Polymer Composites Filled with Metal Derivatives: A Review of Flame Retardants
Abstract
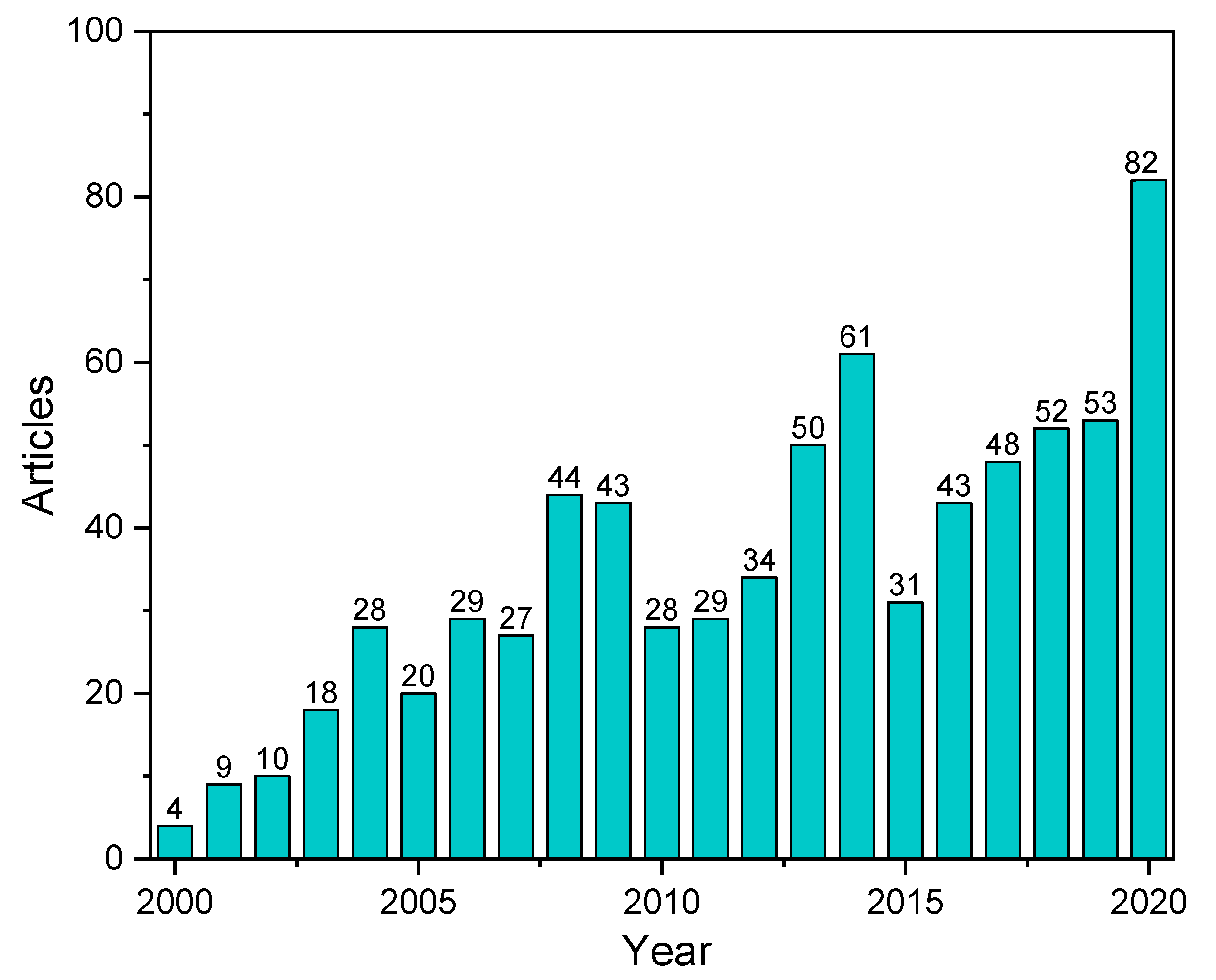
:1. Introduction
2. Flame Retardancy of Polymer Reinforced Composites
3. Combustion Mechanism and Flame Retardancy of Composites
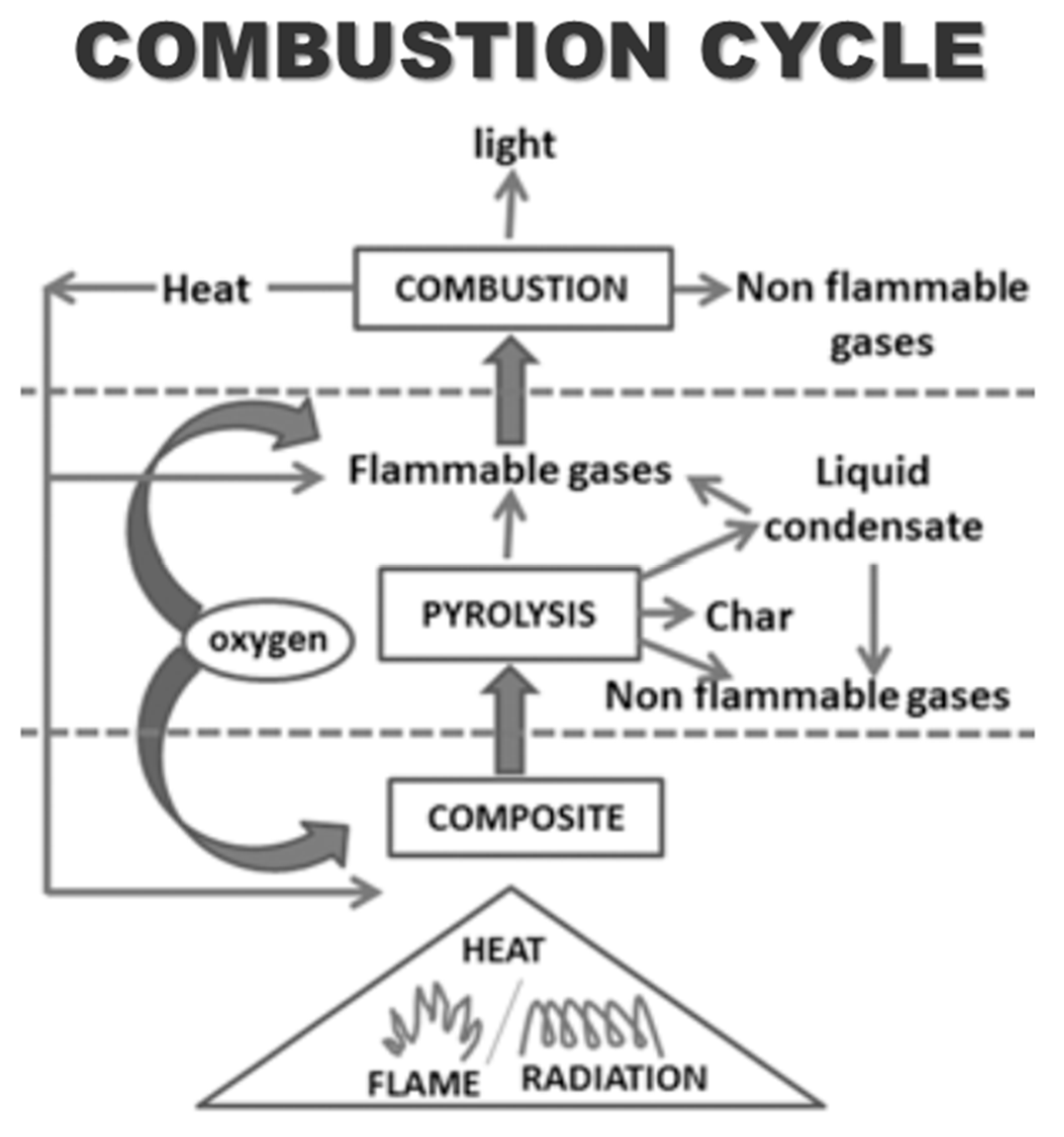
3.1. Combustion Mechanism
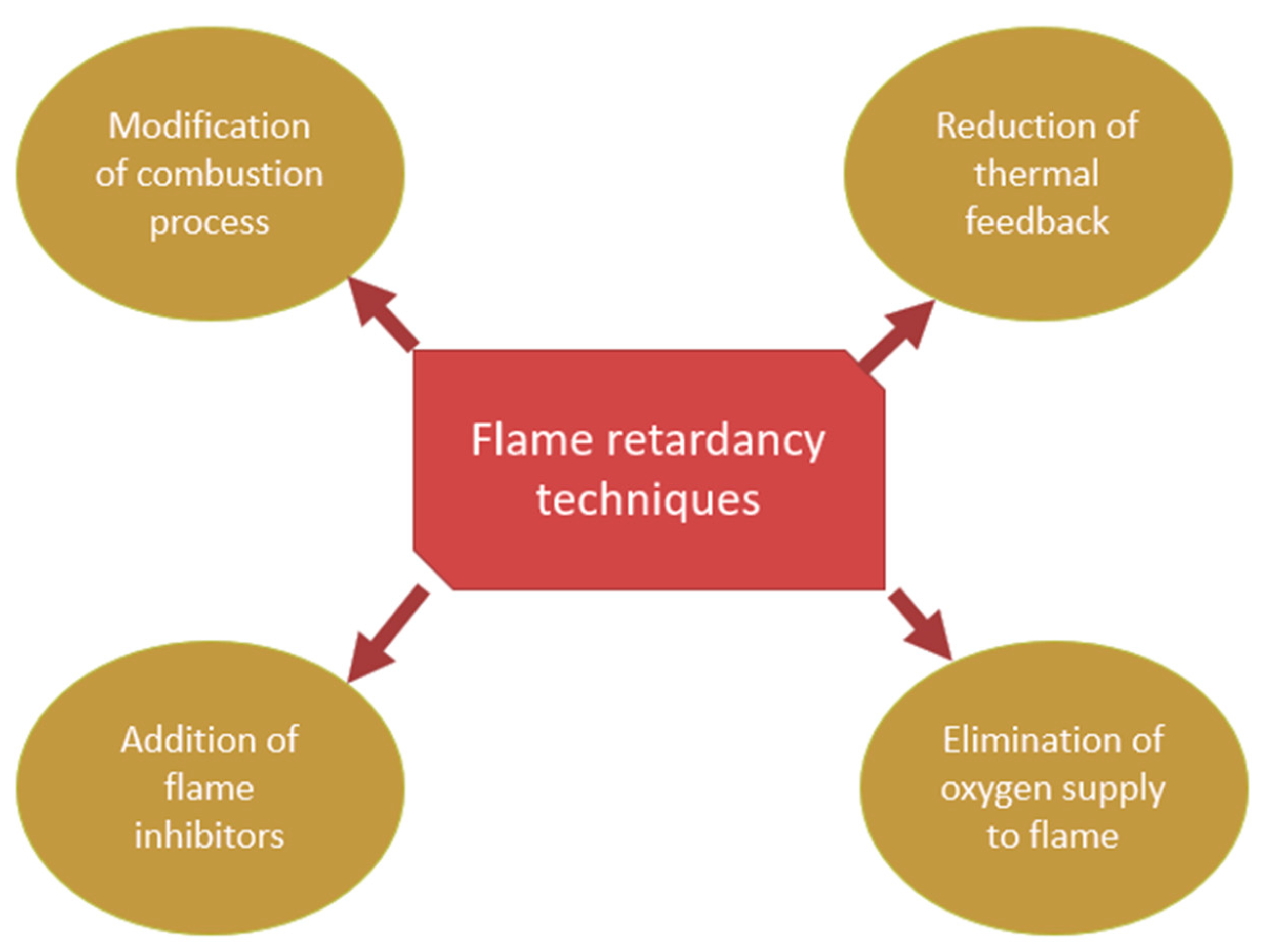
3.2. Flame Retardant Techniques
4. Characterization of Composites after Flame Retardant Treatment
5. The Economic Analysis of Metal-Filled Polymer Composites
6. Drawbacks and Challenges
7. Conclusions and Future Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ye, T.-P.; Liao, S.-F.; Zhang, Y.; Chen, M.-J.; Xiao, Y.; Liu, X.-Y.; Liu, Z.-G.; Wang, D.-Y. Cu(0) and Cu(II) decorated graphene hybrid on improving fireproof efficiency of intumescent flame-retardant epoxy resins. Compos. Part B Eng. 2019, 175, 107189. [Google Scholar] [CrossRef]
- Mamunya, Y.P.; Davydenko, V.V.; Pissis, P.; Lebedev, E.V. Electrical and thermal conductivity of polymers filled with metal powders. Eur. Polym. J. 2002, 38, 1887–1897. [Google Scholar] [CrossRef]
- Suriani, M.J.; Radzi, F.S.M.; Ilyas, R.A.; Petrů, M.; Sapuan, S.M.; Ruzaidi, C.M. Flammability, Tensile, and Morphological Properties of Oil Palm Empty Fruit Bunches Fiber/Pet Yarn-Reinforced Epoxy Fire Retardant Hybrid Polymer Composites. Polymers 2021, 13, 1282. [Google Scholar] [CrossRef] [PubMed]
- Suriani, M.J.; Sapuan, S.M.; Ruzaidi, C.M.; Nair, D.S.; Ilyas, R.A. Flammability, morphological and mechanical properties of sugar palm fiber/polyester yarn-reinforced epoxy hybrid biocomposites with magnesium hydroxide flame retardant filler. Text. Res. J. 2021, 1–12. [Google Scholar] [CrossRef]
- Suriani, M.J.; Rapi, H.Z.; Ilyas, R.A.; Petrů, M.; Sapuan, S.M. Delamination and Manufacturing Defects in Natural Fiber-Reinforced Hybrid Composite: A Review. Polymers 2021, 13, 1323. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.; Sofian, N.; Rusu, D. Mechanical and thermal properties of zinc powder filled high density polyethylene composites. Polym. Test. 2001, 20, 409–417. [Google Scholar] [CrossRef]
- Nurazreena; Hussain, L.B.; Ismail, H.; Mariatti, M. Metal filled high density polyethylene composites—Electrical and tensile properties. J. Thermoplast. Compos. Mater. 2006, 19, 413–425. [Google Scholar] [CrossRef]
- Omran, A.A.B.; Mohammed, A.A.B.A.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Koloor, S.S.R.; Petrů, M. Micro- and Nanocellulose in Polymer Composite Materials: A Review. Polymers 2021, 13, 231. [Google Scholar] [CrossRef]
- Asyraf, M.R.M.; Ishak, M.R.; Sapuan, S.M.; Yidris, N. Utilization of Bracing Arms as Additional Reinforcement in Pultruded Glass Fiber-Reinforced Polymer Composite Cross-Arms: Creep Experimental and Numerical Analyses. Polymers 2021, 13, 620. [Google Scholar] [CrossRef]
- Gonzalez-Gutierrez, J.; Cano, S.; Schuschnigg, S.; Kukla, C.; Sapkota, J.; Holzer, C. Additive manufacturing of metallic and ceramic components by the material extrusion of highly-filled polymers: A review and future perspectives. Materials 2018, 11, 840. [Google Scholar] [CrossRef] [Green Version]
- Asyraf, M.R.M.; Ishak, M.R.; Sapuan, S.M.; Yidris, N.; Rafidah, M.; Ilyas, R.A.; Razman, M.R. Potential application of green composites for cross arm component in transmission tower: A brief review. Int. J. Polym. Sci. 2020. [CrossRef]
- Amir, A.L.; Ishak, M.R.; Yidris, N.; Zuhri, M.Y.M.; Asyraf, M.R.M. Potential of Honeycomb-Filled Composite Structure in Composite Cross-Arm Component: A Review on Recent Progress and Its Mechanical Properties. Polymers 2021, 13, 1341. [Google Scholar] [CrossRef] [PubMed]
- Alsubari, S.; Zuhri, M.Y.M.; Sapuan, S.M.; Ishak, M.R.; Ilyas, R.A.; Asyraf, M.R.M. Potential of Natural Fiber Reinforced Polymer Composites in Sandwich Structures: A Review on Its Mechanical Properties. Polymers 2021, 13, 423. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Harussani, M.M.; Hakimi, M.Y.A.Y.; Haziq, M.Z.M.; Atikah, M.S.N.; Asyraf, M.R.M.; Ishak, M.R.; Razman, M.R.; Nurazzi, N.M.; et al. Polylactic Acid (PLA) Biocomposite: Processing, Additive Manufacturing and Advanced Applications. Polymers 2021, 13, 1326. [Google Scholar] [CrossRef] [PubMed]
- Özgür, S.M.; Amar, M.K.; Manjusri, M. Fiber Technology for Fiber-Reinforced Composites, 1st ed.; Özgür, S.M., Amar, M.K., Manjusri, M., Eds.; Elsevier: Duxford, UK, 2017; ISBN 9780081018712. [Google Scholar]
- Ilyas, R.A.; Sapuan, M.S.; Norizan, M.N.; Norrrahim, M.N.F.; Ibrahim, R.; Atikah, M.S.N.; Huzaifah, M.R.M.; Radzi, A.M.; Izwan, S.; Azammi, A.M.N.; et al. Macro to nanoscale natural fiber composites for automotive components: Research, development, and application. In Biocomposite and Synthetic Composites for Automotive Applications; Sapuan, M.S., Ilyas, R.A., Eds.; Woodhead Publishing Series: Amsterdam, The Netherland, 2020. [Google Scholar]
- Asyraf, M.R.M.; Ishak, M.R.; Sapuan, S.M.; Yidris, N.; Ilyas, R.A. Woods and composites cantilever beam: A comprehensive review of experimental and numerical creep methodologies. J. Mater. Res. Technol. 2020, 9, 6759–6776. [Google Scholar] [CrossRef]
