Synthesis of Sulfonated Poly(Arylene Ether Sulfone)s Containing Aliphatic Moieties for Effective Membrane Electrode Assembly Fabrication by Low-Temperature Decal Transfer Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization and Methods
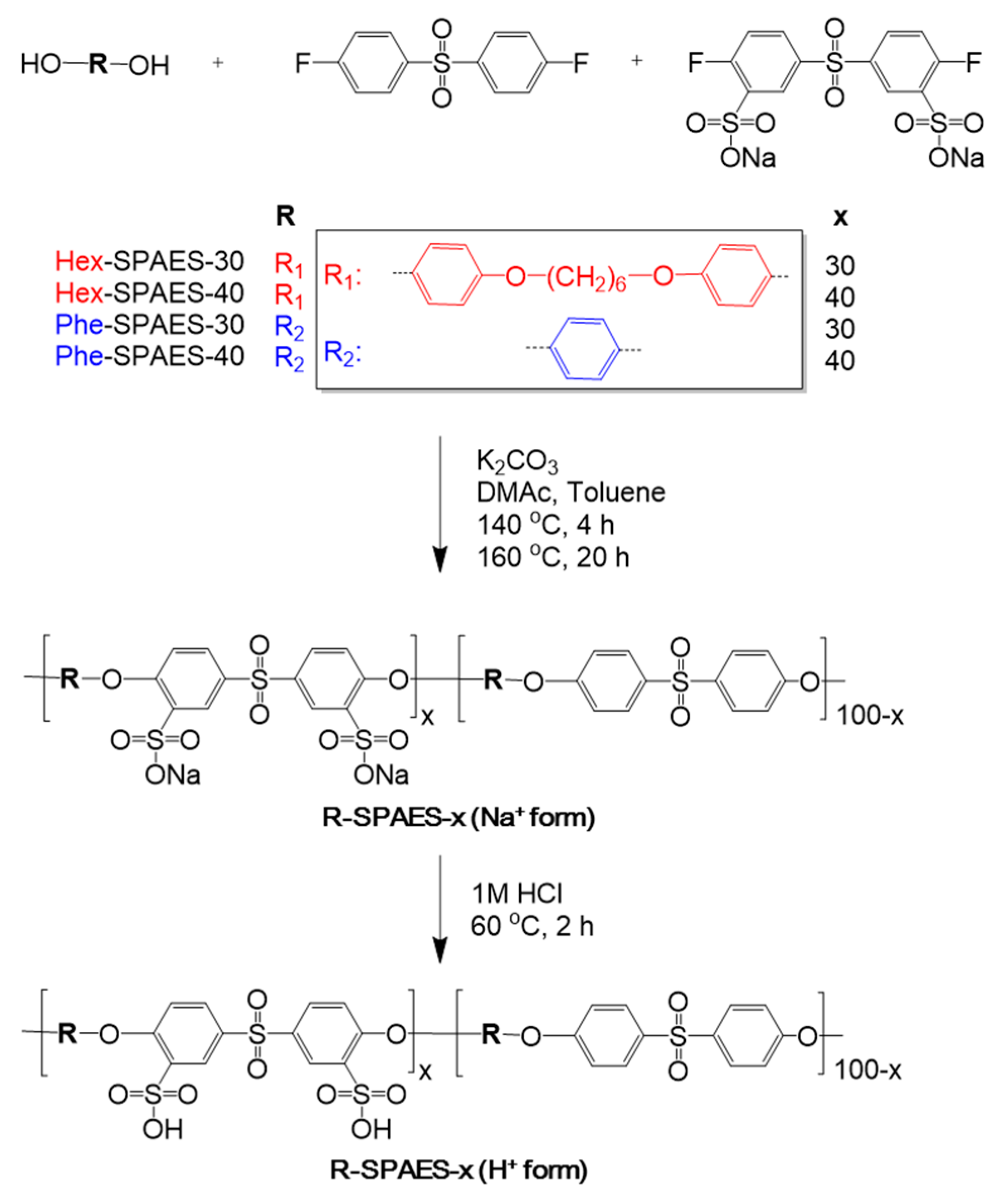
2.3. Synthesis of Sulfonated Poly(Arylene Ether Sulfone)s with Hexyl Aliphatic Chains (Hex-SPAES-30 Salt Form)
2.4. Membrane Preparation and Counter Ion Exchange from the Sodium Form to the Proton Form
2.5. Decal Transfer and MEA Fabrication
2.6. Fuel Cell Tests of Membrane Electrode Assemblies (MEAs)
3. Results and Discussion
3.1. Chemical Structure Analysis of the Synthetic Polymers
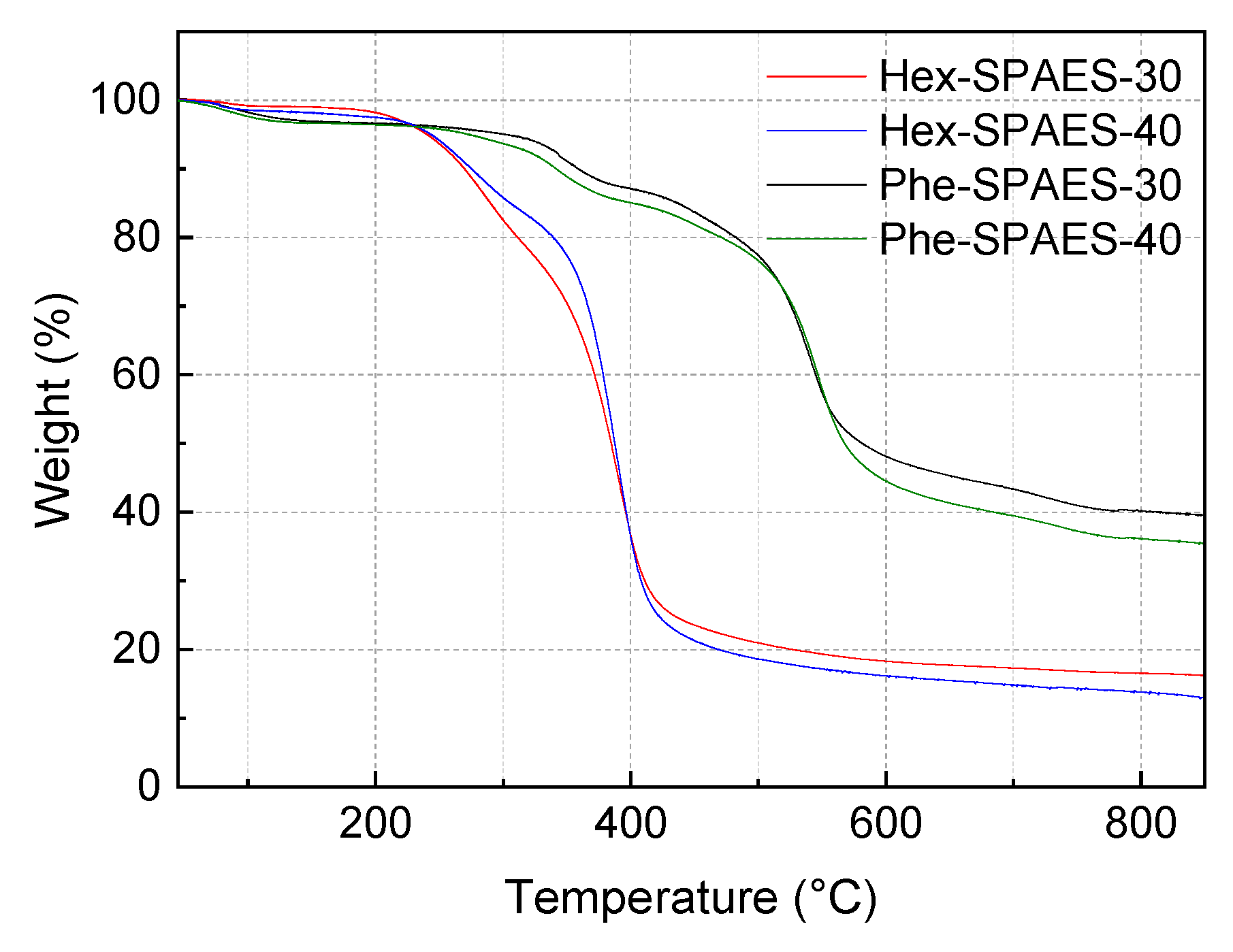
3.2. Effects of the Aliphatic Chain Contents in Polymers on the Thermal Properties
3.3. Mechanical Properties of the Synthetic Polymeric Membranes
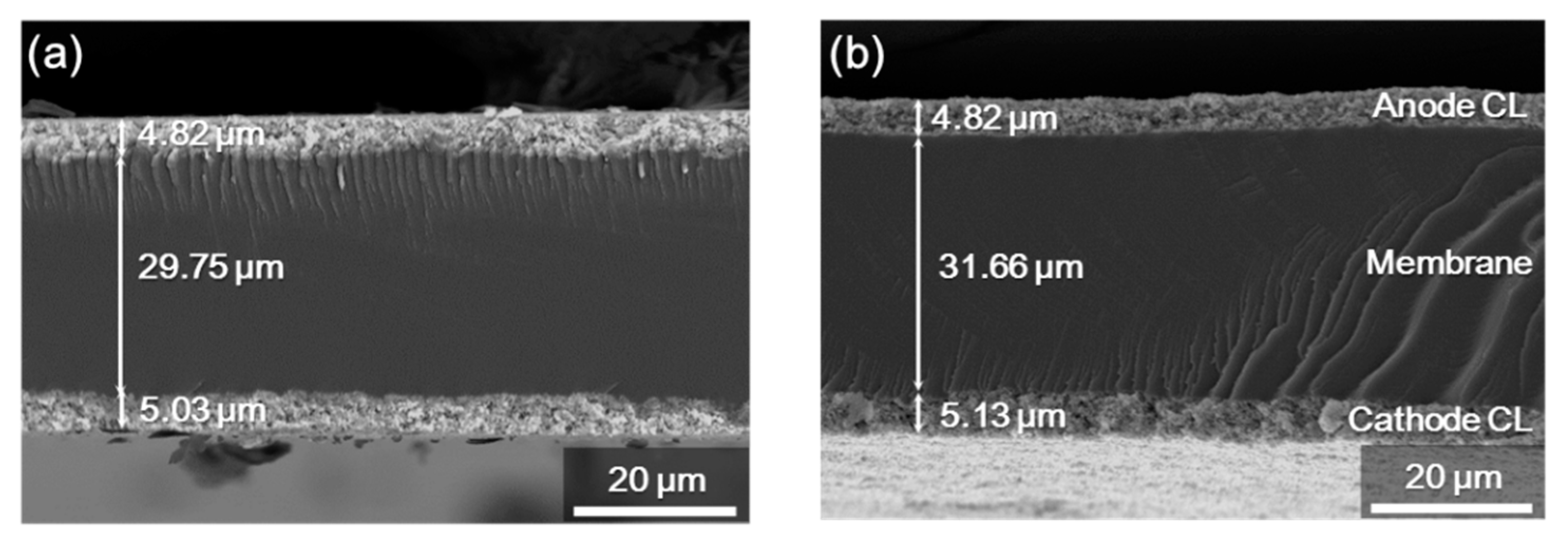
3.4. Morphology of the Synthetic Polymeric Membranes
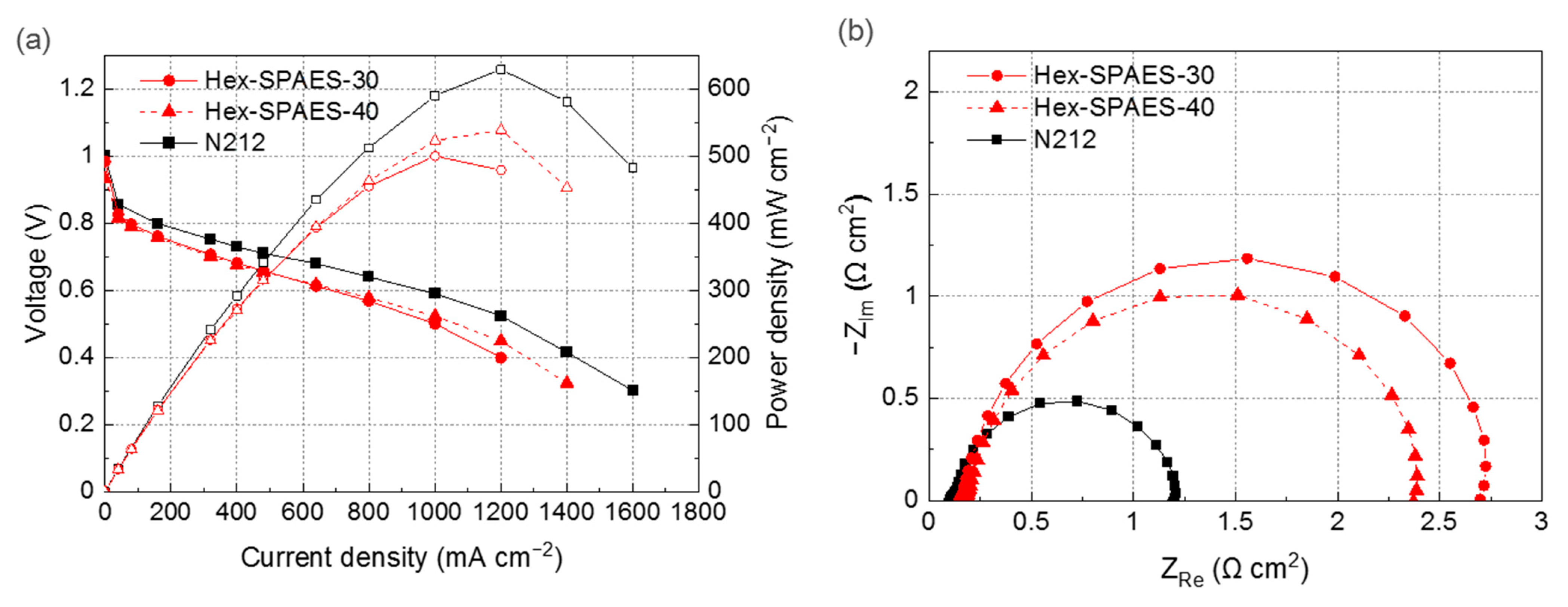
3.5. Proton-Exchange Membrane Fuel Cell Test of the Fabricated Membrane Electrode Assemblies (MEAs)
3.6. Long-Term Test of the Fabricated Membrane Electrode Assemblies (MEAs)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Kraytsberg, A.; Ein-Eli, Y. Review of advanced materials for proton exchange membrane fuel cells. Energy Fuels 2014, 28, 7303–7330. [Google Scholar] [CrossRef]
- Esmaeili, N.; Gray, E.M.; Webb, C.J. Non-fluorinated polymer composite proton exchange membranes for fuel cell applications—A review. ChemPhysChem 2019, 20, 2016–2053. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.; Nam, K.H.; Han, H. Proton transport in aluminum-substituted mesoporous silica channel-embedded high-temperature anhydrous proton-exchange membrane fuel cells. Sci. Rep. 2020, 10, 10352. [Google Scholar] [CrossRef]
