Thermal Effect and Mechanism Analysis of Flame-Retardant Modified Polymer Electrolyte for Lithium-Ion Battery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
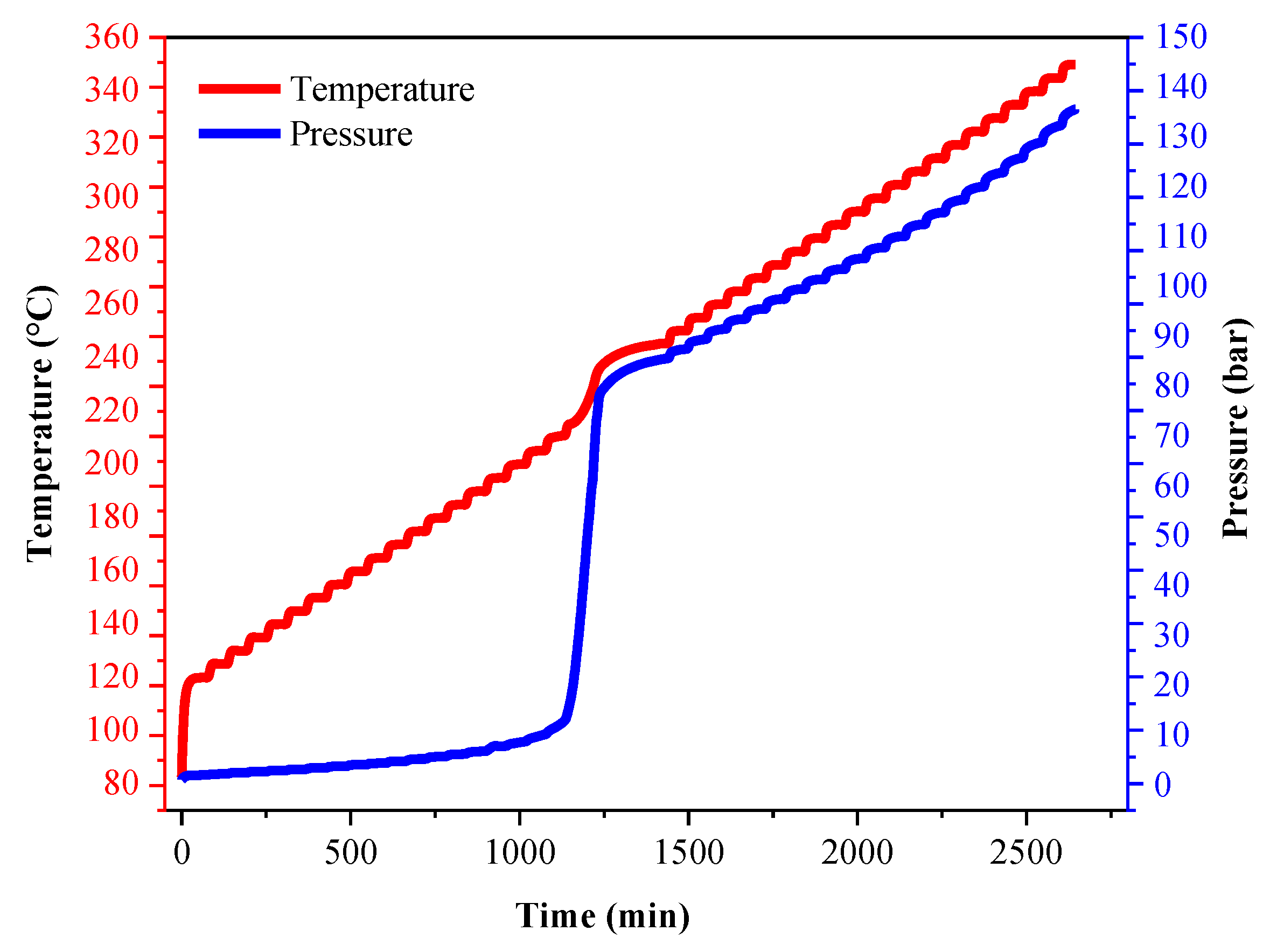
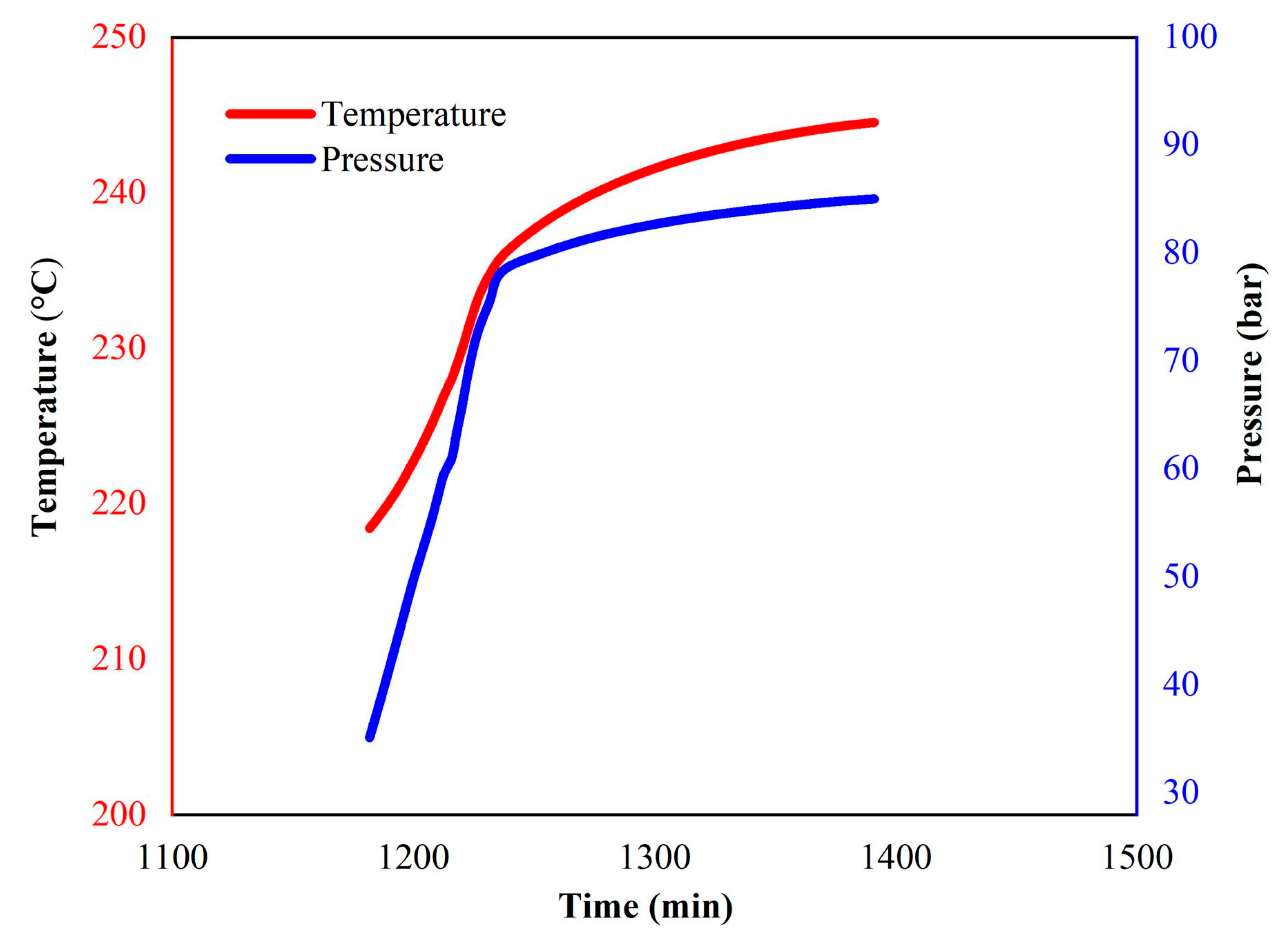
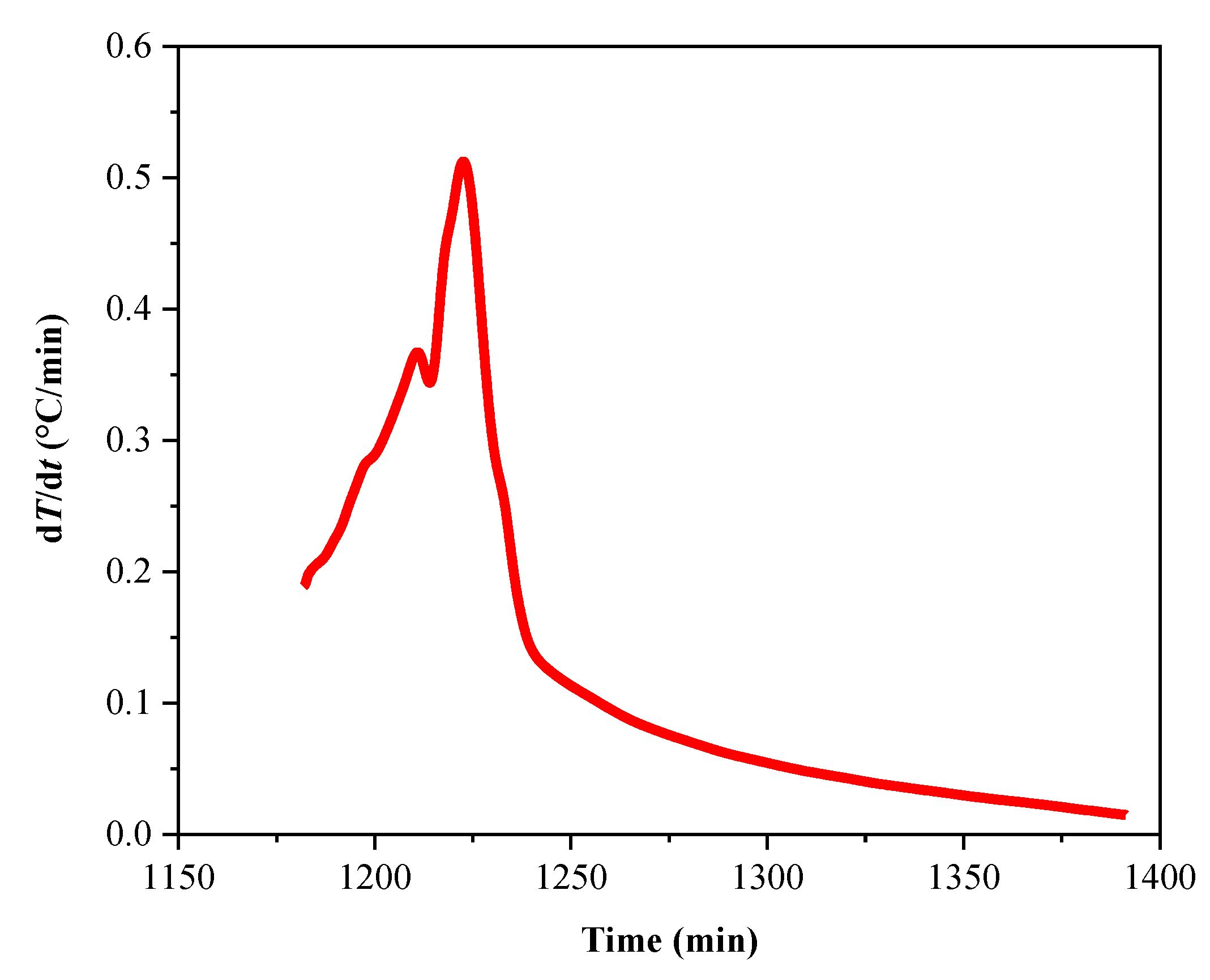
2.2. Adiabatic Reaction Calorimetry (ARC) Measurement
2.3. Differential Scanning Calorimetry (DSC) Measurement
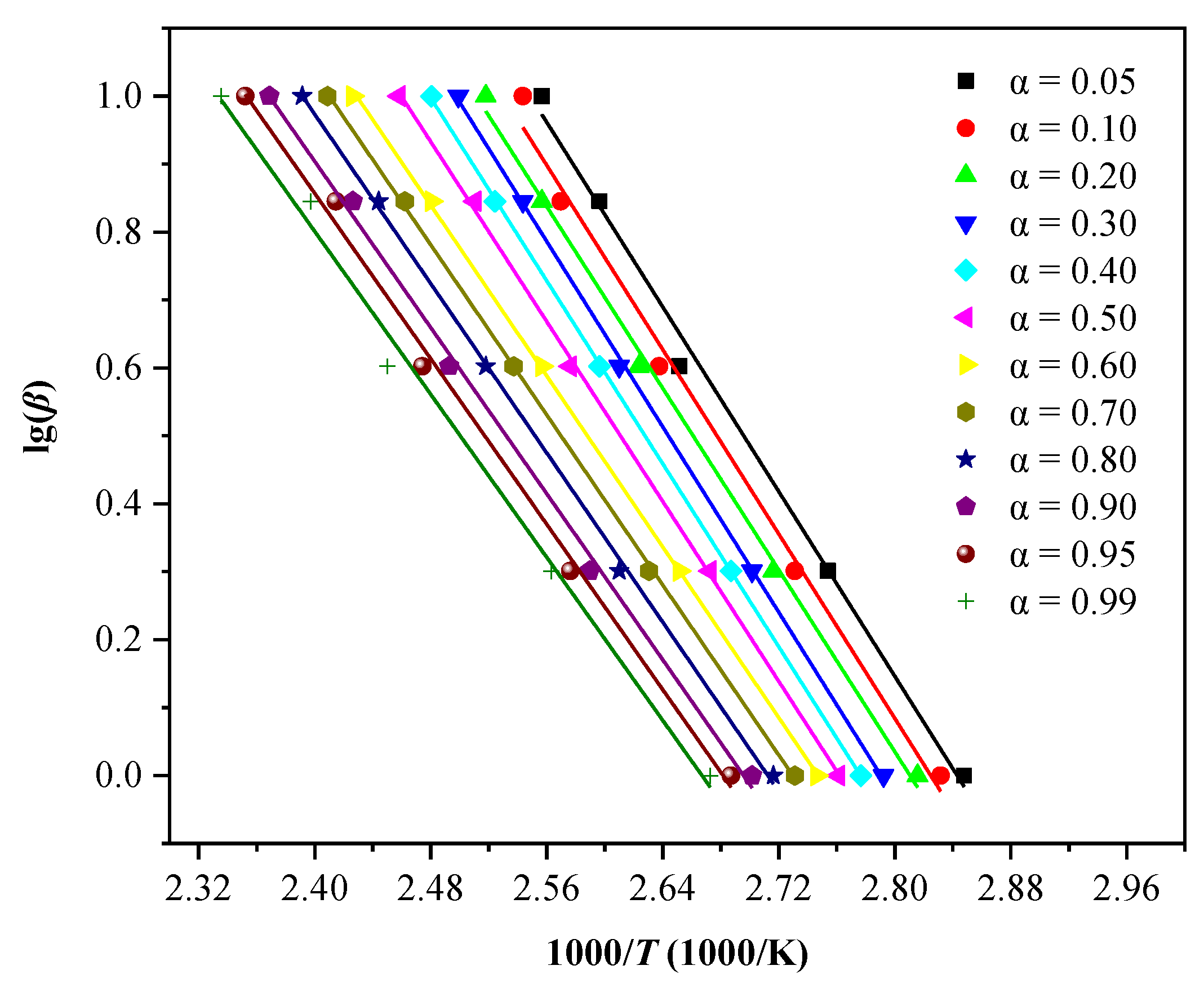
2.4. Kinetic Analysis
2.4.1. Kissinger Model
2.4.2. KAS Method
2.4.3. FWO Method
3. Results and Discussion
3.1. Analysis of Results
3.2. Analysis of Thermodynamic Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, M.; Liu, K. Rational design on separators and liquid electrolytes for safer lithium-ion batteries. J. Energy Chem. 2020, 43, 58–70. [Google Scholar] [CrossRef] [Green Version]
- Dagger, T.; Meier, V.; Hildebrand, S.; Brüggemann, D.; Winter, M.; Schappacher, F.M. Safety Performance of 5 Ah Lithium Ion Battery Cells Containing the Flame Retardant Electrolyte Additive (Phenoxy) Pentafluorocyclotriphosphazene. Energy Technol. 2018, 6, 2001–2010. [Google Scholar] [CrossRef]
- Xu, H.Y.; Xie, S.; Wang, Q.Y.; Yao, X.L.; Wang, Q.S.; Chen, C.H. Electrolyte additive trimethyl phosphite for improving electrochemical performance and thermal stability of LiCoO2 cathode. Electrochim. Acta 2006, 52, 636–642. [Google Scholar] [CrossRef]
- Xiang, H.F.; Jin, Q.Y.; Chen, C.H.; Ge, X.W.; Guo, S.; Sun, J.H. Dimethyl methylphosphonate-based nonflammable electrolyte and high safety lithium–ion batteries. J. Power Sources 2007, 174, 335–341. [Google Scholar] [CrossRef]
- Wang, X.; Yasukawa, E.; Kasuya, S. Nonflammable trimethyl phosphate solvent-containing electrolytes for lithium-ion batteries: I. fundamental properties. J. Electrochem. Soc. 2001, 148. [Google Scholar] [CrossRef]