- Mohd Nurazzi, N.; Asyraf, M.R.M.; Khalina, A.; Abdullah, N.; Sabaruddin, F.A.; Kamarudin, S.H.; Ahmad, S.; Mahat, A.M.; Lee, C.L.; Aisyah, H.A.; et al. Fabrication, Functionalization, and Application of Carbon Nanotube-Reinforced Polymer Composite: An Overview. Polymers 2021, 13, 1047. [Google Scholar] [CrossRef] [PubMed]
- Asyraf, M.R.M.; Ishak, M.R.; Sapuan, S.M.; Yidris, N.; Ilyas, R.A.; Rafidah, M.; Razman, M.R. Evaluation of design and simulation of creep test rig for full-scale cross arm structure. Adv. Civ. Eng. 2020. [Google Scholar] [CrossRef]
- Asyraf, M.R.M.; Rafidah, M.; Ishak, M.R.; Sapuan, S.M.; Ilyas, R.A.; Razman, M.R. Integration of TRIZ, Morphological Chart and ANP method for development of FRP composite portable fire extinguisher. Polym. Compos. 2020, 1–6. [Google Scholar] [CrossRef]
- Asyraf, M.R.M.; Ishak, M.R.; Sapuan, S.M.; Yidris, N. Comparison of Static and Long-term Creep Behaviors between Balau Wood and Glass Fiber Reinforced Polymer Composite for Cross-arm Application. Fibers Polym. 2021, 22. [Google Scholar] [CrossRef]
- Asyraf, M.R.M.; Ishak, M.R.; Sapuan, S.M.; Yidris, N. Conceptual design of creep testing rig for full-scale cross arm using TRIZ-Morphological chart-analytic network process technique. J. Mater. Res. Technol. 2019, 8, 5647–5658. [Google Scholar] [CrossRef]
- Asyraf, M.R.M.; Ishak, M.R.; Sapuan, S.M.; Yidris, N. Conceptual design of multi-operation outdoor flexural creep test rig using hybrid concurrent engineering approach. J. Mater. Res. Technol. 2020, 9, 2357–2368. [Google Scholar] [CrossRef]
- Johari, A.N.; Ishak, M.R.; Leman, Z.; Yusoff, M.Z.M.; Asyraf, M.R.M. Influence of CaCO3 in pultruded glass fibre/unsaturated polyester composite on flexural creep behaviour using conventional and TTSP methods. Polimery 2020, 65, 46–54. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Asyraf, M.R.M.; Khalina, A.; Abdullah, N.; Aisyah, H.A.; Rafiqah, S.A.; Sabaruddin, F.A.; Kamarudin, S.H.; Norrrahim, M.N.F.; Ilyas, R.A.; et al. A Review on Natural Fiber Reinforced Polymer Composite for Bullet Proof and Ballistic Applications. Polymers 2021, 13, 646. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Sapuan, S.M.; Atikah, M.S.N.; Asyraf, M.R.M.; Rafiqah, S.A.; Aisyah, H.A.; Nurazzi, N.M.; Norrrahim, M.N.F. Effect of hydrolysis time on the morphological, physical, chemical, and thermal behavior of sugar palm nanocrystalline cellulose (Arenga pinnata (Wurmb.) Merr). Text. Res. J. 2021, 91, 152–167. [Google Scholar] [CrossRef]
- Woodrow, B. “Fire as Vulnerability”: The Value Added from Adopting a Vulnerability Approach. World Fire Stat. Bull. 2012, 28, 1–26. [Google Scholar]
- Sari, N.H.; Pruncu, C.I.; Sapuan, S.M.; Ilyas, R.A.; Catur, A.D.; Suteja, S.; Sutaryono, Y.A.; Pullen, G. The effect of water immersion and fibre content on properties of corn husk fibres reinforced thermoset polyester composite. Polym. Test. 2020, 91, 106751. [Google Scholar] [CrossRef]
- Syafri, E.; Sudirman; Mashadi; Yulianti, E.; Deswita; Asrofi, M.; Abral, H.; Sapuan, S.M.; Ilyas, R.A.; Fudholi, A. Effect of sonication time on the thermal stability, moisture absorption, and biodegradation of water hyacinth (Eichhornia crassipes) nanocellulose-filled bengkuang (Pachyrhizus erosus) starch biocomposites. J. Mater. Res. Technol. 2019, 8, 6223–6231. [Google Scholar] [CrossRef]
- Siakeng, R.; Jawaid, M.; Asim, M.; Saba, N.; Sanjay, M.R.; Siengchin, S.; Fouad, H. Alkali treated coir/pineapple leaf fibres reinforced PLA hybrid composites: Evaluation of mechanical, morphological, thermal and physical properties. Polym. Lett. 2020, 14, 717–730. [Google Scholar] [CrossRef]
- Abral, H.; Ariksa, J.; Mahardika, M.; Handayani, D.; Aminah, I.; Sandrawati, N.; Sapuan, S.M.; Ilyas, R.A. Highly transparent and antimicrobial PVA based bionanocomposites reinforced by ginger nanofiber. Polym. Test. 2019, 106186. [Google Scholar] [CrossRef]
- Abral, H.; Ariksa, J.; Mahardika, M.; Handayani, D.; Aminah, I.; Sandrawati, N.; Pratama, A.B.; Fajri, N.; Sapuan, S.M.; Ilyas, R.A. Transparent and antimicrobial cellulose film from ginger nanofiber. Food Hydrocoll. 2020, 98, 105266. [Google Scholar] [CrossRef]
- Prachayawarakorn, J.; Limsiriwong, N.; Kongjindamunee, R.; Surakit, S. Effect of Agar and Cotton Fiber on Properties of Thermoplastic Waxy Rice Starch Composites. J. Polym. Environ. 2012, 20, 88–95. [Google Scholar] [CrossRef]
- Kumar, T.S.M.; Chandrasekar, M.; Senthilkumar, K.; Ilyas, R.A.; Sapuan, S.M.; Hariram, N.; Rajulu, A.V.; Rajini, N.; Siengchin, S. Characterization, Thermal and Antimicrobial Properties of Hybrid Cellulose Nanocomposite Films with in-Situ Generated Copper Nanoparticles in Tamarindus indica Nut Powder. J. Polym. Environ. 2020, 1–10. [Google Scholar] [CrossRef]
- Aisyah, H.A.; Paridah, M.T.; Sapuan, S.M.; Khalina, A.; Berkalp, O.B.; Lee, S.H.; Lee, C.H.; Nurazzi, N.M.; Ramli, N.; Wahab, M.S.; et al. Thermal Properties of Woven Kenaf/Carbon Fibre-Reinforced Epoxy Hybrid Composite Panels. Int. J. Polym. Sci. 2019, 2019, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Mazani, N.; Sapuan, S.M.; Sanyang, M.L.; Atiqah, A.; Ilyas, R.A. Design and Fabrication of a Shoe Shelf from Kenaf Fiber Reinforced Unsaturated Polyester Composites. In Lignocellulose for Future Bioeconomy; Ariffin, H., Sapuan, S.M., Hassan, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 315–332. ISBN 9780128163542. [Google Scholar]
