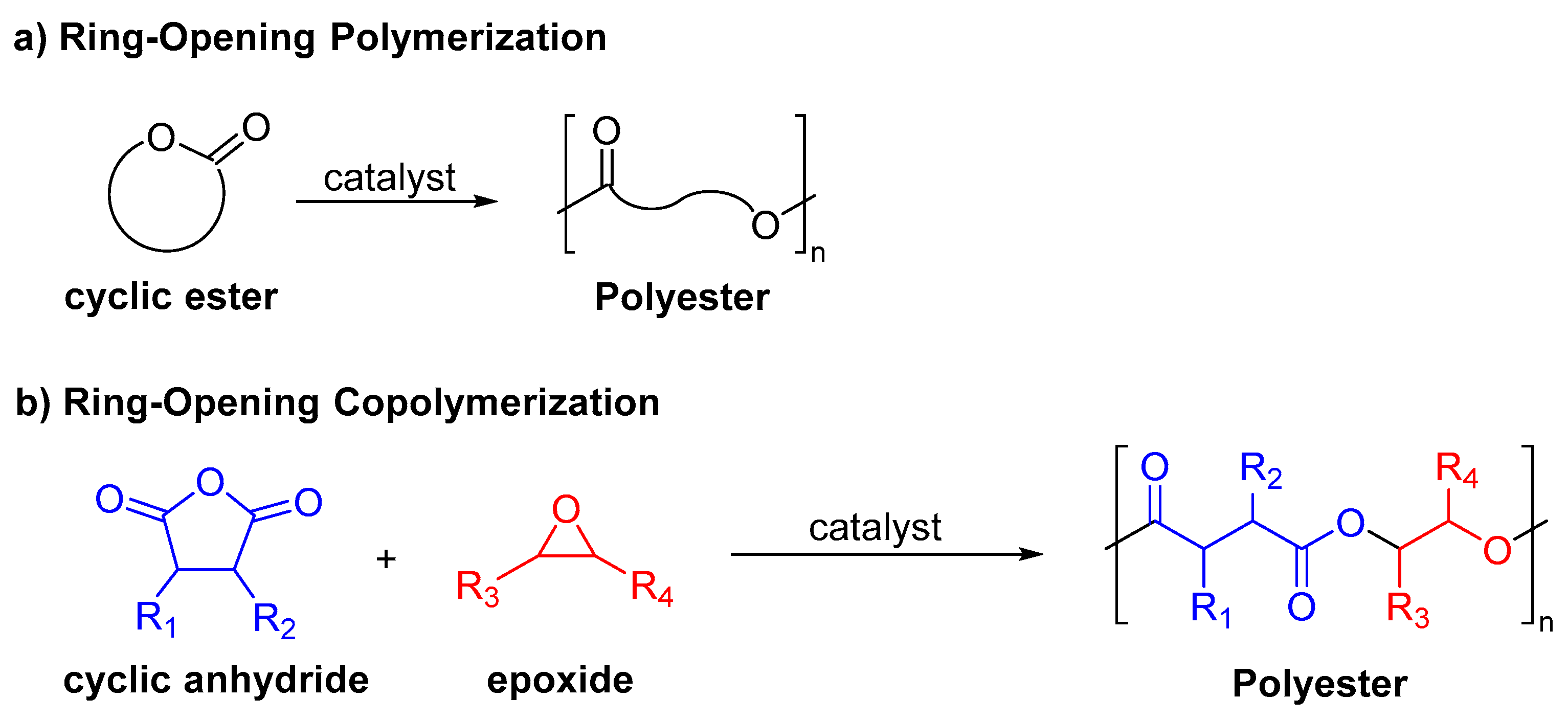
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. General Procedure for the ROCOP of CHO with Cyclic Anhydrides
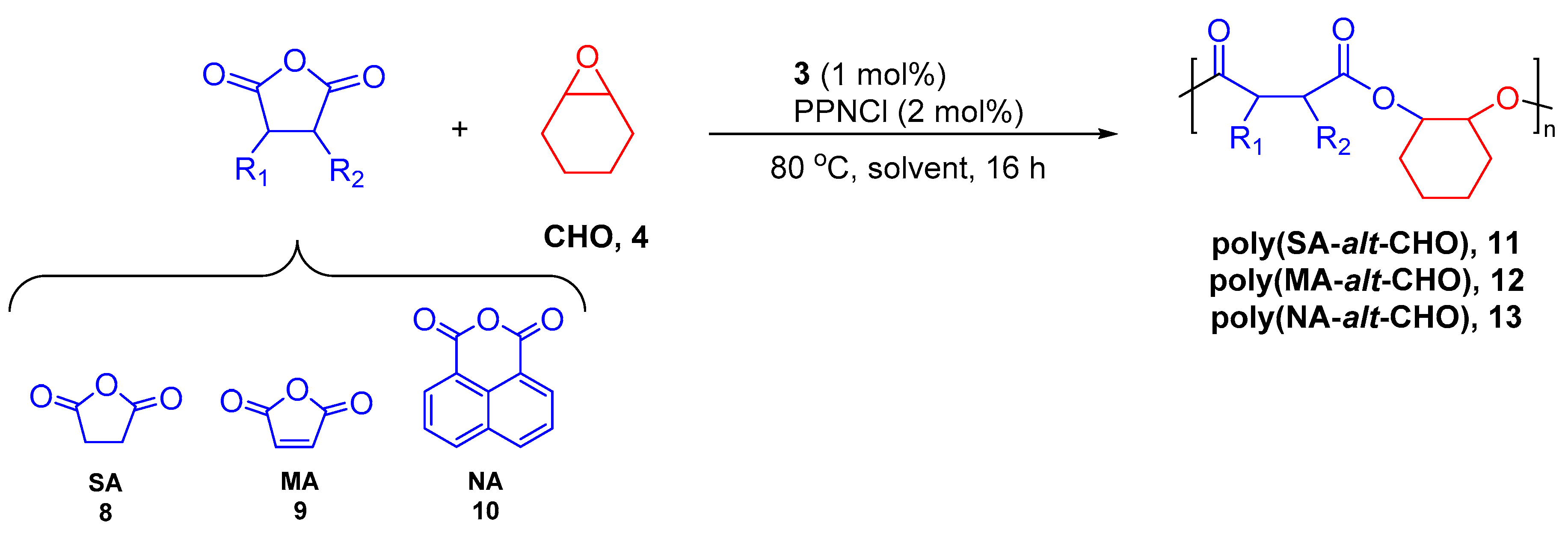
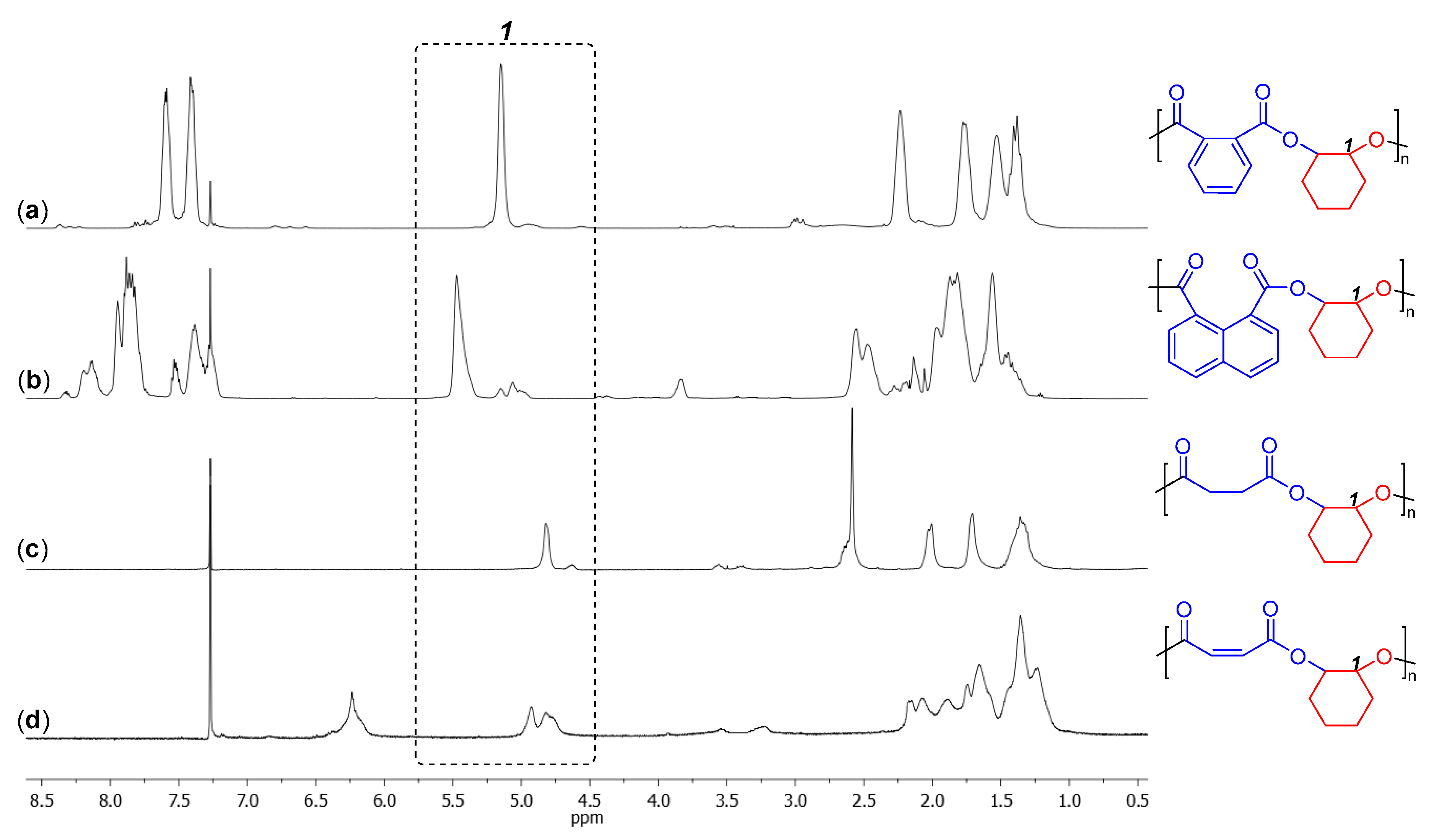
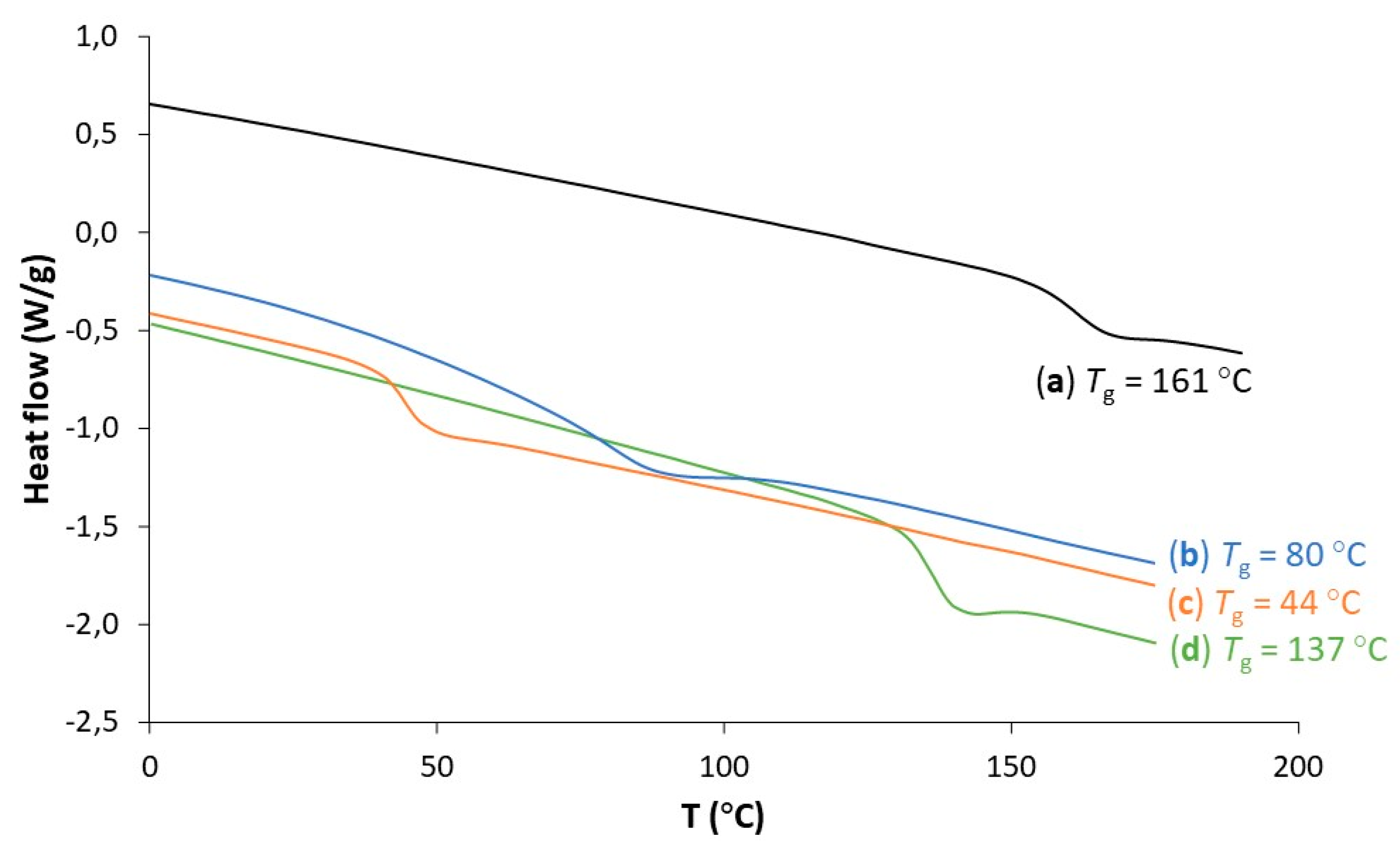
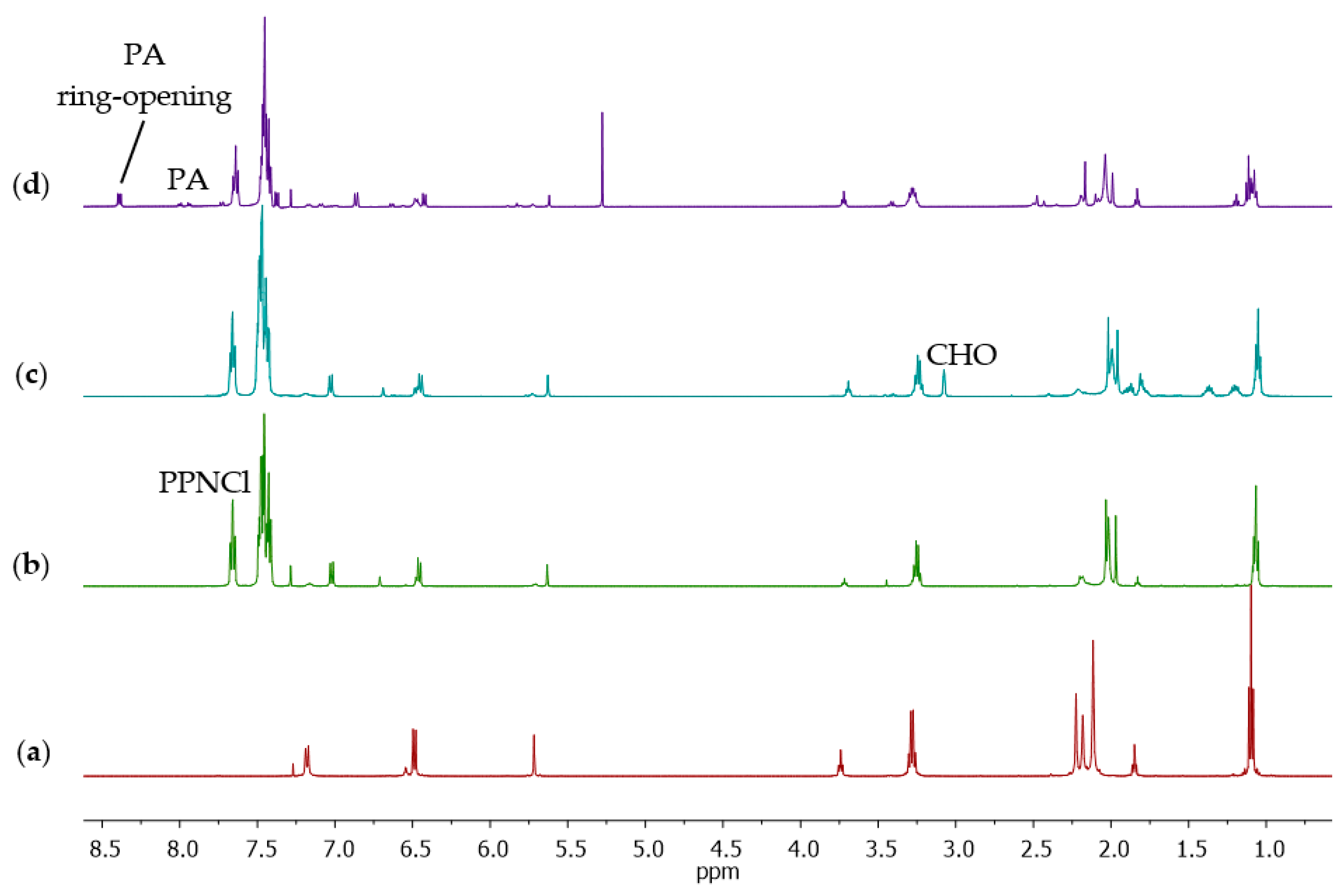
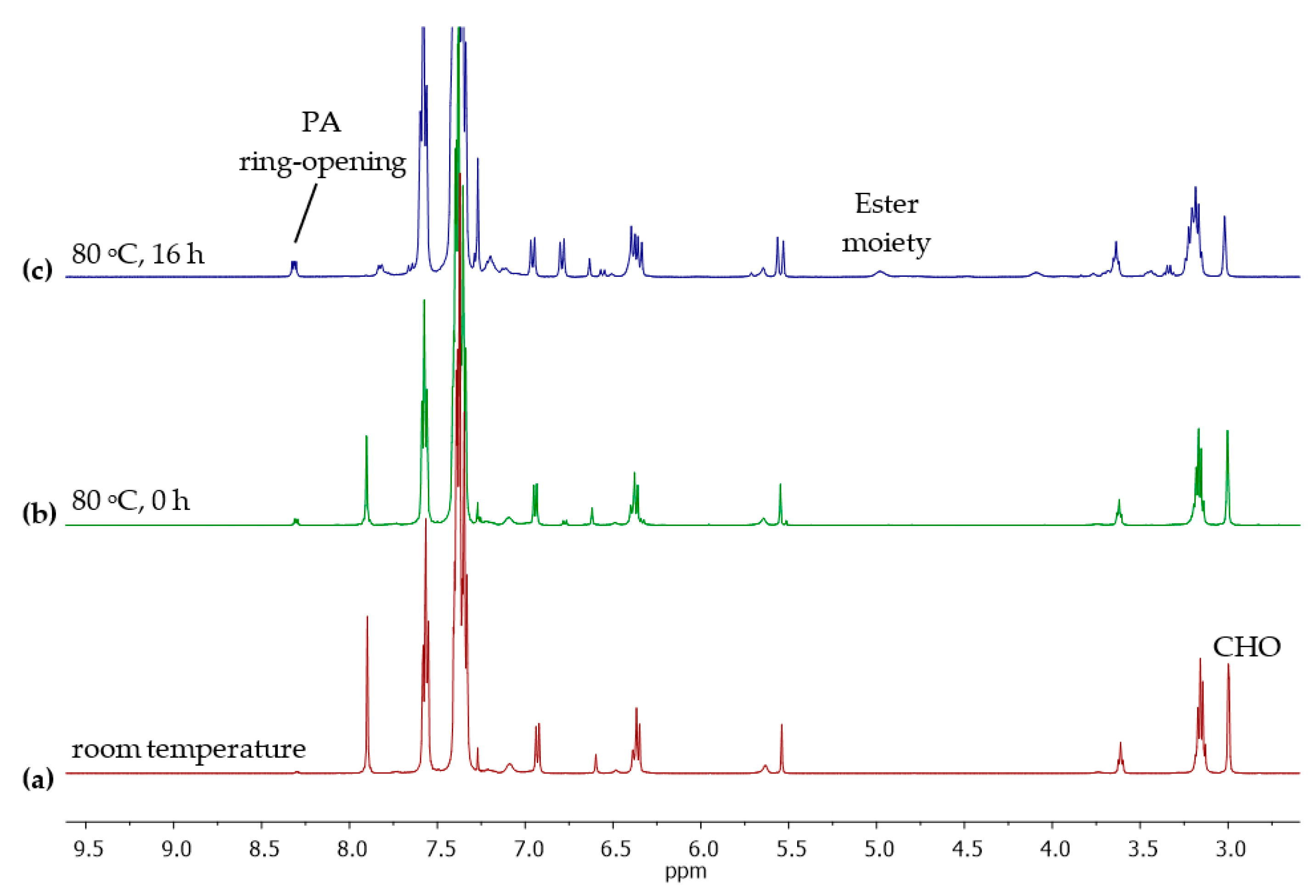
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pearce, E.M. Synthetic Methods in Step-Growth Polymers; Rogers, M.E., Long, T.E., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; ISBN 9780471220527. [Google Scholar]
- Stempfle, F.; Ortmann, P.; Mecking, S. Long-Chain Aliphatic Polymers to Bridge the Gap between Semicrystalline Polyolefins and Traditional Polycondensates. Chem. Rev. 2016, 116, 4597–4641. [Google Scholar] [CrossRef] [Green Version]
- Martínez, J.; Martínez De Sarasa Buchaca, M.; De La Cruz-Martínez, F.; Alonso-Moreno, C.; Sánchez-Barba, L.F.; Fernandez-Baeza, J.; Rodríguez, A.M.; Rodríguez-Diéguez, A.; Castro-Osma, J.A.; Otero, A.; et al. Versatile organoaluminium catalysts based on heteroscorpionate ligands for the preparation of polyesters. Dalton Trans. 2018, 47, 7471–7479. [Google Scholar] [CrossRef]
- Redshaw, C. Use of Metal Catalysts Bearing Schiff Base Macrocycles for the Ring Opening Polymerization (ROP) of Cyclic Esters. Catalysts 2017, 7, 165. [Google Scholar] [CrossRef] [Green Version]
- Nifant’ev, I.; Komarov, P.; Ovchinnikova, V.; Kiselev, A.; Minyaev, M.; Ivchenko, P. Comparative Experimental and Theoretical Study of Mg, Al and Zn Aryloxy Complexes in Copolymerization of Cyclic Esters: The Role of the Metal Coordination in Formation of Random Copolymers. Polymers 2020, 12, 2273. [Google Scholar] [CrossRef] [PubMed]
- Corneillie, S.; Smet, M. PLA architectures: The role of branching. Polym. Chem. 2015, 6, 850–867. [Google Scholar] [CrossRef] [Green Version]
- Carpentier, J.F.; Sarazin, Y. Alkaline-earth metal complexes in homogeneous polymerization catalysis. Top. Organomet. Chem. 2013, 45, 141–190. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, S.; Zhou, S. Aluminum alkyl complexes: Synthesis, structure, and application in ROP of cyclic esters. Dalton Trans. 2016, 45, 4471–4485. [Google Scholar] [CrossRef]