- Yeh, F.-M.; Volli, V.; Laiwang, B.; Tung, P.-H.; Shu, C.-M. Oxidative stability and thermal performance of ester based lube oil with lithium salt additives. Appl. Therm. Eng. 2019, 150, 1328–1336. [Google Scholar] [CrossRef]
- Li, Y.; An, Y.; Tian, Y.; Fei, H.; Xiong, S.; Qian, Y.; Feng, J. Stable and Safe Lithium Metal Batteries with Ni-Rich Cathodes Enabled by a High Efficiency Flame Retardant Additive. J. Electrochem. Soc. 2019, 166, A2736–A2740. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Chen, C. Enhancing the thermal stability of LiCoO2 electrode by 4–isopropyl phenyl diphenyl phosphate in lithium ion batteries. J. Power Sources 2006, 162, 1363–1366. [Google Scholar] [CrossRef]
- Yao, X.L.; Xie, S.; Chen, C.H.; Wang, Q.S.; Sun, J.H.; Li, Y.L.; Lu, S.X. Comparative study of trimethyl phosphite and trimethyl phosphate as electrolyte additives in lithium ion batteries. J. Power Sources 2005, 144, 170–175. [Google Scholar] [CrossRef]
- Wang, W.; Liao, C.; Liu, L.; Cai, W.; Yuan, Y.; Hou, Y.; Guo, W.; Zhou, X.; Qiu, S.; Song, L.; et al. Comparable investigation of tervalent and pentavalent phosphorus based flame retardants on improving the safety and capacity of lithium-ion batteries. J. Power Sources 2019, 420, 143–151. [Google Scholar] [CrossRef]
- Liu, L.; Du, C.; Wang, S.; Chen, S. Three new bifunctional additives for safer nickel-cobalt-aluminum based lithium ion batteries. Chin. Chem. Lett. 2018, 29, 1781–1784. [Google Scholar] [CrossRef]
- Hyung, Y.E.; Vissers, D.R.; Amine, K. Flame-retardant additives for lithiumion batteries. J. Power Sources 2003, 119–121, 383–387. [Google Scholar] [CrossRef]
- Xia, L.; Xia, Y.; Liu, Z. A novel fluorocyclophosphazene as bifunctional additive for safer lithium-ion batteries. J. Power Sources 2015, 278, 190–196. [Google Scholar] [CrossRef]