- Aiza Jaafar, C.N.; Zainol, I.; Ishak, N.S.; Ilyas, R.A.; Sapuan, S.M. Effects of the Liquid Natural Rubber (LNR) on Mechanical Properties and Microstructure of Epoxy/Silica/Kenaf Hybrid Composite for Potential Automotive Applications. J. Mater. Res. Technol. 2021, 12, 1026–1038. [Google Scholar] [CrossRef]
- Sabaruddin, F.A.; Paridah, M.T.; Sapuan, S.M.; Ilyas, R.A.; Lee, S.H.; Abdan, K.; Mazlan, N.; Roseley, A.S.M.; Abdul Khalil, H.P.S. The effects of unbleached and bleached nanocellulose on the thermal and flammability of polypropylene-reinforced kenaf core hybrid polymer bionanocomposites. Polymers 2020, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Suriani, M.J.; Zainudin, H.A.; Ilyas, R.A.; Petrů, M.; Sapuan, S.M.; Ruzaidi, C.M.; Mustapha, R. Kenaf Fiber/Pet Yarn Reinforced Epoxy Hybrid Polymer Composites: Morphological, Tensile, and Flammability Properties. Polymers 2021, 13, 1532. [Google Scholar] [CrossRef]
- Jumaidin, R.; Adam, N.W.; Ilyas, R.A.; Hussin, M.S.F.; Taha, M.M.; Mansor, M.R.; Azlan, U.A.-A.; Yob, M.S. Water Transport and Physical Properties of Sugarcane Bagasse Fibre Reinforced Thermoplastic Potato Starch Biocomposite. J. Adv. Res. Fluid Mech. Therm. Sci. 2019, 61, 273–281. [Google Scholar]
- Asrofi, M.; Sujito; Syafri, E.; Sapuan, S.M.; Ilyas, R.A. Improvement of Biocomposite Properties Based Tapioca Starch and Sugarcane Bagasse Cellulose Nanofibers. Key Eng. Mater. 2020, 849, 96–101. [Google Scholar] [CrossRef]
- Asrofi, M.; Sapuan, S.M.; Ilyas, R.A.; Ramesh, M. Characteristic of composite bioplastics from tapioca starch and sugarcane bagasse fiber: Effect of time duration of ultrasonication (Bath-Type). Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Correa-Aguirre, J.P.; Luna-Vera, F.; Caicedo, C.; Vera-Mondragón, B.; Hidalgo-Salazar, M.A. The Effects of Reprocessing and Fiber Treatments on the Properties of Polypropylene-Sugarcane Bagasse Biocomposites. Polymers 2020, 12, 1440. [Google Scholar] [CrossRef] [PubMed]
- Nassiopoulos, E.; Njuguna, J. Thermo-mechanical performance of poly(lactic acid)/flax fibre-reinforced biocomposites. Mater. Des. 2015, 66, 473–485. [Google Scholar] [CrossRef]
- Syafri, E.; Kasim, A.; Abral, H.; Asben, A. Cellulose nanofibers isolation and characterization from ramie using a chemical-ultrasonic treatment. J. Nat. Fibers 2018, 1–11. [Google Scholar] [CrossRef]
- Battegazzore, D.; Noori, A.; Frache, A. Hemp hurd and alfalfa as particle filler to improve the thermo-mechanical and fire retardant properties of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate). Polym. Compos. 2019, 40. [Google Scholar] [CrossRef]
- Prachayawarakorn, J.; Chaiwatyothin, S.; Mueangta, S.; Hanchana, A. Effect of jute and kapok fibers on properties of thermoplastic cassava starch composites. Mater. Des. 2013, 47, 309–315. [Google Scholar] [CrossRef]
- Gupta, M.; Singh, R. PLA-coated sisal fibre-reinforced polyester composite: Water absorption, static and dynamic mechanical properties. J. Compos. Mater. 2019, 53, 65–72. [Google Scholar] [CrossRef]
- Ayu, R.S.; Khalina, A.; Harmaen, A.S.; Zaman, K.; Isma, T.; Liu, Q.; Ilyas, R.A.; Lee, C.H. Characterization Study of Empty Fruit Bunch (EFB) Fibers Reinforcement in Poly(Butylene) Succinate (PBS)/Starch/Glycerol Composite Sheet. Polymers 2020, 12, 1571. [Google Scholar] [CrossRef] [PubMed]
- Jumaidin, R.; Diah, N.A.; Ilyas, R.A.; Alamjuri, R.H.; Yusof, F.A.M. Processing and Characterisation of Banana Leaf Fibre Reinforced Thermoplastic Cassava Starch Composites. Polymers 2021, 13, 1420. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Atiqah, A.; Ibrahim, R.; Abral, H.; Ishak, M.R.; Zainudin, E.S.; Nurazzi, N.M.; Atikah, M.S.N.; Ansari, M.N.M.; et al. Sugar palm (Arenga pinnata [Wurmb.] Merr) starch films containing sugar palm nanofibrillated cellulose as reinforcement: Water barrier properties. Polym. Compos. 2020, 41, 459–467. [Google Scholar] [CrossRef]
- Rozilah, A.; Jaafar, C.N.A.; Sapuan, S.M.; Zainol, I.; Ilyas, R.A. The Effects of Silver Nanoparticles Compositions on the Mechanical, Physiochemical, Antibacterial, and Morphology Properties of Sugar Palm Starch Biocomposites for Antibacterial Coating. Polymers 2020, 12, 2605. [Google Scholar] [CrossRef]
- Atiqah, A.; Jawaid, M.; Sapuan, S.M.; Ishak, M.R.; Ansari, M.N.M.; Ilyas, R.A. Physical and thermal properties of treated sugar palm/glass fibre reinforced thermoplastic polyurethane hybrid composites. J. Mater. Res. Technol. 2019, 8, 3726–3732. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R. Isolation and characterization of nanocrystalline cellulose from sugar palm fibres (Arenga Pinnata). Carbohydr. Polym. 2018, 181, 1038–1051. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S. Development and characterization of sugar palm nanocrystalline cellulose reinforced sugar palm starch bionanocomposites. Carbohydr. Polym. 2018, 202, 186–202. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Sapuan, S.M.; Ibrahim, R.; Abral, H.; Ishak, M.R.; Zainudin, E.S.; Atiqah, A.; Atikah, M.S.N.; Syafri, E.; Asrofi, M.; et al. Thermal, Biodegradability and Water Barrier Properties of Bio-Nanocomposites Based on Plasticised Sugar Palm Starch and Nanofibrillated Celluloses from Sugar Palm Fibres. J. Biobased Mater. Bioenergy 2020, 14, 234–248. [Google Scholar] [CrossRef]