- Saito, T.; Aizawa, Y.; Yamamoto, T.; Tajima, K.; Isono, T.; Satoh, T. Alkali Metal Carboxylate as an Efficient and Simple Catalyst for Ring-Opening Polymerization of Cyclic Esters. Macromolecules 2018, 51, 689–696. [Google Scholar] [CrossRef]
- Melendez, D.O.; Castro-Osma, J.A.; Lara-Sanchez, A.; Rojas, R.S.; Otero, A. Ring-Opening Polymerization and Copolymerization of Cyclic Esters Catalyzed by Amidinate Aluminum Complexes. J. Polym. Sci. Part A Polym. Chem. 2017. [Google Scholar] [CrossRef]
- Muiruri, J.K.; Liu, S.; Teo, W.S.; Kong, J.; He, C. Highly Biodegradable and Tough Polylactic Acid-Cellulose Nanocrystal Composite. ACS Sustain. Chem. Eng. 2017, 5, 3929–3937. [Google Scholar] [CrossRef]
- Niza, E.; Castro-Osma, J.A.; Posadas, I.; Alonso-Moreno, C.; Bravo, I.; Garzón, A.; Canales-Vázquez, J.; Ceña, V.; Lara-Sánchez, A.; Albaladejo, J.; et al. Assessment of doxorubicin delivery devices based on tailored bare polycaprolactone against glioblastoma. Int. J. Pharm. 2019, 558, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, Applications, Nanocomposites, and Release Studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Longo, J.M.; Sanford, M.J.; Coates, G.W. Ring-Opening Copolymerization of Epoxides and Cyclic Anhydrides with Discrete Metal Complexes: Structure-Property Relationships. Chem. Rev. 2016, 116, 15167–15197. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, J.Z.; Lu, H.W.; Wang, H.B.; Lu, X.B. Making Various Degradable Polymers from Epoxides Using a Versatile Dinuclear Chromium Catalyst. Macromolecules 2018, 51, 771–778. [Google Scholar] [CrossRef]
- Ryu, H.K.; Bae, D.Y.; Lim, H.; Lee, E.; Son, K.S. Ring-opening copolymerization of cyclic epoxide and anhydride using a five-coordinate chromium complex with a sterically demanding amino triphenolate ligand. Polym. Chem. 2020, 11, 3756–3761. [Google Scholar] [CrossRef]
- Liu, B.; Chen, J.; Liu, N.; Ding, H.; Wu, X.; Dai, B.; Kim, I. Bio-based polyesters synthesized by ring-opening copolymerizations of eugenyl glycidyl ether and cyclic anhydrides using a binuclear [OSSO]CrCl complex. Green Chem. 2020, 22, 5742–5750. [Google Scholar] [CrossRef]
- Bester, K.; Bukowska, A.; Myśliwiec, B.; Hus, K.; Tomczyk, D.; Urbaniak, P.; Bukowski, W. Alternating ring-opening copolymerization of phthalic anhydride with epoxides catalysed by salophen chromium(iii) complexes. An effect of substituents in salophen ligands. Polym. Chem. 2018, 9, 2147–2156. [Google Scholar] [CrossRef]