- Liu, J.; Song, X.; Zhou, L.; Wang, S.; Song, W.; Liu, W.; Long, H.; Zhou, L.; Wu, H.; Feng, C.; et al. Fluorinated phosphazene derivative—A promising electrolyte additive for high voltage lithium ion batteries: From electrochemical performance to corrosion mechanism. Nano Energy 2018, 46, 404–414. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, W.; Chen, L.; Lu, Y.; Su, Y.; Bao, L.; Wang, J.; Chen, R.; Chen, S.; Wu, F. Ethoxy (pentafluoro) cyclotriphosphazene (PFPN) as a multi-functional flame retardant electrolyte additive for lithium-ion batteries. J. Power Sources 2018, 378, 707–716. [Google Scholar] [CrossRef]
- Huang, A.-C.; Huang, C.-F.; Tang, Y.; Xing, Z.-X.; Jiang, J.-C. Evaluation of multiple reactions in dilute benzoyl peroxide concentrations with additives using calorimetric technology. J. Loss Prev. Process Ind. 2021, 69, 104373–104378. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, S.-H.; Huang, A.-C.; Huang, C.-F.; Chuang, Y.-K.; Shu, C.-M. Effects of mixing malic acid and salicylic acid with metal oxides in medium- to low-temperature isothermal conditions, as determined using the thermal activity monitor IV. J. Therm. Anal. Calorim. 2018, 133, 779–784. [Google Scholar] [CrossRef]
- Huang, A.-C.; Liao, F.-C.; Huang, C.-F.; Tang, Y.; Zhang, Y.; Shu, C.-M.; Xing, Z.-X.; Jiang, J.-C.; Hsieh, W.-Y. Calorimetric approach to establishing thermokinetics for cosmeceutical benzoyl peroxides containing metal ions. J. Therm. Anal. Calorim. 2021, 144, 373–382. [Google Scholar] [CrossRef]
- Yang, Y.P.; Huang, A.C.; Tang, Y.; Liu, Y.C.; Wu, Z.H.; Zhou, H.L.; Li, Z.P.; Shu, C.M.; Jiang, J.C.; Xing, Z.X. Thermal Stability Analysis of Lithium-Ion Battery Electrolytes Based on Lithium Bis(trifluoromethanesulfonyl)imide-Lithium Difluoro(oxalato)Borate Dual-Salt. Polymers 2021, 13, 707. [Google Scholar] [CrossRef]
