Cellulose-Chitosan-Nanohydroxyapatite Hybrid Composites by One-Pot Synthesis for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Nano-Hydroxyapatite, nHAp
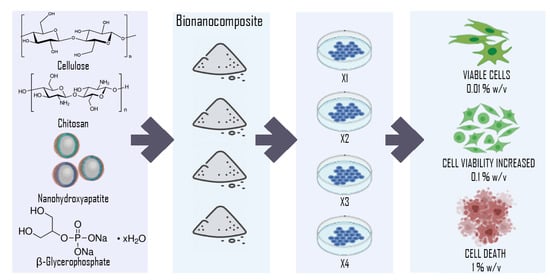
2.2. Bio-Nanocomposites Preparation
2.3. Bio-Nanocomposites Characterization
2.4. In Vitro Cytotoxicity
3. Results and Discussion
3.1. FTIR Spectrophotometry
3.2. X-ray Diffraction
3.3. Scanning Electron Microscopy
3.4. In Vitro Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darder, M.; Aranda, P.; Ruiz-Hitzky, E. Bionanocomposites: A new concept of ecological, bioinspired, and functional hybrid materials. Adv. Mater. 2007, 19, 1309–1319. [Google Scholar] [CrossRef]
- Catauro, M.; Renella, R.A.; Papale, F.; Vecchio Ciprioti, S. Investigation of bioactivity, biocompatibility and thermal behavior of sol–gel silica glass containing a high PEG percentage. Mater. Sci. Eng. C Mater Biol. Appl. 2016, 61, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Aliramaji, S.; Zamanian, A.; Mozafari, M. Super-paramagnetic responsive Silk Fibroin/chitosan/magnetite scaffolds with tunable pore structures for bone tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Delgado, A.; Hernández-Cocoletzi, H.; Rubio-Rosas, E.; Escobedo-Morales, A.; Chigo-Anota, E.; Olarte-Paredes, A.; Salgado-Delgado, R.; Trejo-Duran, M.; Castaño, V. Electrospinning production of PVA/CS/HEMA/nHA bionanocomposite. Int. J. Nano Biomater. 2019, 8, 93–105. [Google Scholar] [CrossRef]
- Ali, S.; Sangi, L.; Kumar, N.; Kumar, B.; Khurshid, Z.; Zafar, M.S. Evaluating antibacterial and surface mechanical properties of chitosan modified dental resin composites. Technol. Health Care 2020, 28, 165–173. [Google Scholar] [CrossRef]
- Husain, S.; Al-Samadani, K.H.; Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Qasim, S.B. Chitosan biomaterials for current and potential dental applications. Materials 2017, 10, 602. [Google Scholar] [CrossRef]
- She, Z.; Zhang, B.; Jin, C.; Feng, Q.; Xu, Y. Preparation and in vitro degradation of porous three-dimensional silk fibroin/chitosan scaffold. Polym. Degrad. Stab. 2008, 93, 1316–1322. [Google Scholar] [CrossRef]
- Tavakol, S.; Ragerdi Kashani, I.; Azami, M.; Khoshzaban, A.; Tavakol, B.; Kharrazi, S.; Ebrahimi, S.; Rezayat Sorkhabadi, S.M. In vitro and in vivo investigations on bone regeneration potential of laminated hydroxyapatite/gelatin nanocomposite scaffold along with DBM. J. Nanopart. Res. 2012, 14, 1265. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kozlowska, J. Characterization of collagen/hydroxyapatite composite sponges as a potential bone substitute. Int. J. Biol. Macromol. 2010, 47, 483–487. [Google Scholar] [CrossRef]
- Tavakol, S.; Khoshzaban, A.; Azami, M.; Kashani, I.R.; Tavakol, H.; Yazdanifar, M.; Sorkhabadi, S.M. The effect of carrier type on bone regeneration of demineralized bone matrix in vivo. J. Craniofac. Surg. 2013, 24, 2135–2140. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, T.; Chen, J.; Su, J.; Zhi, X.; Pan, P.; Zou, L.; Zhang, Q. Magnetic bioinspired micro/nanostructured composite scaffold for bone regeneration. Colloid Surface B 2019, 174, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Tavakol, S.; Nikpour, M.R.; Hoveizi, E.; Tavakol, B.; Rezayat, S.M.; Adabi, M.; Abokheili, S.; Jahanshahi, M. Investigating the effects of particle size and chemical structure on cytotoxicity and bacteriostatic potential of nano hydroxyapatite/chitosan/silica and nano hydroxyapatite/chitosan/silver; as antibacterial bone substitutes. J. Nanopart. Res 2014, 16, 2622. [Google Scholar] [CrossRef]