- Sassin, M.B.; Garsany, Y.; Gould, B.D.; Swider-Lyons, K.E. Fabrication Method for Laboratory-Scale High-Performance Membrane Electrode Assemblies for Fuel Cells. Anal. Chem. 2017, 89, 511–518. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Alaefour, I.; Li, X. Impact of manufacturing processes on proton exchange membrane fuel cell performance. Appl. Energy 2018, 225, 1022–1032. [Google Scholar] [CrossRef]
- Choi, M.; Kim, J.K.; Kim, J.; Yang, S.; Park, J.-E.; Kim, O.-H.; Cho, Y.-H. PtRu/C catalyst slurry preparation for large-scale decal transfer with high performance of proton exchange membrane fuel cells. RSC Adv. 2018, 8, 36313–36322. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Alaefour, I.; Unsworth, G.; Li, X. Development of a low temperature decal transfer method for the fabrication of proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2017, 42, 11813–11822. [Google Scholar] [CrossRef]
- Jung, H.-Y.; Kim, J.W. Role of the glass transition temperature of Nafion 117 membrane in the preparation of the membrane electrode assembly in a direct methanol fuel cell (DMFC). Int. J. Hydrog. Energy 2012, 37, 12580–12585. [Google Scholar] [CrossRef]
- Chae, J.E.; Kim, B.H.; Noh, J.H.; Jung, J.; Kim, J.-Y.; Jang, J.H.; Yoo, S.J.; Kim, H.-J.; Lee, S.Y. Effect of the spirobiindane group in sulfonated poly(arylene ether sulfone) copolymer as electrode binder for polymer electrolyte membrane fuel cells. J. Ind. Eng. Chem. 2017, 47, 315–322. [Google Scholar] [CrossRef]