- Uhlemann, M.; Madian, M.; Leones, R.; Oswald, S.; Maletti, S.; Eychmuller, A.; Mikhailova, D. In-Depth Study of Li4Ti5O12 Performing beyond Conventional Operating Conditions. ACS Appl. Mater. Interfaces 2020, 12, 37227–37238. [Google Scholar] [CrossRef]
- Dagger, T.; Lürenbaum, C.; Schappacher, F.M.; Winter, M. Electrochemical performance evaluations and safety investigations of pentafluoro(phenoxy)cyclotriphosphazene as a flame retardant electrolyte additive for application in lithium ion battery systems using a newly designed apparatus for improved self-extinguishing time measurements. J. Power Sources 2017, 342, 266–272. [Google Scholar] [CrossRef]
- Yan, P.; Zhu, Y.; Pan, X.; Ji, H. A novel flame-retardant electrolyte additive for safer lithium-ion batteries. Int. J. Energy Res. 2020, 45, 2776–2784. [Google Scholar] [CrossRef]
- Lang, P.; Wojcik, T.; Povoden-Karadeniz, E.; Falahati, A.; Kozeschnik, E. Thermo-kinetic prediction of metastable and stable phase precipitation in Al–Zn–Mg series aluminium alloys during non-isothermal DSC analysis. J. Alloys Compd. 2014, 609, 129–136. [Google Scholar] [CrossRef]
- Geng, P.; Zore, A.; Van De Mark, M.R. Thermodynamic characterization of free and surface water of colloidal unimolecular polymer (CUP) particles utilizing DSC. Polymers (Basel) 2020, 12, 1417. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.-C.; Huang, C.-F.; Xing, Z.-X.; Jiang, J.-C.; Shu, C.-M. Thermal hazard assessment of the thermal stability of acne cosmeceutical therapy using advanced calorimetry technology. Process Saf. Environ. Prot. 2019, 131, 197–204. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Yang, Y.; Huang, H.C.; Shu, C.M. Inhibitory effects of three chemical dust suppressants on nitrocellulose dust cloud explosion. AlChE J. 2020, 66, 16888–16899. [Google Scholar] [CrossRef]