- Atikah, M.S.N.; Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S.; Ibrahim, R.; Atiqah, A.; Ansari, M.N.M.; Jumaidin, R. Degradation and physical properties of sugar palm starch/sugar palm nanofibrillated cellulose bionanocomposite. Polimery 2019, 64, 680–689. [Google Scholar] [CrossRef] [Green Version]
- Ilyas, R.A.; Sapuan, S.M.; Ibrahim, R.; Abral, H.; Ishak, M.R.; Zainudin, E.S.; Atikah, M.S.N.; Mohd Nurazzi, N.; Atiqah, A.; Ansari, M.N.M.; et al. Effect of sugar palm nanofibrillated cellulose concentrations on morphological, mechanical and physical properties of biodegradable films based on agro-waste sugar palm (Arenga pinnata (Wurmb.) Merr) starch. J. Mater. Res. Technol. 2019, 8, 4819–4830. [Google Scholar] [CrossRef]
- Hazrol, M.D.; Sapuan, S.M.; Ilyas, R.A.; Othman, M.L.; Sherwani, S.F.K. Electrical properties of sugar palm nanocrystalline cellulose reinforced sugar palm starch nanocomposites. Polimery 2020, 65, 363–370. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S. Sugar palm nanofibrillated cellulose (Arenga pinnata (Wurmb.) Merr): Effect of cycles on their yield, physic-chemical, morphological and thermal behavior. Int. J. Biol. Macromol. 2019, 123, 379–388. [Google Scholar] [CrossRef]
- Asyraf, M.R.M.; Rafidah, M.; Azrina, A.; Razman, M.R. Dynamic mechanical behaviour of kenaf cellulosic fibre biocomposites: A comprehensive review on chemical treatments. Cellulose 2021. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Fang, Z.; Huang, Y.; Xu, H.; Liu, Y.; Wu, D.; Zhuang, J.; Sun, J. Recent Advances in Preparation, Mechanisms, and Applications of Thermally Conductive Polymer Composites: A Review. J. Compos. Sci. 2020, 4, 180. [Google Scholar] [CrossRef]
- Shrigondekar, H.; Chowdhury, A.; Prabhu, S.V. Performance of water mist system with base injection in extinguishing small container fires. J. Loss Prev. Process Ind. 2021, 71, 104448. [Google Scholar] [CrossRef]
- Kalyon, D.M.; Birinci, E.; Yazici, R.; Karuv, B.; Walsh, S. Electrical properties of composites as affected by the degree of mixedness of the conductive filler in the polymer matrix. Polym. Eng. Sci. 2002, 42, 1609–1617. [Google Scholar] [CrossRef]
- Feng, N.L.; Malingam, S.D.; Ping, C.W. Mechanical characterisation of kenaf/PALF reinforced composite-metal laminates: Effects of hybridisation and weaving architectures. J. Reinf. Plast. Compos. 2021, 40, 193–205. [Google Scholar] [CrossRef]
- Chandrasekar, M.; Ishak, M.R.; Sapuan, S.M.; Leman, Z.; Jawaid, M.; Shahroze, R.M. Fabrication of Fibre Metal Laminate with Flax and Sugar Palm Fibre based Epoxy Composite and Evaluation of their Fatigue Properties. J. Polym. Mater. 2019, 35, 463–473. [Google Scholar] [CrossRef]
- Krishnasamy, P.; Rajamurugan, G.; Thirumurugan, M. Performance of fiber metal laminate composites embedded with AL and CU wire mesh. J. Ind. Text. 2020. [Google Scholar] [CrossRef]
- El-Sabbagh, A.; Steuernagel, L.; Ziegmann, G. Low combustible polypropylene/flax/magnesium hydroxide composites: Mechanical, flame retardation characterization and recycling effect. J. Reinf. Plast. Compos. 2013, 32, 1030–1043. [Google Scholar] [CrossRef] [Green Version]
- Bar, M.; Alagirusamy, R.; Das, A. Flame retardant polymer composites. Fibers Polym. 2015, 16, 705–717. [Google Scholar] [CrossRef]
- Marosi, G.; Szolnoki, B.; Bocz, K.; Toldy, A. Fire-retardant recyclable and biobased polymer composites. In Novel Fire Retardant Polymers and Composite Materials; Wang, D.-Y., Ed.; Elsevier: Duxford, UK, 2017; pp. 117–146. [Google Scholar]
- Kim, N.K.; Dutta, S.; Bhattacharyya, D. A review of flammability of natural fibre reinforced polymeric composites. Compos. Sci. Technol. 2018, 162, 64–78. [Google Scholar] [CrossRef]
- Akash; Girisha, K.G.; Venkatesha Gupta, N.S.; Sreenivas Rao, K.V. A study on flammability and moisture absorption behavior of sisal/coir fiber reinforced hybrid composites. IOP Conf. Ser. Mater. Sci. Eng. 2017, 191. [Google Scholar] [CrossRef]
- Zhao, C.-S.; Huang, F.-L.; Xiong, W.-C.; Wang, Y.-Z. A novel halogen-free flame retardant for glass-fiber-reinforced poly(ethylene terephthalate). Polym. Degrad. Stab. 2008, 93, 1188–1193. [Google Scholar] [CrossRef]
- Zadeh, K.M.; Ponnamma, D.; Al Ali Al-Maadeed, M. Date palm fibre filled recycled ternary polymer blend composites with enhanced flame retardancy. Polym. Test. 2017, 61, 341–348. [Google Scholar] [CrossRef]
- Yuan, B.; Sheng, H.; Mu, X.; Song, L.; Tai, Q.; Shi, Y.; Liew, K.M.; Hu, Y. Enhanced flame retardancy of polypropylene by melamine-modified graphene oxide. J. Mater. Sci. 2015, 50, 5389–5401. [Google Scholar] [CrossRef]
- Vieira, L.M.G.; dos Santos, J.C.; Panzera, T.H.; Rubio, J.C.C.; Scarpa, F. Novel fibre metal laminate sandwich composite structure with sisal woven core. Ind. Crops Prod. 2017, 99, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Ishak, N.M.; Malingam, S.D.; Mansor, M.R.; Razali, N.; Mustafa, Z.; Ghani, A.F.A. Investigation of natural fibre metal laminate as car front hood. Mater. Res. Express 2021, 8. [Google Scholar] [CrossRef]