- Lee, J.; Ahn, Y.; Kim, D. Binder effect on fuel cell performance and interfacial stability of membrane electrode assembly fabricated with sulfonated poly(ether ether ketone) membrane. Macromol. Res. 2019, 27, 175–181. [Google Scholar] [CrossRef]
- Kim, S.-U.; Yu, D.M.; Kim, T.-H.; Hong, Y.T.; Nam, S.Y.; Choi, J.-H. Effect of sulfonated poly(arylene ether sulfone) binder on the performance of polymer electrolyte membrane fuel cells. J. Ind. Eng. Chem. 2015, 23, 316–320. [Google Scholar] [CrossRef]
- Oh, H.J.; Freeman, B.D.; McGrath, J.E.; Lee, C.H.; Paul, D.R. Thermal analysis of disulfonated poly(arylene ether sulfone) plasticized with poly(ethylene glycol) for membrane formation. Polymer 2014, 55, 235–247. [Google Scholar] [CrossRef]
- Lee, K.H.; Chu, J.Y.; Kim, A.R.; Yoo, D.J. Facile fabrication and characterization of improved proton conducting sulfonated poly(arylene biphenylether sulfone) blocks containing fluorinated hydrophobic units for proton exchange membrane fuel cell applications. Polymers 2018, 10, 1367. [Google Scholar] [CrossRef] [PubMed]
- Vlad-Bubulac, T.; Hamciuc, C. Aliphatic–aromatic copolyesters containing phosphorous cyclic bulky groups. Polymer 2009, 50, 2220–2227. [Google Scholar] [CrossRef]
- Zhao, B.; He, G.; El Hamouti, I.; Gao, L.; Liu, Y.; Deng, R.; Yan, X. A novel strategy for constructing a highly conductive and swelling-resistant semi-flexible aromatic polymer based anion exchange membranes. Int. J. Hydrogen Energy 2017, 42, 10228–10237. [Google Scholar] [CrossRef]
- Yan, X.; Zhao, B.; Liu, J.; Zhang, X.; He, G. Tailoring the nanophase-separated morphology of anion exchange membrane by embedding aliphatic chains of different lengths into aromatic main chains. J. Membr. Sci. 2018, 564, 436–443. [Google Scholar] [CrossRef]