- Si, G.; Zhang, L.; Han, B.; Duan, Z.; Li, B.; Dong, J.; Li, X.; Liu, B. Novel chromium complexes with a [OSSO]-type bis(phenolato) dianionic ligand mediate the alternating ring-opening copolymerization of epoxides and phthalic anhydride. Polym. Chem. 2015, 6, 6372–6377. [Google Scholar] [CrossRef]
- Saini, P.K.; Romain, C.; Zhu, Y.; Williams, C.K. Di-magnesium and zinc catalysts for the copolymerization of phthalic anhydride and cyclohexene oxide. Polym. Chem. 2014, 5, 6068–6075. [Google Scholar] [CrossRef] [Green Version]
- Wannipurage, D.; Hollingsworth, T.S.; Santulli, F.; Cozzolino, M.; Lamberti, M.; Groysman, S.; Mazzeo, M. Synthesis of a mononuclear magnesium bis(alkoxide) complex and its reactivity in the ring-opening copolymerization of cyclic anhydrides with epoxides. Dalton Trans. 2020, 49, 2715–2723. [Google Scholar] [CrossRef] [PubMed]
- Saini, P.K.; Fiorani, G.; Mathers, R.T.; Williams, C.K. Zinc versus Magnesium: Orthogonal Catalyst Reactivity in Selective Polymerizations of Epoxides, Bio-derived Anhydrides and Carbon Dioxide. Chem. Eur. J. 2017, 23, 4260–4265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiranoi, Y.; Nakano, K. Copolymerization of epoxides with cyclic anhydrides catalyzed by dinuclear cobalt complexes. Beilstein J. Org. Chem. 2018, 14, 2779–2788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.Y.; Chuang, H.J.; Ko, B.T. Bimetallic bis(benzotriazole iminophenolate) cobalt, nickel and zinc complexes as versatile catalysts for coupling of carbon dioxide with epoxides and copolymerization of phthalic anhydride with cyclohexene oxide. Catal. Sci. Technol. 2016, 6, 1779–1791. [Google Scholar] [CrossRef]
- Liu, D.; Wu, J.; Yang, Z.; Kang, J.; Gong, M.; Lü, X. Novel binuclear manganese(III), cobalt(III) and chromium(III) complexes for the alternating ring-opening copolymerization of cyclohexene oxide and maleic anhydride. Inorg. Chim. Acta 2016, 453, 222–229. [Google Scholar] [CrossRef]
- Chang, C.H.; Tsai, C.Y.; Lin, W.J.; Su, Y.C.; Chuang, H.J.; Liu, W.L.; Chen, C.T.; Chen, C.K.; Ko, B.T. Alternating copolymerization of epoxides with carbon dioxide or cyclic anhydrides using bimetallic nickel and cobalt catalysts: Preparation of hydrophilic nanofibers from functionalized polyesters. Polymer 2018, 141, 1–11. [Google Scholar] [CrossRef]