- Levchik, S.V. Introduction to Flame Retardancy and Polymer Flammability. In Flame Retardant Polymer Nanocomposites; Morgan, A.B., Wilkie, C.A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 1–29. ISBN 9780471734260. [Google Scholar]
- Zhang, P.; Song, L.; Lu, H.; Wang, J.; Hu, Y. The thermal property and flame retardant mechanism of intumescent flame retardant paraffin system with metal. Ind. Eng. Chem. Res. 2010. [Google Scholar] [CrossRef]
- Gao, W.; Yu, Y.; Chen, T.; Zhang, Q.; Chen, Z.; Chen, Z.; Jiang, J. Enhanced flame retardancy of unsaturated polyester resin composites containing ammonium polyphosphate and metal oxides. J. Appl. Polym. Sci. 2020, 137, 49148. [Google Scholar] [CrossRef]
- Jia, Y.-W.; Zhao, X.; Fu, T.; Li, D.-F.; Guo, Y.; Wang, X.-L.; Wang, Y.-Z. Synergy effect between quaternary phosphonium ionic liquid and ammonium polyphosphate toward flame retardant PLA with improved toughness. Compos. Part B Eng. 2020, 197, 108192. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, T.; Zhang, W.; Dou, Y. Influence of surface flame-retardant layer containing ammonium polyphosphate and expandable graphite on the performance of jute/polypropylene composites. J. Therm. Anal. Calorim. 2019, 135, 2367–2375. [Google Scholar] [CrossRef]
- Pang, X.; Xin, Y.; Shi, X.; Xu, J. Effect of different size-modified expandable graphite and ammonium polyphosphate on the flame retardancy, thermal stability, physical, and mechanical properties of rigid polyurethane foam. Polym. Eng. Sci. 2019, 59, 1381–1394. [Google Scholar] [CrossRef]
- 8Shi, X.; Pan, Y.; Wang, Y.; Jia, Z.; Chen, T.; Gong, J.; Jiang, J. Synergistic Effects of Graphene and Ammonium Polyphosphate Modified with Vinyltrimethoxysilane on the Properties of High-Impact Polystyrene Composites. Polymers 2021, 13, 881. [Google Scholar] [CrossRef]
- Zhou, R.; Ming, Z.; He, J.; Ding, Y.; Jiang, J. Effect of Magnesium Hydroxide and Aluminum Hydroxide on the Thermal Stability, Latent Heat and Flammability Properties of Paraffin/HDPE Phase Change Blends. Polymers 2020, 12, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, W.; Song, P.; Yu, B.; Fang, Z.; Wang, H. Flame retardant polymeric nanocomposites through the combination of nanomaterials and conventional flame retardants. Prog. Mater. Sci. 2020, 114, 100687. [Google Scholar] [CrossRef]
- Kalali, E.N.; Zhang, L.; Shabestari, M.E.; Croyal, J.; Wang, D.-Y. Flame-retardant wood polymer composites (WPCs) as potential fire safe bio-based materials for building products: Preparation, flammability and mechanical properties. Fire Saf. J. 2019, 107, 210–216. [Google Scholar] [CrossRef]
- Yen, Y.Y.; Wang, H.T.; Guo, W.J. Synergistic flame retardant effect of metal hydroxide and nanoclay in EVA composites. Polym. Degrad. Stab. 2012, 97, 863–869. [Google Scholar] [CrossRef]
- Zhang, S.; Horrocks, A.R. A review of flame retardant polypropylene fibres. Prog. Polym. Sci. 2003, 28, 1517–1538. [Google Scholar] [CrossRef]
- Lin, M.; Li, B.; Li, Q.; Li, S.; Zhang, S. Synergistic effect of metal oxides on the flame retardancy and thermal degradation of novel intumescent flame-retardant thermoplastic polyurethanes. J. Appl. Polym. Sci. 2011, 121, 1951–1960. [Google Scholar] [CrossRef]
- Sain, M.; Park, S.H.; Suhara, F.; Law, S. Flame retardant and mechanical properties of natural fibre-PP composites containing magnesium hydroxide. Polym. Degrad. Stab. 2004, 83, 363–367. [Google Scholar] [CrossRef]
- Jeencham, R.; Suppakarn, N.; Jarukumjorn, K. Effect of flame retardants on flame retardant, mechanical, and thermal properties of sisal fiber/polypropylene composites. Compos. Part B Eng. 2014. [Google Scholar] [CrossRef]
- Wang, B.; Sheng, H.; Shi, Y.; Hu, W.; Hong, N.; Zeng, W.; Ge, H.; Yu, X.; Song, L.; Hu, Y. Recent advances for microencapsulation of flame retardant. Polym. Degrad. Stab. 2015, 113, 96–109. [Google Scholar] [CrossRef]
- Wang, D.Y.; Liu, Y.; Wang, Y.Z.; Artiles, C.P.; Hull, T.R.; Price, D. Fire retardancy of a reactively extruded intumescent flame retardant polyethylene system enhanced by metal chelates. Polym. Degrad. Stab. 2007, 92, 1592–1598. [Google Scholar] [CrossRef]
- Wang, D.L.; Liu, Y.; Wang, D.Y.; Zhao, C.X.; Mou, Y.R.; Wang, Y.Z. A novel intumescent flame-retardant system containing metal chelates for polyvinyl alcohol. Polym. Degrad. Stab. 2007. [Google Scholar] [CrossRef]
- Rothon, R.; Hornsby, P. Fire Retardant Fillers for Polymers. In Polymer Green Flame Retardants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 289–321. ISBN 9780444538093. [Google Scholar]
- Braun, U.; Bahr, H.; Sturm, H.; Schartel, B. Flame retardancy mechanisms of metal phosphinates and metal phosphinates in combination with melamine cyanurate in glass-fiber reinforced poly(1,4-butylene terephthalate): The influence of metal cation. Polym. Adv. Technol. 2008, 19, 680–692. [Google Scholar] [CrossRef]
- Wu, N.; Yang, R. Effects of metal oxides on intumescent flame-retardant polypropylene. Polym. Adv. Technol. 2011. [Google Scholar] [CrossRef]