- Kwon, Y.; Lee, S.Y.; Hong, S.; Jang, J.H.; Henkensmeier, D.; Yoo, S.J.; Kim, H.-J.; Kim, S.-H. Novel sulfonated poly(arylene ether sulfone) containing hydroxyl groups for enhanced proton exchange membrane properties. Polym. Chem. 2015, 6, 233–239. [Google Scholar] [CrossRef]
- Lee, C.H.; Park, H.B.; Lee, Y.M.; Lee, R.D. Importance of proton conductivity measurement in polymer electrolyte membrane for fuel cell application. Ind. Eng. Chem. Res. 2005, 44, 7617–7626. [Google Scholar] [CrossRef]
- Griffin, A.C.; Havens, S.J. Mesogenic polymers. III. Thermal properties and synthesis of three homologous series of thermotropic liquid crystalline “backbone” polyesters. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 951–969. [Google Scholar] [CrossRef]
- Xie, R.; Weisen, A.R.; Lee, Y.; Aplan, M.A.; Fenton, A.M.; Masucci, A.E.; Kempe, F.; Sommer, M.; Pester, C.W.; Colby, R.H.; et al. Glass transition temperature from the chemical structure of conjugated polymers. Nat. Commun. 2020, 11, 893. [Google Scholar] [CrossRef]
- Blanchard, L.-P.; Hesse, J.; Malhotra, S.L. Effect of molecular weight on glass transition by differential scanning calorimetry. Can. J. Chem. 1974, 52, 3170–3175. [Google Scholar] [CrossRef]
- Park, J.; Seo, M.; Choi, H.; Kim, S.Y. Synthesis and physical gelation induced by self-assembly of well-defined poly(arylene ether sulfone)s with various numbers of arms. Polym. Chem. 2011, 2, 1174–1179. [Google Scholar] [CrossRef]