- Diciccio, A.M.; Longo, J.M.; Rodríguez-Calero, G.G.; Coates, G.W. Development of Highly Active and Regioselective Catalysts for the Copolymerization of Epoxides with Cyclic Anhydrides: An Unanticipated Effect of Electronic Variation. J. Am. Chem. Soc. 2016, 138, 7107–7113. [Google Scholar] [CrossRef]
- Liu, D.F.; Zhu, L.Q.; Wu, J.; Wu, L.Y.; Lü, X.Q. Ring-opening copolymerization of epoxides and anhydrides using manganese(iii) asymmetrical Schiff base complexes as catalysts. RSC Adv. 2015, 5, 3854–3859. [Google Scholar] [CrossRef]
- Robert, C.; Ohkawara, T.; Nozaki, K. Manganese-corrole complexes as versatile catalysts for the ring-opening homo- and co-polymerization of epoxide. Chem. Eur. J. 2014, 20, 4789–4795. [Google Scholar] [CrossRef]
- Buchard, A.; Kember, M.R.; Sandeman, K.G.; Williams, C.K. A bimetallic iron(iii) catalyst for CO2/epoxide coupling. Chem. Commun. 2011, 47, 212–214. [Google Scholar] [CrossRef] [Green Version]
- Peña Carrodeguas, L.; Martín, C.; Kleij, A.W. Semiaromatic Polyesters Derived from Renewable Terpene Oxides with High Glass Transitions. Macromolecules 2017, 50, 5337–5345. [Google Scholar] [CrossRef]
- Champouret, Y.; Hashmi, O.H.; Visseaux, M. Discrete iron-based complexes: Applications in homogeneous coordination-insertion polymerization catalysis. Coord. Chem. Rev. 2019, 390, 127–170. [Google Scholar] [CrossRef]
- Shi, Z.; Jiang, Q.; Song, Z.; Wang, Z.; Gao, C. Dinuclear iron(iii) complexes bearing phenylene-bridged bis(amino triphenolate) ligands as catalysts for the copolymerization of cyclohexene oxide with carbon dioxide or phthalic anhydride. Polym. Chem. 2018, 9, 4733–4743. [Google Scholar] [CrossRef]
- Proverbio, M.; Galotto Galotto, N.; Losio, S.; Tritto, I.; Boggioni, L. Boggioni Influence of Co-Catalysts and Polymerization Conditions on Properties of Poly(anhydride-alt-epoxide)s from ROCOP Using Salen Complexes with Different Metals. Polymers 2019, 11, 1222. [Google Scholar] [CrossRef] [Green Version]
- Martínez de Sarasa Buchaca, M.; de la Cruz-Martínez, F.; Martínez, J.; Alonso-Moreno, C.; Fernández-Baeza, J.; Tejeda, J.; Niza, E.; Castro-Osma, J.A.; Otero, A.; Lara-Sánchez, A. Alternating Copolymerization of Epoxides and Anhydrides Catalyzed by Aluminum Complexes. ACS Omega 2018, 3, 17581–17589. [Google Scholar] [CrossRef]