- Lujan-Acosta, R.; Sánchez-Valdes, S.; Ramírez-Vargas, E.; Ramos-DeValle, L.F.; Espinoza-Martinez, A.B.; Rodriguez-Fernandez, O.S.; Lozano-Ramirez, T.; Lafleur, P.G. Effect of Amino alcohol functionalized polyethylene as compatibilizer for LDPE/EVA/clay/flame-retardant nanocomposites. Mater. Chem. Phys. 2014, 146, 437–445. [Google Scholar] [CrossRef]
- Wang, Z.; Han, E.; Ke, W. Effect of nanoparticles on the improvement in fire-resistant and anti-ageing properties of flame-retardant coating. Surf. Coat. Technol. 2006. [Google Scholar] [CrossRef]
- Xiao, F.; Wu, K.; Luo, F.; Yao, S.; Lv, M.; Zou, H.; Lu, M. Influence of Ionic Liquid-Based Metal–Organic Hybrid on Thermal Degradation, Flame Retardancy, and Smoke Suppression Properties of Epoxy Resin Composites. J. Mater. Sci. 2018, 53, 10135–10146. [Google Scholar] [CrossRef]
- Gallo, E.; Schartel, B.; Acierno, D.; Russo, P. Flame retardant biocomposites: Synergism between phosphinate and nanometric metal oxides. Eur. Polym. J. 2011. [Google Scholar] [CrossRef]
- Beyer, G. Flame retardant properties of EVA-nanocomposites and improvements by combination of nanofillers with aluminium trihydrate. Fire Mater. 2001, 25, 193–197. [Google Scholar] [CrossRef]
- Murphy, J. Flame retardants: Trends and new developments. Reinf. Plast. 2001, 45, 42–46. [Google Scholar] [CrossRef]
- Sharma, S.K.; Saxena, N.K. Flame Retardant Smoke Suppressant Protection for Poly Vinylchloride. Fire Technol. 2004, 40, 385–398. [Google Scholar] [CrossRef]
- Beyer, G. Flame retardancy of nanocomposites from research to technical products. J. Fire Sci. 2005. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.Z. A review on flame retardant technology in China. Part I: Development of flame retardants. Polym. Adv. Technol. 2010, 21, 1–26. [Google Scholar] [CrossRef]
- Dogan, M.; Murat Unlu, S. Flame retardant effect of boron compounds on red phosphorus containing epoxy resins. Polym. Degrad. Stab. 2014, 99, 12–17. [Google Scholar] [CrossRef]
- Topfer, O.; Clauss, M.; Futterer, T.; Schmitt, E. Flame retardants for engineering thermoplastics used in electric and electronic equipment like connectors. In Proceedings of the Electronics Goes Green 2012+, ECG 2012—Joint International Conference and Exhibition, Proceedings; Lang, K.-D., Nissen, N.F., Middendorf, A., Chancerel, P., Eds.; Fraunhofer Verlag: Berlin, Germany, 2012. [Google Scholar]
- Kusakli, S.; Kocaman, S.; Ceyhan, A.A.; Ahmetli, G. Improving the flame retardancy and mechanical properties of epoxy composites using flame retardants with red mud waste. J. Appl. Polym. Sci. 2020, 1–15. [Google Scholar] [CrossRef]
- Song, P.; Fang, Z.; Tong, L.; Jin, Y.; Lu, F. Effects of metal chelates on a novel oligomeric intumescent flame retardant system for polypropylene. J. Anal. Appl. Pyrolysis 2008, 82, 286–291. [Google Scholar] [CrossRef]
- Malkapuram, R.; Kumar, V.; Singh Negi, Y. Recent development in natural fiber reinforced polypropylene composites. J. Reinf. Plast. Compos. 2009, 28, 1169–1189. [Google Scholar] [CrossRef]
- Müller, P.; Morys, M.; Sut, A.; Jäger, C.; Illerhaus, B.; Schartel, B. Melamine poly(zinc phosphate) as flame retardant in epoxy resin: Decomposition pathways, molecular mechanisms and morphology of fire residues. Polym. Degrad. Stab. 2016. [Google Scholar] [CrossRef]
- Hu, C.; Xue, J.; Dong, L.; Jiang, Y.; Wang, X.; Qu, L.; Dai, L. Scalable preparation of multifunctional fire-retardant ultralight graphene foams. ACS Nano 2016. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.J.; Horrocks, A.R.; Alderson, A. The sensitisation of thermal decomposition of ammonium polyphosphate by selected metal ions and their potential for improved cotton fabric flame retardancy. Polym. Degrad. Stab. 2005. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Kuila, T. Fire Retardancy of Elastomers and Elastomer Nanocomposites. In Polymer Green Flame Retardants; Papaspyrides, C.D., Kiliaris, P., Eds.; Elsevier: Oxford, UK, 2014; pp. 597–651. ISBN 9780444538093. [Google Scholar]
- Holdsworth, A.F.; Horrocks, A.R.; Kandola, B.K.; Price, D. The potential of metal oxalates as novel flame retardants and synergists for engineering polymers. Polym. Degrad. Stab. 2014. [Google Scholar] [CrossRef]
- Otsuki, M.; Ogino, T. Flame-retardant additives for lithium-ion batteries. In Lithium-Ion Batteries: Science and Technologies; Yoshio, M., Brodd, R.J., Kozawa, A., Eds.; Springer-Verlag New York: New York, NY, USA, 2009; pp. 275–290. ISBN 9780387344447. [Google Scholar]
- Abd El-Wahab, H.; Abd El-Fattah, M.; El-alfy, H.M.Z.; Owda, M.E.; Lin, L.; Hamdy, I. Synthesis and characterisation of sulphonamide (Schiff base) ligand and its copper metal complex and their efficiency in polyurethane varnish as flame retardant and antimicrobial surface coating additives. Prog. Org. Coat. 2020. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. A review of recent progress in phosphorus-based flame retardants. J. Fire Sci. 2006. [Google Scholar] [CrossRef]
- Chang, M.K.; Hwang, S.S.; Liu, S.P. Flame retardancy and thermal stability of ethylene-vinyl acetate copolymer nanocomposites with alumina trihydrate and montmorillonite. J. Ind. Eng. Chem. 2014. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, T.; Yan, H.; Peng, M.; Fang, Z. Modification of ramie fabric with a metal-ion-doped flame-retardant coating. J. Appl. Polym. Sci. 2013. [Google Scholar] [CrossRef]