- Pirali-Hamedani, M.; Mehdipour-Ataei, S. Effect of sulfonation degree on molecular weight, thermal stability, and proton conductivity of poly(arylene ether sulfone)s membrane. Des. Monomers Polym. 2017, 20, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.W.; Guiver, M.D.; Lee, Y.M. Hydrocarbon-based polymer electrolyte membranes: Importance of morphology on ion transport and membrane stability. Chem. Rev. 2017, 117, 4759–4805. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, S.; Wang, L.; Li, M.; Gao, C. Understanding of nanophase separation and hydrophilic morphology in Nafion and SPEEK membranes: A combined experimental and theoretical studies. Nanomaterials 2019, 9, 869. [Google Scholar] [CrossRef]
- Lee, S.W.; Chen, J.C.; Wu, J.A.; Chen, K.H. Synthesis and properties of poly(ether sulfone)s with clustered sulfonic groups for PEMFC applications under various relative humidity. ACS Appl. Mater. Interfaces 2017, 9, 9805–9814. [Google Scholar] [CrossRef]
- Wang, C.; Shin, D.W.; Lee, S.Y.; Kang, N.R.; Robertson, G.P.; Lee, Y.M.; Guiver, M.D. A clustered sulfonated poly(ether sulfone) based on a new fluorene-based bisphenol monomer. J. Mater. Chem. 2012, 22, 25093. [Google Scholar] [CrossRef]
- Prabhuram, J.; Krishnan, N.N.; Choi, B.; Lim, T.-H.; Ha, H.Y.; Kim, S.-K. Long-term durability test for direct methanol fuel cell made of hydrocarbon membrane. Int. J. Hydrog. Energy 2010, 35, 6924–6933. [Google Scholar] [CrossRef]
- Choi, J.; Kim, M.-H.; Han, J.Y.; Chae, J.E.; Lee, W.H.; Lee, Y.M.; Lee, S.Y.; Jang, J.H.; Kim, J.Y.; Henkensmeier, D.; et al. Application of spirobiindane-based microporous poly(ether sulfone)s as polymeric binder on solid alkaline exchange membrane fuel cells. J. Membr. Sci. 2018, 568, 67–75. [Google Scholar] [CrossRef]
- Choi, J.; Jang, J.-H.; Chae, J.E.; Park, H.-Y.; Lee, S.Y.; Jang, J.H.; Kim, J.Y.; Henkensmeier, D.; Yoo, S.J.; Lee, K.Y.; et al. Spirobiindane-based poly(arylene ether sulfone) ionomers for alkaline anion exchange membrane fuel cells. Macromol. Res. 2020, 28, 275–281. [Google Scholar] [CrossRef]