- Gholizadeh, S.; Moztarzadeh, F.; Haghighipour, N.; Ghazizadeh, L.; Baghbani, F.; Shokrgozar, M.A.; Allahyari, Z. Preparation and characterization of novel functionalized multiwalled carbon nanotubes/chitosan/β-Glycerophosphate scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2017, 97, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Hua, K.; Rocha, I.; Zhang, P.; Gustafsson, S.; Ning, Y.; Strømme, M.; Mihranyan, A.; Ferraz, N. Transition from Bioinert to Bioactive Material by Tailoring the Biological Cell Response to Carboxylated Nanocellulose. Biomacromolecules 2016, 17, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Capadona, J.R.; Van Den Berg, O.; Capadona, L.A.; Schroeter, M.; Rowan, S.J.; Tyler, D.J.; Weder, C. A versatile approach for the processing of polymer nanocomposites with self-assambled nanofibre templates. Nat. Nanotechnol. 2007, 2, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef]
- Rodríguez-Robledo, M.C.; González-Lozano, M.A.; Ponce-Peña, P.; Quintana Owen, P.; Aguilar-González, M.A.; Nieto-Castañeda, G.; Bazán-Mora, E.; López-Martínez, R.; Ramírez-Galicia, G.; Poisot, M. Cellulose-Silica Nanocomposites of High Reinforcing Content with Fungi Decay Resistance by One-Pot Synthesis. Materials 2018, 11, 575. [Google Scholar] [CrossRef]
- Chen, F.; Huang, P.; Zhu, Y.J.; Wu, J.; Zhang, C.L.; Cui, D.X. The photoluminescence, drug delivery and imaging properties of multifunctional Eu3+/Gd3+ dual-doped hydroxyapatite nanorods. Biomaterials 2011, 32, 9031–9039. [Google Scholar] [CrossRef]
- Lak, A.; Mazloumi, M.; Mohajerani, M.S.; Zanganeh, S.; Shayegh, M.R.; Kajbafvala, A.; Arami, H.; Sadrnezhaad, S.K. Rapid Formation of Mono-Dispersed Hydroxyapatite Nanorods with Narrow-Size Distribution via Microwave Irradiation. J. Am. Ceram. Soc. 2008, 91, 3580–3584. [Google Scholar] [CrossRef]
- Delgado Jiménez, J.F. Síntesis de Nanopartículas de Hidroxiapatita Dopada con Eu3+ por Irradiación de Microondas. Bacherol’s Thesis, Universidad de las Americas Puebla, Puebla, México, 2013. [Google Scholar]
- Skwarczynska, A.; Kaminska, M.; Owczarz, P.; Bartoszek, N.; Walkowiak, B.; Modrzejewska, Z. The structural (FTIR, XRD, and XPS) and biological studies of thermosensitive chitosan chloride gels with β-glycerophosphate disodium. J. Appl. Polym. Sci. 2018, 135, 46459. [Google Scholar] [CrossRef]
- Tabaght, F.E.; El Idrissi, A.; Bellaouchi, R.; Asehraou, A.; Aqil, M.; El Barkany, S.; Benarbia, A.; Achalhi, N.; Tahani, A. Cellulose grafted aliphatic polyesters: Synthesis, characterization and biodegradation under controlled conditions in a laboratory test system. J. Mol. Struct. 2020, 1205, 127582. [Google Scholar] [CrossRef]
- Lin, L.; Hao, R.; Xiong, W.; Zhong, J. Quantitative analyses of the effect of silk fibroin/nano-hydroxyapatite composites on osteogenic differentiation of MG-63 human osteosarcoma cells. J. Biosci. Bioeng. 2015, 119, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Pascu, E.I.; Stokes, J.; McGuinness, G.B. Electrospun composites of PHBV, silk fibroin and nano-hydroxyapatite for bone tissue engineering. Mater. Sci. Eng. C Mater Biol. Appl. 2013, 33, 4905–4916. [Google Scholar] [CrossRef]
- Mobika, J.; Rajkumar, M.; Nithya Priya, V.; Linto Sibi, S.P. Substantial effect of Silk fibroin reinforcement on properties of Hydroxyapatite/Silk fibroin nanocomposite for bone tissue engineering application. J. Mol. Struct. 2020, 1206, 127739. [Google Scholar] [CrossRef]