- Üreyen, M.E.; Kaynak, E. Effect of Zinc Borate on Flammability of PET Woven Fabrics. Adv. Polym. Technol. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Suppakarn, N.; Jarukumjorn, K. Mechanical properties and flammability of sisal/PP composites: Effect of flame retardant type and content. Compos. Part B Eng. 2009, 40, 613–618. [Google Scholar] [CrossRef]
- Li, H.; Ning, N.; Zhang, L.; Wang, Y.; Liang, W.; Tian, M. Different flame retardancy effects and mechanisms of aluminium phosphinate in PPO, TPU and PP. Polym. Degrad. Stab. 2014, 105, 86–95. [Google Scholar] [CrossRef]
- Bulei, C.; Kiss, I.; Alexa, V. Development of metal matrix composites using recycled secondary raw materials from aluminium wastes. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Vo Dong, P.A.; Azzaro-Pantel, C.; Boix, M.; Jacquemin, L.; Domenech, S. Modelling of Environmental Impacts and Economic Benefits of Fibre Reinforced Polymers Composite Recycling Pathways; Elsevier: Amsterdam, The Netherlands, 2015; Volume 37, ISBN 9780444635761. [Google Scholar]
- Tahir, F.; Mabrouk, A.; Al-Ghamdi, S.G.; Krupa, I.; Sedlacek, T.; Abdala, A.; Koc, M. Sustainability Assessment and Techno-Economic Analysis of Thermally Enhanced Polymer Tube for Multi-Effect Distillation (MED) Technology. Polymers 2021, 13, 681. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Cheng, H.M. The fabrication, properties, and uses of graphene/polymer composites. Macromol. Chem. Phys. 2012, 213, 1060–1077. [Google Scholar] [CrossRef]
- Pan, Y.T.; Zhang, Z.; Yang, R. The rise of MOFs and their derivatives for flame retardant polymeric materials: A critical review. Compos. Part B Eng. 2020, 199. [Google Scholar] [CrossRef]
- Su, Z.; Wang, H.; Ye, X.; Tian, K.; Huang, W.; He, J.; Guo, Y.; Tian, X. Anisotropic thermally conductive flexible polymer composites filled with hexagonal born nitride (h-BN) platelets and ammine carbon nanotubes (CNT-NH2): Effects of the filler distribution and orientation. Compos. Part A Appl. Sci. Manuf. 2018, 109, 402–412. [Google Scholar] [CrossRef]
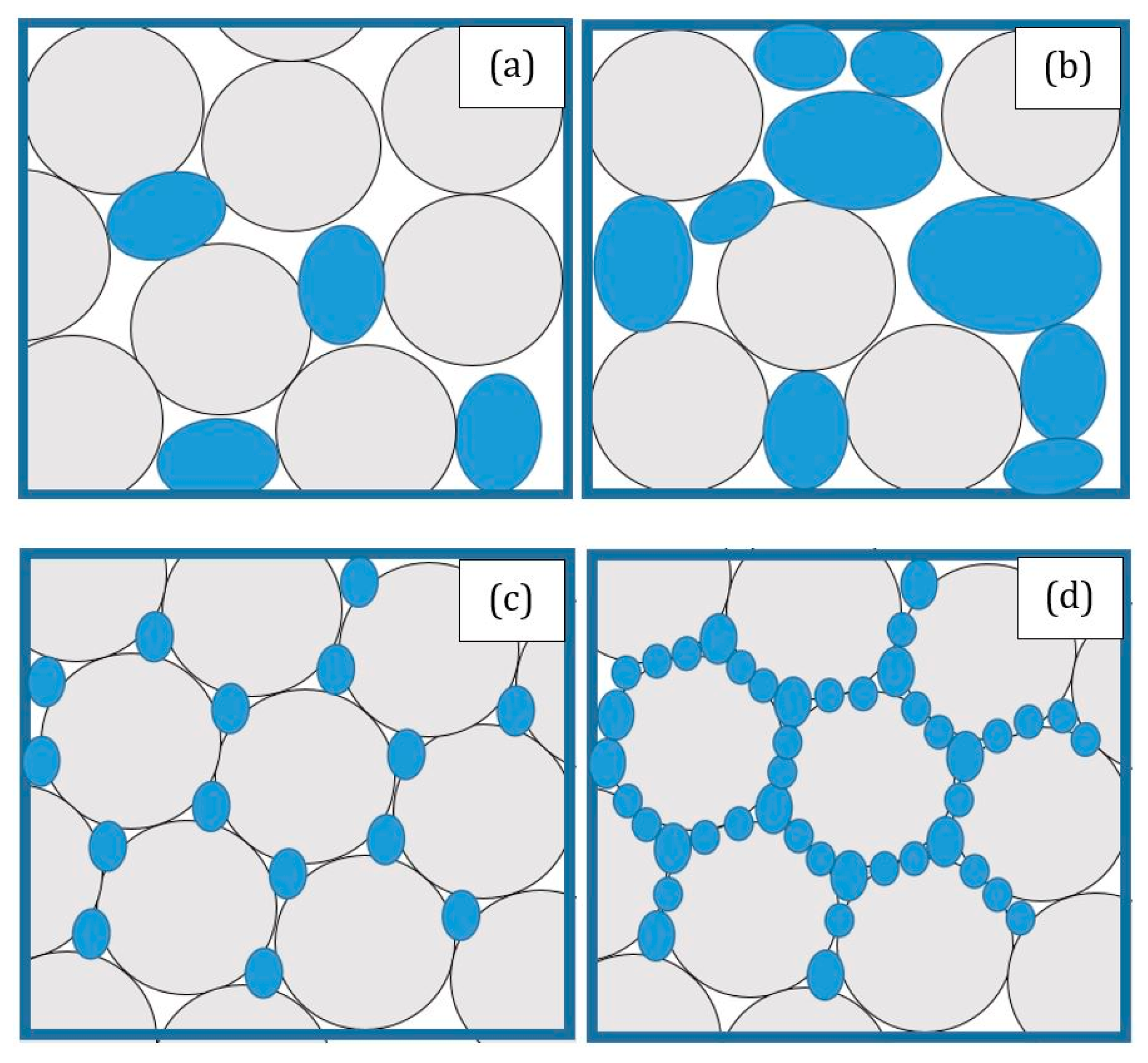
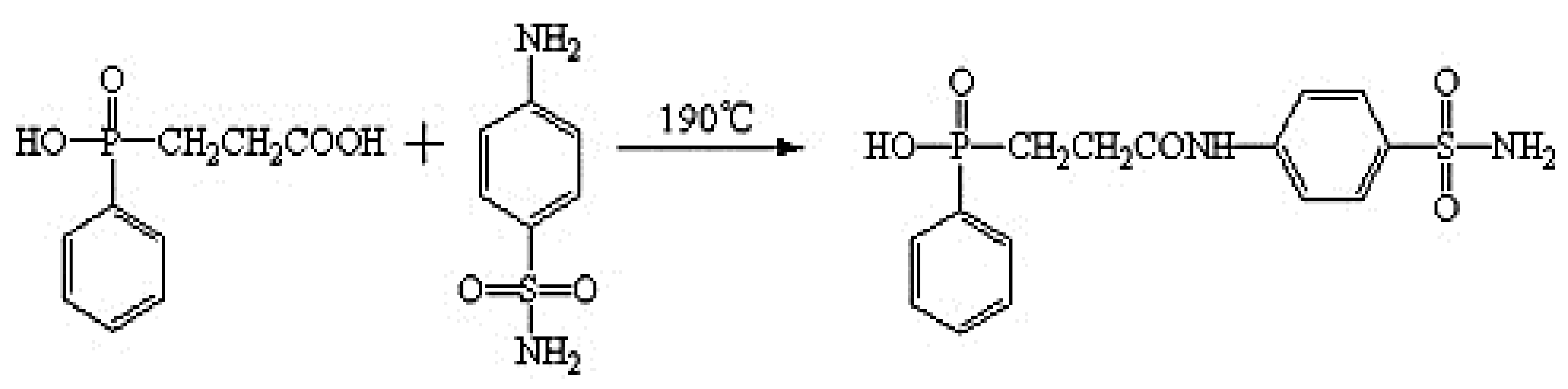

| Flame Retardant Chemical Nature | Example of Flame Retardants | Working Mechanism |
|---|---|---|
| Metal oxides and hydroxide | Magnesium hydroxide, Aluminum hydroxide, alumina trihydrate, calcium carbonate | Heat sink |
| Boron based | Boric acid, borax, Zink borate, boron phosphate | By forming the insulating layer |
| Halogen based | TCPA, TBPA, Polybrominated diphenyl ethers, Polybrominated biphenyl | Gas-phase |
| Phosphorus based | THPC | Condense phase |
| Synergistic | P/N, Halogen/Antimony tri-oxide, P/halogen | The presence of other compounds would increase the slowness of the flame emitted by the major compound. |
| Intumescent | Acid donor (ex-phosphoric acid, ammonium polyphosphate), carbonizing agent (ex-pentaerythritol), bowling agent (ex-melamine, urea) | Both in the gas and condensed phase |
| Metal Components | Composites | Effect of Reinforcement | Reference |
|---|---|---|---|
| Metal hydroxide | Ethylene-vinyl acetate (EVA) | Form new layer that acts as insulation to flame | [88] |
| Silicon-containing, metal hydrate and oxide | Polypropylene (PP) | Decreasing the flow rate of the burning surface | [89] |
| Metal oxides | Thermoplastic polyurethane (TPU) | Low flammability and smoke emission | [90] |
| Zinc borate and magnesium hydroxide | Sawdust/rice husk filled polypropylene | A marginal reduction in mechanical properties and reduce flammability | [91] |
| Magnesium hydroxide (Mg(OH)2 and zinc borate (Zb) | Fiber/polypropylene | Improved thermal stability and flame retardancy | [92] |
| Magnesium hydroxide | Ethylene-vinyl acetate (EVA) | Better water resistance, flame retardancy, and higher pyrolysis temperature | [93] |
| Salicylaldoxime and chelated copper(II)salicylaldehyde | Polyethylene (PE) | Provide good flame retardant behavior | [94] |
| Metal chelates | Polyvinyl alcohol (PVA) | Promotes thermal stability and improve flame-retardant | [95] |
| Aluminum and magnesium hydroxides | Rubbers and ethylene-vinyl acetate (EVA) | No corrosive or potentially toxic substances occur and reducing the smoke level | [96] |
| Zinc phosphonate | Glass-fiber reinforced poly(butylene terephthalate) | No improvement on fire behavior satisfactorily | [97] |
| Manganese (IV) oxide (MnO2), zinc oxide (ZnO), and nickel(III) oxide (Ni2O3) | Polypropylene (PP) | Enhance the charring and corresponds well to the gas release with increasing temperature | [98] |
| Magnesium hydroxide and alumina trihydrate | Low-density polyethylene (LDPE) and ethylene-vinyl acetate (EVA) | Superior thermal stability and reduction of gases produced during burning | [99] |
| Nanometer titanium dioxide (nano-TiO2), aluminum oxide (Al2O3), and magnesium aluminate spinel (MgAl2O4) | Ammonium polyphosphate-pentaerythritol-melamine (APP-PER-MEL) | Enhance fire-resistant and anti-aging properties of the APP-PER-MEL coating | [100] |
| Ionic liquid-based metal-organic hybrid (PMAIL) | Epoxy resin (EP) | Total smoke production was reduced | [101] |