- Isnard, F.; Carratù, M.; Lamberti, M.; Venditto, V.; Mazzeo, M. Copolymerization of cyclic esters, epoxides and anhydrides: Evidence of the dual role of the monomers in the reaction mixture. Catal. Sci. Technol. 2018, 8, 5034–5043. [Google Scholar] [CrossRef]
- Isnard, F.; Lamberti, M.; Pellecchia, C.; Mazzeo, M. Ring-Opening Copolymerization of Epoxides with Cyclic Anhydrides Promoted by Bimetallic and Monometallic Phenoxy-Imine Aluminum complexes. ChemCatChem 2017, 9, 2972–2979. [Google Scholar] [CrossRef]
- Fieser, M.E.; Sanford, M.J.; Mitchell, L.A.; Dunbar, C.R.; Mandal, M.; Van Zee, N.J.; Urness, D.M.; Cramer, C.J.; Coates, G.W.; Tolman, W.B. Mechanistic Insights into the Alternating Copolymerization of Epoxides and Cyclic Anhydrides Using a (Salph)AlCl and Iminium Salt Catalytic System. J. Am. Chem. Soc. 2017, 139, 15222–15231. [Google Scholar] [CrossRef]
- Sanford, M.J.; Peña Carrodeguas, L.; Van Zee, N.J.; Kleij, A.W.; Coates, G.W. Alternating Copolymerization of Propylene Oxide and Cyclohexene Oxide with Tricyclic Anhydrides: Access to Partially Renewable Aliphatic Polyesters with High Glass Transition Temperatures. Macromolecules 2016, 49, 6394–6400. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Selective Polymerization Catalysis: Controlling the Metal Chain End Group to Prepare Block Copolyesters. J. Am. Chem. Soc. 2015, 137, 12179–12182. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.F.; Wu, L.Y.; Feng, W.X.; Zhang, X.M.; Wu, J.; Zhu, L.Q.; Fan, D.D.; Lü, X.Q.; Shi, Q. Ring-opening copolymerization of CHO and MA catalyzed by mononuclear [Zn(L2)(H2O)] or trinuclear [Zn3(L 2)2(OAc)2] complex based on the asymmetrical bis-Schiff-base ligand precursor. J. Mol. Catal. A Chem. 2014, 382, 136–145. [Google Scholar] [CrossRef]
- Abbina, S.; Chidara, V.K.; Du, G. Ring-Opening Copolymerization of Styrene Oxide and Cyclic Anhydrides by using Highly Effective Zinc Amido-Oxazolinate Catalysts. ChemCatChem 2017, 9, 1343–1348. [Google Scholar] [CrossRef]
- Thevenon, A.; Garden, J.A.; White, A.J.P.; Williams, C.K. Dinuclear Zinc Salen Catalysts for the Ring Opening Copolymerization of Epoxides and Carbon Dioxide or Anhydrides. Inorg. Chem. 2015, 54, 11906–11915. [Google Scholar] [CrossRef] [Green Version]