| Sample | Mn a (g mol−1) | PDI b |
|---|---|---|
| Hex-SPAES-30 | 32,378 | 2.23 |
| Hex-SPAES-40 | 23,999 | 1.74 |
| Phe-SPAES-30 | 54,956 | 2.51 |
| Phe-SPAES-40 | 42,401 | 2.26 |
| Sample | IECTheoretical a (meq g−1) | IECTitrated (meq g−1) | Water Uptake (%) | Dimensional Stability (%) | Conductivity (S/cm) | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| x–y Plane | z-Axis | 30 °C | 40 °C | 60 °C | 80 °C | |||||
| Hex-SPAES-30 | 1.1 | 1.1 ± 0.1 | 6.6 ± 0.9 | 4.4 ± 0.3 | 3.2 ± 1.9 | 0.05 | 0.08 | 0.10 | 0.11 | |
| Hex-SPAES-40 | 1.4 | 1.4 ± 0.1 | 10.1 ± 3.2 | 4.6 ± 1.1 | 8.5 ± 1.2 | 0.15 | 0.18 | 0.22 | 0.29 | |
| Phe-SPAES-30 | 1.3 | 1.3 ± 0.1 | 9.8 ± 3.0 | 2.2 ± 1.3 | 6.2 ± 1.9 | 0.04 | 0.05 | 0.07 | 0.09 | |
| Phe-SPAES-40 | 1.7 | 1.4 ± 0.2 | 19.3 ± 5.5 | 5.0 ± 0.7 | 8.1 ± 2.1 | 0.07 | 0.13 | 0.19 | 0.27 | |
| Nafion 212 | 1.0 | - | 20 ± 2 | 9.5 ± 1.9 | 12.7 ± 2.5 | 0.09 | 0.13 a | 0.16 | 0.18 | [27,28] |
| Sample | Yield Strength (MPa) | Tensile Strength (MPa) | Elongation (%) | Young’s Modulus (GPa) | Reference |
|---|---|---|---|---|---|
| Hex-SPAES-30 | 38.2 ± 2.7 | 41.9 ± 6.9 | 184.0 ± 37.7 | 0.55 ± 0.06 | |
| Hex-SPAES-40 | 34.3 ± 4.0 | 41.1 ± 4.6 | 198.6 ± 8.6 | 0.51 ± 0.06 | |
| Phe-SPAES-30 | 40.7 ± 1.4 | 41.7 ± 2.2 | 151.1 ± 18.1 | 0.68 ± 0.01 | |
| Phe-SPAES-40 | 40.4 ± 0.9 | 41.0 ± 0.1 | 162.2 ± 28.6 | 0.61 ± 0.03 | |
| Nafion 212 | - | 25.6 ± 1.4 | 365.2 ± 63.1 | 0.12 ± 0.01 | [27] |
| Sample | Photographic Images After the Decal Transfer Process | Catalyst Transfer Yield (%) | ||||
|---|---|---|---|---|---|---|