- Tahmasebi, A.; Yu, J.; Su, H.; Han, Y.; Lucas, J.; Zheng, H.; Wall, T. A differential scanning calorimetric (DSC) study on the characteristics and behavior of water in low-rank coals. Fuel 2014, 135, 243–252. [Google Scholar] [CrossRef]
- Cucos, A.; Budrugeac, P.; Miu, L. DMA and DSC studies of accelerated aged parchment and vegetable–tanned leather samples. Thermochim. Acta 2014, 583, 86–93. [Google Scholar] [CrossRef]
- Duh, Y.-S.; Ho, T.-C.; Chen, J.-R.; Kao, C.-S. Study on exothermic oxidation of acrylonitrile-butadiene-styrene (ABS) resin powder with application to ABS processing safety. Polymers 2010, 2, 174–187. [Google Scholar] [CrossRef] [Green Version]
- Tsai, Y.T.; Huang, G.T.; Zhao, J.Q.; Shu, C.M. Dust cloud explosion characteristics and mechanisms in MgH2 based hydrogen storage materials. AlChE J. 2021. [Google Scholar] [CrossRef]
- Wu, W.-Q.; Feng, W.; Lin, Q.-H.; Wang, S.-Y.; Guo, Z.-C.; Chen, L.-P.; Chen, W.-H. Synthesis and thermal decomposition of TNPG. Thermochim. Acta 2020, 683, 178396. [Google Scholar] [CrossRef]
- Vyazovkin, S. Kissinger method in kinetics of materials: Things to beware and be aware of. Molecules 2020, 25, 2813. [Google Scholar] [CrossRef] [PubMed]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Ferrer, N.; Serra, E.; Sempere, J.; Nomen, R. Non-parametric kinetic analysis of autocatalytic reactions. J. Loss Prev. Process Ind. 2017, 49, 357–366. [Google Scholar] [CrossRef]
- Deng, J.; Zhao, J.-Y.; Huang, A.-C.; Zhang, Y.-N.; Wang, C.-P.; Shu, C.-M. Thermal behavior and microcharacterization analysis of second-oxidized coal. J. Therm. Anal. Calorim. 2017, 439–448. [Google Scholar] [CrossRef]