- Winkler, M.; Romain, C.; Meier, M.A.R.; Williams, C.K. Renewable polycarbonates and polyesters from 1,4-cyclohexadiene. Green Chem. 2014, 17, 300–306. [Google Scholar] [CrossRef] [Green Version]
- Shaik, M.; Chidara, V.K.; Abbina, S.; Du, G. Zinc Amido-Oxazolinate Catalyzed Ring Opening Copolymerization and Terpolymerization of Maleic Anhydride and Epoxides. Molecules 2020, 25, 4044. [Google Scholar] [CrossRef]
- Virachotikul, A.; Laiwattanapaisarn, N.; Wongmahasirikun, P.; Piromjitpong, P.; Chainok, K.; Phomphrai, K. Ring-Opening Copolymerizaton of Cyclohexene Oxide and Succinic Anhydride by Zinc and Magnesium Schiff-Base Complexes Containing Alkoxy Side Arms. Inorg. Chem. 2020, 59, 8983–8994. [Google Scholar] [CrossRef]
- Jeske, R.C.; DiCiccio, A.M.; Coates, G.W. Alternating copolymerization of epoxides and cyclic anhydrides: An improved route to aliphatic polyesters. J. Am. Chem. Soc. 2007, 129, 11330–11331. [Google Scholar] [CrossRef]
- Chen, T.T.D.; Zhu, Y.; Williams, C.K. Pentablock Copolymer from Tetracomponent Monomer Mixture Using a Switchable Dizinc Catalyst. Macromolecules 2018, 51, 5346–5351. [Google Scholar] [CrossRef] [Green Version]
- Romain, C.; Zhu, Y.; Dingwall, P.; Paul, S.; Rzepa, H.S.; Buchard, A.; Williams, C.K. Chemoselective Polymerizations from Mixtures of Epoxide, Lactone, Anhydride, and Carbon Dioxide. J. Am. Chem. Soc. 2016, 138, 4120–4131. [Google Scholar] [CrossRef] [Green Version]
- Gaona, M.A.; de la Cruz-Martínez, F.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Alonso-Moreno, C.; Rodríguez, A.M.; Rodríguez-Diéguez, A.; Castro-Osma, J.A.; Otero, A.; Lara-Sánchez, A. Synthesis of helical aluminium catalysts for cyclic carbonate formation. Dalton Trans. 2019, 48, 4218–4227. [Google Scholar] [CrossRef]
- Martínez, J.; Castro-Osma, J.A.; Lara-Sánchez, A.; Otero, A.; Fernández-Baeza, J.; Tejeda, J.; Sánchez-Barba, L.F.; Rodríguez-Diéguez, A. Ring-opening copolymerisation of cyclohexene oxide and carbon dioxide catalysed by scorpionate zinc complexes. Polym. Chem. 2016, 7, 6475–6484. [Google Scholar] [CrossRef]
- de la Cruz-Martínez, F.; Martínez De Sarasa Buchaca, M.; Martínez, J.; Martínez, J.; Tejeda, J.; Fernández-Baeza, J.; Alonso-Moreno, C.; Rodríguez, A.M.; Castro-Osma, J.A.; Lara-Sánchez, A. Bimetallic Zinc Catalysts for Ring-Opening Copolymerization Processes. Inorg. Chem. 2020, 59, 8412–8423. [Google Scholar] [CrossRef]