- Rivera-Munoz, E.M. Hydroxyapatite-Based Materials: Synthesis and Characterization, Biomedical Engineering—Frontiers and Challenges; Fazel, R., Ed.; InTech: London, UK, 2011; ISBN 978-953-307-309-5. [Google Scholar]
- Pereira, R.M.; Andrade, G.S.S.; Castro, H.F.D.; Campos, M.G.N. Performance of chitosan/glycerol phosphate hydrogel as a support for lipase immobilization. Mat. Res. 2017, 20, 190–201. [Google Scholar] [CrossRef]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A Quality Materials Characterization Database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Fawcett, T.G.; Crowder, C.E.; Kabekkodu, S.N.; Needham, F.; Kaduk, J.A.; Blanton, T.N.; Petkov, V.; Bucher, E.; Shpanchenko, R. Reference materials for the study of polymorphism and crystallinity in cellulosics. Powder Diffr. 2013, 28, 18–31. [Google Scholar] [CrossRef]
- Hammond, C. The Basics of Crystallography and Diffraction, IUCr Texts on Crystallography, No 3; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Niranjan, R.; Koushik, C.; Saravanan, S.; Moorthi, A.; Vairamani, M.; Selvamurugan, N. A novel injectable temperature-sensitive zinc doped chitosan/β-glycerophosphate hydrogel for bone tissue engineering. Int. J. Biol. Macromol. 2013, 54, 24–29. [Google Scholar] [CrossRef]
- Matoug-Elwerfelli, M.; Nazzal, H.; Raif, E.M.; Wilshaw, S.P.; Esteves, F.; Duggal, M. Ex-vivo recellularisation and stem cell differentiation of a decellularised rat dental pulp matrix. Sci. Rep. 2020, 10, 21553. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, B.A.; Fliesler, S.J. Streamlined duplex live-dead microplate assay for cultured cells. Exp. Eye Res. 2017, 161, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Ayyagari, V.N.; Diaz-Sylvester, P.L.; Jeff Hsieh, T.H.; Brard, L. Evaluation of the cytotoxicity of the Bithionol-paclitaxel combination in a panel of human ovarian cancer cell lines. PLoS ONE 2017, 12, e0185111. [Google Scholar] [CrossRef] [PubMed]








| Label | C g | BG g | Ch g | Ch/C + BG% | BG/Ch | nHAp g |
|---|---|---|---|---|---|---|
| X1 | 5 | 5 | 2.5 | 25 | 2 | 0.5 |
| X2 | 5 | 5 | 1.25 | 12.5 | 4 | 0.5 |
| X3 | 5 | 5 | 1 | 10 | 5 | 0.5 |
| X4 | 5 | 5 | 0.75 | 7.5 | 6.66 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarquin-Yáñez, K.; Rubio-Rosas, E.; Piñón-Zárate, G.; Castell-Rodríguez, A.; Poisot, M. Cellulose-Chitosan-Nanohydroxyapatite Hybrid Composites by One-Pot Synthesis for Biomedical Applications. Polymers 2021, 13, 1655. https://doi.org/10.3390/polym13101655
Jarquin-Yáñez K, Rubio-Rosas E, Piñón-Zárate G, Castell-Rodríguez A, Poisot M. Cellulose-Chitosan-Nanohydroxyapatite Hybrid Composites by One-Pot Synthesis for Biomedical Applications. Polymers. 2021; 13(10):1655. https://doi.org/10.3390/polym13101655
Chicago/Turabian StyleJarquin-Yáñez, Katia, Efrain Rubio-Rosas, Gabriela Piñón-Zárate, Andrés Castell-Rodríguez, and Martha Poisot. 2021. "Cellulose-Chitosan-Nanohydroxyapatite Hybrid Composites by One-Pot Synthesis for Biomedical Applications" Polymers 13, no. 10: 1655. https://doi.org/10.3390/polym13101655
APA StyleJarquin-Yáñez, K., Rubio-Rosas, E., Piñón-Zárate, G., Castell-Rodríguez, A., & Poisot, M. (2021). Cellulose-Chitosan-Nanohydroxyapatite Hybrid Composites by One-Pot Synthesis for Biomedical Applications. Polymers, 13(10), 1655. https://doi.org/10.3390/polym13101655