- Farjas, J.; Roura, P. Exact analytical solution for the Kissinger equation: Determination of the peak temperature and general properties of thermally activated transformations. Thermochim. Acta 2014, 598, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Tankov, I.; Yankova, R.; Mitkova, M.; Stratiev, D. Non-isothermal decomposition kinetics of pyridinium nitrate under nitrogen atmosphere. Thermochim. Acta 2018, 665, 85–91. [Google Scholar] [CrossRef]
- Bianchi, O.; Oliveira, R.V.B.; Fiorio, R.; De Martins, J.N.; Zattera, A.J.; Canto, L.B. Assessment of Avrami, Ozawa and Avrami–Ozawa equations for determination of EVA crosslinking kinetics from DSC measurements. Polym. Test. 2008, 27, 722–729. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Sbirrazzuoli, N. Isoconversional kinetic analysis of thermally stimulated processes in polymers. Macromol. Rapid Commun. 2006, 27, 1515–1532. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.; Huang, G.; Liu, H.; Yang, S.; Zhang, A. Thermal degradation behaviors and reaction mechanism of carbon fibre-epoxy composite from hydrogen tank by TG-FTIR. J. Hazard. Mater. 2018, 357, 73–80. [Google Scholar] [CrossRef]
- Campion, C.L.; Li, W.; Lucht, B.L. Thermal decomposition of LiPF6-based electrolytes for lithium-ion batteries. J. Electrochem. Soc. 2005, 152, 35–42. [Google Scholar] [CrossRef]
- Sloop, S.E.; Pugh, J.K.; Wang, S.; Kerr, J.B.; Kinoshita, K. Chemical reactivity of PF5 and LiPF6 in ethylene carbonate/dimethyl carbonate solutions. Electrochem. Solid State Lett. 2001, 4, 357–364. [Google Scholar] [CrossRef]
- Venkatesh, M.; Ravi, P.; Tewari, S.P. Isoconversional kinetic analysis of decomposition of nitroimidazoles: Friedman method vs. Flynn–Wall–Ozawa method. J. Phys. Chem. A 2013, 117, 10162–10181. [Google Scholar] [CrossRef] [PubMed]