- de la Cruz-Martínez, F.; Martínez, J.; Gaona, M.A.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Rodríguez, A.M.; Castro-Osma, J.A.; Otero, A.; Lara-Sánchez, A. Bifunctional Aluminum Catalysts for the Chemical Fixation of Carbon Dioxide into Cyclic Carbonates. ACS Sustain. Chem. Eng. 2018, 6, 5322–5332. [Google Scholar] [CrossRef]
- Martínez, J.; Castro-Osma, J.A.; Alonso-Moreno, C.; Rodríguez-Diéguez, A.; North, M.; Otero, A.; Lara-Sánchez, A. One-Component Aluminum(heteroscorpionate) Catalysts for the Formation of Cyclic Carbonates from Epoxides and Carbon Dioxide. ChemSusChem 2017, 10, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
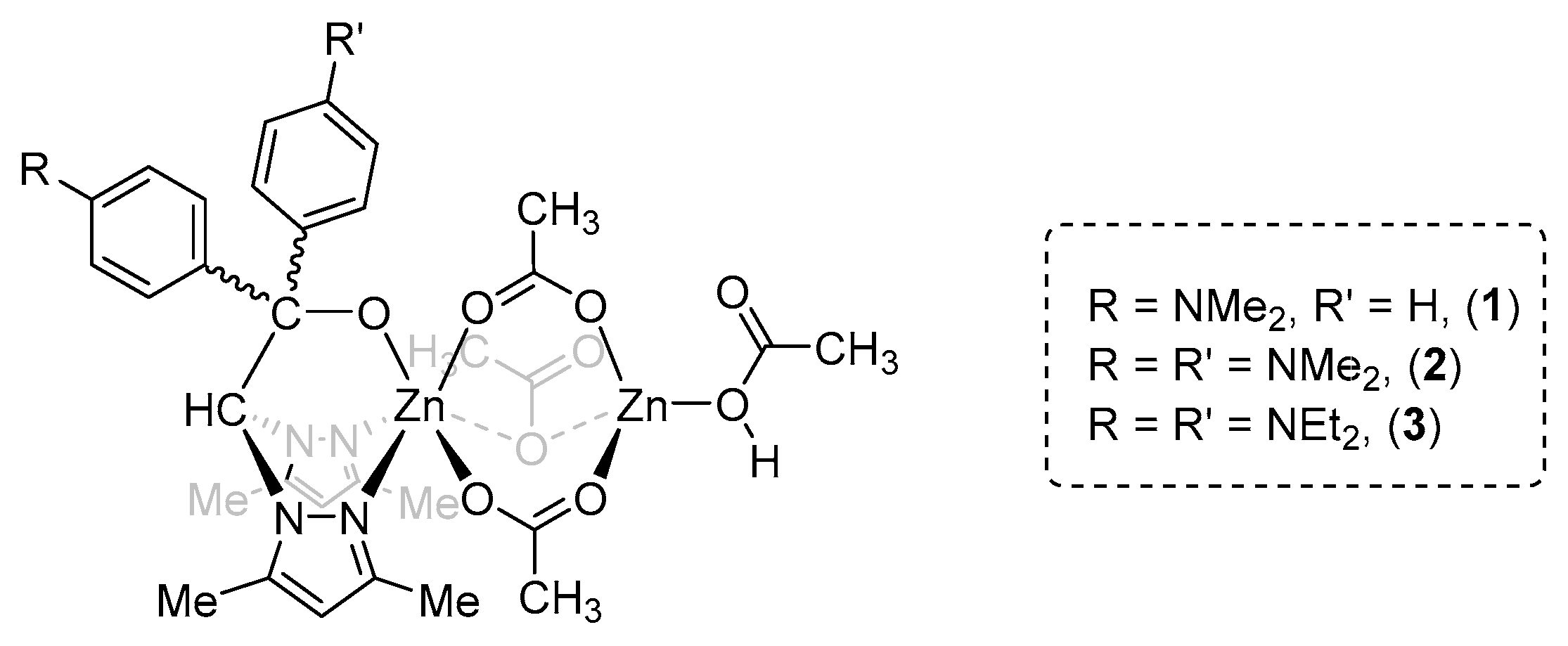



| Entry | [Cat]:[CHO]:[PA] | Catalyst | Solvent | Conversion (%) 2 | Polyester, 6 (%) 2 | Polyether, 7 (%) 2 |
|---|---|---|---|---|---|---|
| 1 | 1:100:100 | 1 | - | 70 | 22 | 78 |
| 2 | 1:100:100 | 2 | - | 75 | 20 | 80 |
| 3 | 1:100:100 | 3 | - | 76 | 28 | 72 |
| 4 | 1:200:100 | 1 | - | 75 | 20 | 80 |
| 5 | 1:200:100 | 2 | - | 77 | 18 | 82 |
| 6 | 1:200:100 | 3 | - | 78 | 21 | 79 |
| 7 | 1:100:0 | 1 | - | 0 | - | - |
| 8 | 1:100:0 | 2 | - | 0 | - | - |
| 9 | 1:100:0 | 3 | - | 0 | - | - |
| 10 | 1:100:100 | 1 | Toluene | 42 | 42 | 58 |
| 11 | 1:100:100 | 2 | Toluene | 42 | 43 | 58 |
| 12 | 1:100:100 | 3 | Toluene | 50 | 43 | 57 |
| 13 | 1:100:100 | 3 | THF | 35 | 46 | 54 |
| 14 3 | 1:100:100 | 3 | Toluene | 35 | 45 | 55 |
| 15 4 | 1:100:100 | 3 | Toluene | 57 | 90 | 10 |
| 16 5 | 1:100:100 | 3 | Toluene | 90 | 95 | 5 |
| Entry | [3]:[Cocat]:[CHO]:[PA] | Cocatalyst | Conversion (%) 2 | Polyester, 6 (%) 2 | Polyether, 7 (%) 2 |
|---|---|---|---|---|---|
| 1 | 1:2:100:100 | TBAC | 84 | 97 | 3 |
| 2 | 1:2:100:100 | TBAB | 88 | 93 | 7 |
| 3 | 1:2:100:100 | TBAI | 66 | 97 | 3 |
| 4 | 1:2:100:100 | PPNCl | 96 | 97 | 3 |
| 5 | 1:2:100:100 | PPNN3 | 66 | 95 | 5 |
| 6 | 1:2:100:100 | PPNDNP | 95 | 96 | 4 |
| 7 | 1:2:100:100 | DMAP | 90 | 95 | 5 |
| 8 3 | 1:2:100:100 | PPNCl | 60 | 94 | 6 |
| 9 3 | 1:2:100:100 | PPNDNP | 45 | 93 | 7 |
| 10 4 | 0:2:100:100 | PPNCl | 40 | 96 | 4 |
| Entry | [3]:[PPNCl]:[CHO]:[PA] | Conversion (%) 2 | Polyester, 6 (%) 2 | Mn exp (kg·mol−1) (PDI) 3 |
|---|---|---|---|---|