| Aluminum phosphonate (AlPi), antimony oxide, and nanometric iron oxide | Poly(3-hydroxy-butyrate-co-3-hydroxyvalerate) /poly(butylene adipate-co-terephthalate) (PHBV/PBAT) | Great pyrolysis and the fire retardancy | [102] |
| Aluminum trihydrate | Ethylene-vinyl acetate (EVA) and montmorillonites (MMT) | Improvement of thermal stability and flame retardancy | [103] |
| Iron, magnesium, aluminum, and zinc | Paraffin | Increase the char yield and decrease volatilization for the combustible gases | [79] |
| Metal hydroxides and antimony trioxide | Thermoplastics | Improvements in thermal stability and pigmentation properties | [104] |
| Metal-based organic (MBO) | Polyvinylchloride (PVC) | Improved resistance to ignition, flame spread, and smoke generation | [105] |
| Aluminum trihydrate | Ethylene-vinyl acetate (EVA) | Reduction in heat release rates | [106] |
| Silicon-containing materials and metal oxides | Aliphatic and aromatic phosphonates | Good smoke suppressant effects | [107] |
| Zinc borate (ZnB) | Polyamides, polyesters, polyolefin, and boron Compounds | Lower heat release and lower total heat evolved | [108] |
| Metal Phosphonates and Aluminum Oxide Hydroxide | Polyamide, Polyesters, and phosphorous | Improved flame-retardant and mechanical or electrical performance | [109] |
| Aluminum hydroxide (Al(OH)3) | Cycloaliphatic polyamine, epoxy resins | Small burned area and better tensile strength properties | [110] |
| Metal chelates, chromium acetylacetonate, and zinc acetylacetonate | Polypropylene and poly(4,4-diamino diphenyl methane-O-bicyclicpentaerythritol phosphate-phosphate) | A denser char layer was established on the composite | [111] |
| Metal hydroxides | Silicon | Improve the thermal protective layer build on the polymer’s surface | [78] |
| Alumina trihydrate, montmorillonite (MMT) | Ethylene-vinyl acetate and nanocomposite | Improve the fiber-matrix adhesion | [112] |
| Zinc borate, and magnesium hydroxide (Mg(OH)2) | Polypropylene and ammonium polyphosphate | Thermal stability and fire retardancy were improved | [92] |
| Titanium dioxide | Ammonium polyphosphate-pentaerythritol-melamine (APP-PER-MEL) | Anti-aging properties of the flame-retardant coating were improved | [100] |
| Melamine poly (zinc phosphate) (MPZnP) | Epoxy resin (EP) and polyphosphate | Earlier decomposition and slightly changed evolved gas | [113] |
| Metallic oxide and Metal hydroxide | Graphene foam | Better flame retardant and compressible structure | [114] |
| Manganese and metal salts | Ammonium polyphosphate and cellulose | Enhancing flame retardant efficiency | [115] |
| Zinc hydroxyl stannate and alumina trihydrate | Ethylene-vinyl acetate, polyurethane, styrene-butadiene rubber, silicone rubber, and polychloroprene rubber. | Improvement of fire resistance and better mechanical and thermal properties of the elastomer | [116] |
| Ammonium bromide, manganese(II), iron(II), calcium, zinc oxalate, and metal oxalates | Polyamide and cotton | Reduction of combustion rate for cotton | [117] |
| Nickel-metal hydride, nickel-cadmium (Ni-Cd), and metal oxide | Graphites | Excellent ability for flame-retardance, cell performance, and wettability improvement | [118] |
| Copper metal complex | Polyurethane | Superior flame retardant and antimicrobial properties | [119] |
| Diphenyl phosphates and calcium hypophosphite, | Polycarbonates and polyurethanes | Good thermal stability and low volatility | [120] |
| Metal hydroxides, metal hydrate, and alumina trihydrate | Ethylene-vinyl acetate and octadecylamine | Improvement of tensile and flame-resistance properties | [121] |
| Cupric and zinc ions | Polyethylenimine and ramie fabric | Improved thermal stability and reduced flammability | [122] |
| Zinc Borate and metal hydroxide | Polyethylene terephthalate, woven and organophosphorus | Decrease smoke release but no flammability improvement | [123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilyas, R.A.; Sapuan, S.M.; Asyraf, M.R.M.; Dayana, D.A.Z.N.; Amelia, J.J.N.; Rani, M.S.A.; Norrrahim, M.N.F.; Nurazzi, N.M.; Aisyah, H.A.; Sharma, S.; et al. Polymer Composites Filled with Metal Derivatives: A Review of Flame Retardants. Polymers 2021, 13, 1701. https://doi.org/10.3390/polym13111701
Ilyas RA, Sapuan SM, Asyraf MRM, Dayana DAZN, Amelia JJN, Rani MSA, Norrrahim MNF, Nurazzi NM, Aisyah HA, Sharma S, et al. Polymer Composites Filled with Metal Derivatives: A Review of Flame Retardants. Polymers. 2021; 13(11):1701. https://doi.org/10.3390/polym13111701
Chicago/Turabian StyleIlyas, R. A., S. M. Sapuan, M. R. M. Asyraf, D. A. Z. N. Dayana, J. J. N. Amelia, M. S. A. Rani, Mohd Nor Faiz Norrrahim, N. M. Nurazzi, H. A. Aisyah, Shubham Sharma, and et al. 2021. "Polymer Composites Filled with Metal Derivatives: A Review of Flame Retardants" Polymers 13, no. 11: 1701. https://doi.org/10.3390/polym13111701
APA StyleIlyas, R. A., Sapuan, S. M., Asyraf, M. R. M., Dayana, D. A. Z. N., Amelia, J. J. N., Rani, M. S. A., Norrrahim, M. N. F., Nurazzi, N. M., Aisyah, H. A., Sharma, S., Ishak, M. R., Rafidah, M., & Razman, M. R. (2021). Polymer Composites Filled with Metal Derivatives: A Review of Flame Retardants. Polymers, 13(11), 1701. https://doi.org/10.3390/polym13111701