| Substrate | MEA | |||||
| Anode | Cathode | Anode | Cathode | Anode | Cathode | |
| Hex-SPAES-30 |  |  |  |  | 98 | 100 |
| Phe-SPAES-30 |  |  |  |  | 3 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.; Kyeong, M.; Kim, M.; Lee, S.-S.; Seo, B.; Park, H.S.; Park, H.-Y.; Henkensmeier, D.; Lee, S.Y.; Kim, H.-J. Synthesis of Sulfonated Poly(Arylene Ether Sulfone)s Containing Aliphatic Moieties for Effective Membrane Electrode Assembly Fabrication by Low-Temperature Decal Transfer Methods. Polymers 2021, 13, 1713. https://doi.org/10.3390/polym13111713
Choi J, Kyeong M, Kim M, Lee S-S, Seo B, Park HS, Park H-Y, Henkensmeier D, Lee SY, Kim H-J. Synthesis of Sulfonated Poly(Arylene Ether Sulfone)s Containing Aliphatic Moieties for Effective Membrane Electrode Assembly Fabrication by Low-Temperature Decal Transfer Methods. Polymers. 2021; 13(11):1713. https://doi.org/10.3390/polym13111713
Chicago/Turabian StyleChoi, Jieun, Minkyu Kyeong, Minsung Kim, Sang-Soo Lee, Bora Seo, Hyun Seo Park, Hee-Young Park, Dirk Henkensmeier, So Young Lee, and Hyoung-Juhn Kim. 2021. "Synthesis of Sulfonated Poly(Arylene Ether Sulfone)s Containing Aliphatic Moieties for Effective Membrane Electrode Assembly Fabrication by Low-Temperature Decal Transfer Methods" Polymers 13, no. 11: 1713. https://doi.org/10.3390/polym13111713
APA StyleChoi, J., Kyeong, M., Kim, M., Lee, S.-S., Seo, B., Park, H. S., Park, H.-Y., Henkensmeier, D., Lee, S. Y., & Kim, H.-J. (2021). Synthesis of Sulfonated Poly(Arylene Ether Sulfone)s Containing Aliphatic Moieties for Effective Membrane Electrode Assembly Fabrication by Low-Temperature Decal Transfer Methods. Polymers, 13(11), 1713. https://doi.org/10.3390/polym13111713