| 1 | 1:2:50:50 | 100 | 97 | 2.46 (1.58) |
| 2 | 1:2:100:100 | 96 | 97 | 2.87 (1.49) |
| 3 | 1:2:150:150 | 76 | 95 | 3.29 (1.45) |
| 4 | 1:2:200:200 | 70 | 91 | 3.67 (1.39) |
| Entry | [3]:[PPNCl]:[CHO]:[CA] | CA | Conversion (%) 2 | Polyester, 6 (%) 2 | Mn exp (kg·mol−1)(PDI) 3 |
|---|---|---|---|---|---|
| 1 | 1:2:100:100 | SA | 100 | 97 | 2.95 (1.43) |
| 2 | 1:2:200:200 | SA | 99 | 99 | 4.03 (1.34) |
| 3 | 1:2:100:100 | MA | 100 | 89 | 2.40 (1.51) |
| 4 | 1:2:200:200 | MA | 100 | 90 | 3.52 (1.39) |
| 5 | 1:2:100:100 | NA | 55 | 96 | 2.07 (1.48) |
| 6 4 | 1:2:100:100 | NA | 100 | 96 | 2.92 (1.45) |
| 7 4 | 1:2:200:200 | NA | 98 | 98 | 3.89 (1.37) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Cruz-Martínez, F.; Martínez de Sarasa Buchaca, M.; del Campo-Balguerías, A.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Garcés, A.; Alonso-Moreno, C.; Castro-Osma, J.A.; Lara-Sánchez, A. Ring-Opening Copolymerization of Cyclohexene Oxide and Cyclic Anhydrides Catalyzed by Bimetallic Scorpionate Zinc Catalysts. Polymers 2021, 13, 1651. https://doi.org/10.3390/polym13101651
de la Cruz-Martínez F, Martínez de Sarasa Buchaca M, del Campo-Balguerías A, Fernández-Baeza J, Sánchez-Barba LF, Garcés A, Alonso-Moreno C, Castro-Osma JA, Lara-Sánchez A. Ring-Opening Copolymerization of Cyclohexene Oxide and Cyclic Anhydrides Catalyzed by Bimetallic Scorpionate Zinc Catalysts. Polymers. 2021; 13(10):1651. https://doi.org/10.3390/polym13101651
Chicago/Turabian Stylede la Cruz-Martínez, Felipe, Marc Martínez de Sarasa Buchaca, Almudena del Campo-Balguerías, Juan Fernández-Baeza, Luis F. Sánchez-Barba, Andrés Garcés, Carlos Alonso-Moreno, José A. Castro-Osma, and Agustín Lara-Sánchez. 2021. "Ring-Opening Copolymerization of Cyclohexene Oxide and Cyclic Anhydrides Catalyzed by Bimetallic Scorpionate Zinc Catalysts" Polymers 13, no. 10: 1651. https://doi.org/10.3390/polym13101651
APA Stylede la Cruz-Martínez, F., Martínez de Sarasa Buchaca, M., del Campo-Balguerías, A., Fernández-Baeza, J., Sánchez-Barba, L. F., Garcés, A., Alonso-Moreno, C., Castro-Osma, J. A., & Lara-Sánchez, A. (2021). Ring-Opening Copolymerization of Cyclohexene Oxide and Cyclic Anhydrides Catalyzed by Bimetallic Scorpionate Zinc Catalysts. Polymers, 13(10), 1651. https://doi.org/10.3390/polym13101651