- El Hazzat, M.; Sifou, A.; Arsalane, S.; El Hamidi, A. Novel approach to thermal degradation kinetics of gypsum: Application of peak deconvolution and Model-Free isoconversional method. J. Therm. Anal. Calorim. 2019, 140, 657–671. [Google Scholar] [CrossRef]





| Organic Solvent | Boiling Point (°C) | Flash Point (°C) | Melting Point (°C) | Viscosity (mPa·s) (25 °C) | Dielectric Constant |
|---|---|---|---|---|---|
| Ethylene carbonate (EC) | 248 | 160 | 37 | 1.90 | 89.6 |
| Dimethyl carbonate (DMC) | 90 | 17 | 2 | 0.62 | 2.6 |
| Propylene carbonate (PC) | 242 | 128 | −49 | 2.50 | 69.0 |
| Diethyl carbonate (DEC) | 126 | 25 | −43 | 0.75 | 2.8 |
| Ethyl methyl carbonate (EMC) | 108 | 23 | −55 | 0.65 | 2.9 |
| β (°C/min) | Mass (mg) | LP30 | LP30 + PFPN | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| To1 (°C) | Tp1 (°C) | Te1 (°C) | To2 (°C) | Tp2 (°C) | Te2 (°C) | To1 (°C) | Tp1 (°C) | Te1 (°C) | To2 (°C) | Tp2 (°C) | Te2 (°C) | ||
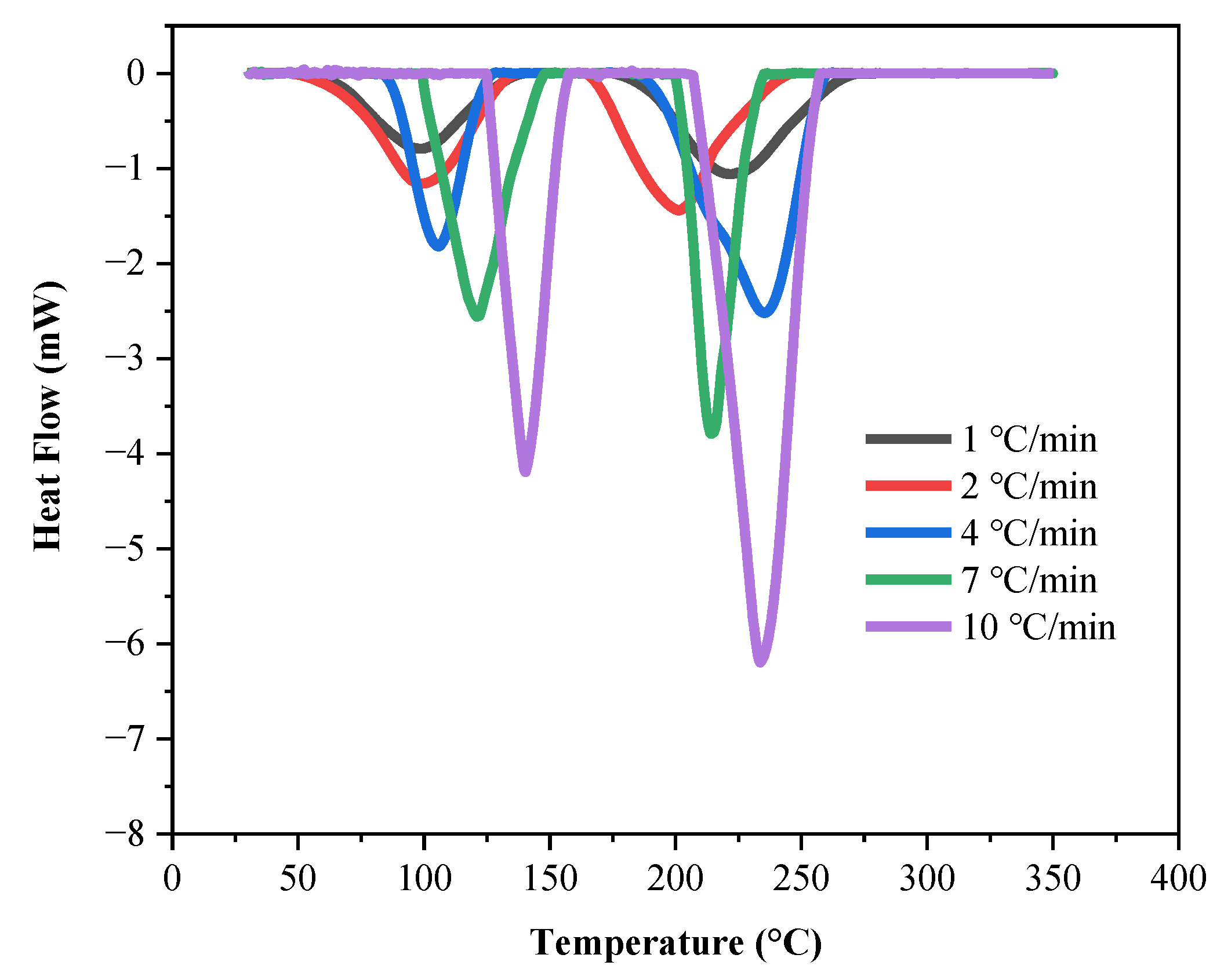
| 1 | 6.5 ± 0.5 | 68.67 | 103.33 | 132.33 | 192.67 | 223.00 | 260.33 | 61.33 | 96.67 | 140.33 | 178.67 | 222.00 | 272.67 |
| 2 | 78.67 | 103.33 | 123.33 | 191.67 | 223.00 | 258.00 | 56.00 | 97.67 | 137.00 | 162.33 | 200.00 | 248.67 | |
| 4 | 89.00 | 104.67 | 122.00 | 193.33 | 239.67 | 262.33 | 81.33 | 105.33 | 129.67 | 183.00 | 234.00 | 264.00 | |
| 7 | 95.45 | 117.85 | 146.55 | 195.90 | 212.70 | 234.47 | 95.45 | 121.00 | 150.058 | 196.25 | 214.10 | 236.85 | |
| 10 | 121.67 | 140.67 | 158.33 | 202.33 | 234.67 | 258.67 | 122.00 | 139.00 | 159.33 | 204.33 | 233.67 | 260.67 | |
| ms (g) | mball (g) | Material | Temperature (°C) | Heating Rate (°C/min) | Exothern Threshold (°C/min) | Waiting Time (min) | Temperature Increment (°C) |
|---|---|---|---|---|---|---|---|
| 3.346 | 21.000 | Hastelloy | 80–350 | 10 | 0.02 | 30 | 5 |
| α | Ea (kJ/mol) | R2 |
|---|---|---|
| 0.05 | 60.5039 | 0.9931 |
| 0.1 | 60.4064 | 0.9909 |
| 0.2 | 59.5369 | 0.9969 |
| 0.3 | 60.7497 | 0.9995 |
| 0.4 | 59.9467 | 0.9999 |
| 0.5 | 58.9380 | 0.9992 |
| 0.6 | 55.9757 | 0.9997 |
| 0.7 | 55.7361 | 0.9996 |
| 0.8 | 55.4792 | 0.9988 |
| 0.9 | 54.4398 | 0.9966 |
| 0.95 | 54.1983 | 0.9948 |
| 0.99 | 53.4124 | 0.9917 |
| (kJ/mol) | ||
|---|---|---|
| Kissinger | 50.8878 | 0.9948 |
| KAS | 54.1431 | 0.9970 |
| FWO | 57.4436 | 0.9967 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.-H.; Huang, A.-C.; Tang, Y.; Yang, Y.-P.; Liu, Y.-C.; Li, Z.-P.; Zhou, H.-L.; Huang, C.-F.; Xing, Z.-X.; Shu, C.-M.; et al. Thermal Effect and Mechanism Analysis of Flame-Retardant Modified Polymer Electrolyte for Lithium-Ion Battery. Polymers 2021, 13, 1675. https://doi.org/10.3390/polym13111675
Wu Z-H, Huang A-C, Tang Y, Yang Y-P, Liu Y-C, Li Z-P, Zhou H-L, Huang C-F, Xing Z-X, Shu C-M, et al. Thermal Effect and Mechanism Analysis of Flame-Retardant Modified Polymer Electrolyte for Lithium-Ion Battery. Polymers. 2021; 13(11):1675. https://doi.org/10.3390/polym13111675
Chicago/Turabian StyleWu, Zhi-Hao, An-Chi Huang, Yan Tang, Ya-Ping Yang, Ye-Cheng Liu, Zhi-Ping Li, Hai-Lin Zhou, Chung-Fu Huang, Zhi-Xiang Xing, Chi-Min Shu, and et al. 2021. "Thermal Effect and Mechanism Analysis of Flame-Retardant Modified Polymer Electrolyte for Lithium-Ion Battery" Polymers 13, no. 11: 1675. https://doi.org/10.3390/polym13111675
APA StyleWu, Z.-H., Huang, A.-C., Tang, Y., Yang, Y.-P., Liu, Y.-C., Li, Z.-P., Zhou, H.-L., Huang, C.-F., Xing, Z.-X., Shu, C.-M., & Jiang, J.-C. (2021). Thermal Effect and Mechanism Analysis of Flame-Retardant Modified Polymer Electrolyte for Lithium-Ion Battery. Polymers, 13(11), 1675. https://doi.org/10.3390/polym13111675









