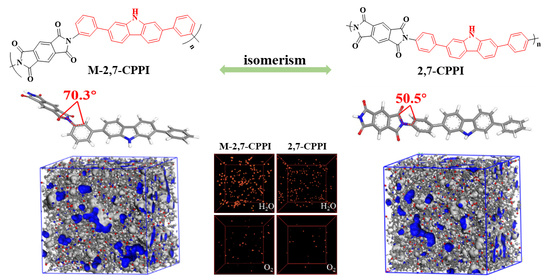
The Effect of Molecular Isomerism on the Barrier Properties of Polyimides: Perspectives from Experiments and Simulations
Abstract
1. Introduction
2. Experimental Section
2.1. Experimental Materials
2.2. Instrumentation
2.3. Molecular Simulation
2.4. Synthesis of 2,7-Bis(3-nitrophenyl)-9H-carbazole (M-2,7-CPDN)
2.5. Synthesis of 3,3′-(9H-Carbazole-2,7-diyl)diamino (M-2,7-CPDA)
2.6. Synthesis of Polyimide M-2,7-CPPI
3. Results and Discussion
3.1. Synthesis and Characterizations of Monomers
3.2. Synthesis and Characterizations of PI
3.3. Thermal and Mechanical Performances
3.4. Barrier Properties
3.5. Aggregation Structure Analysis
3.6. Hydrogen Bonds Analysis
3.7. Free Volumes Analysis
3.7.1. Positron Annihilation Test
3.7.2. Molecular Simulations
3.8. Gas Transport Behavior
3.8.1. Gas Diffusivity
3.8.2. Gas Solubility
3.8.3. Gas Permeability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Windrich, F.; Kappert, E.J.; Malanin, M.; Eichhorn, K.-J.; Häuβler, L.; Benes, N.E.; Voit, B. In-situ imidization analysis in microscale thin films of an ester-type photosensitive polyimide for microelectronic packaging applications. Eur. Polym. J. 2016, 84, 279–291. [Google Scholar] [CrossRef]
- Pakhuruddin, M.Z.; Ibrahim, K.; Aziz, A.A. Properties of polyimide substrate for applications in flexible solar cells. J. Optoelectron. Adv. Mater. 2013, 7, 377–380. [Google Scholar]
- Jagadish, R.S.; Raj, B.; Asha, M.R. Blending of low-density polyethylene with vanillin for improved barrier and aroma-releasing properties in food packaging. J. Appl. Polym. Sci. 2009, 113, 3732–3741. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Wang, K.-L.; Huang, Y.-C.; Lee, K.-R.; Lai, J.-Y.; Ha, C.-S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Yi, C.; Li, W.; Shi, S.; He, K.; Ma, P.; Chen, M.; Yang, C. High-temperature-resistant and colorless polyimide: Preparations, properties, and applications. Sol. Energy 2020, 195, 340–354. [Google Scholar] [CrossRef]
- Choi, M.-C.; Kim, Y.; Ha, C.-S. Polymers for flexible displays: From material selection to device applications. Prog. Polym. Sci. 2008, 33, 581–630. [Google Scholar] [CrossRef]
- Tiwari, A.N.; Romeo, A.; Baetzner, D.; Zogg, H. Flexible CdTe solar cells on polymer films. Prog. Photovolt. 2001, 9, 211–215. [Google Scholar] [CrossRef]
- Salleo, A.; Street, R.A. Light-induced bias stress reversal in polyfluorene thin-film transistors. J. Appl. Phys. 2003, 94, 471–479. [Google Scholar] [CrossRef]
- Street, R.A. Thin-film transistors. Adv. Mater. 2009, 21, 2007–2022. [Google Scholar] [CrossRef]
- Zekriardehani, S.; Jabarin, S.A.; Gidley, D.R.; Coleman, M.R. Effect of chain dynamics, crystallinity, and free volume on the barrier properties of poly(ethylene terephthalate) biaxially oriented films. Macromolecules 2017, 50, 2845–2855. [Google Scholar] [CrossRef]
- Xu, H.; Luo, D.; Li, M.; Xu, M.; Zou, J.; Tao, H.; Lin-Feng, L.; Wang, L.; Peng, J.; Cao, Y. A flexible AMOLED display on the PEN substrate driven by oxide thin-film transistors using anodized aluminium oxide as dielectric. J. Mater. Chem. C 2014, 2, 1255–1259. [Google Scholar] [CrossRef]
- Hu, Y.S.; Mehta, S.; Schiraldi, D.A.; Hiltner, A.; Baer, E. Effect of water sorption on oxygen-barrier properties of aromatic polyamides. J. Polym. Sci. Part B Polym. Phys 2005, 43, 1365–1381. [Google Scholar] [CrossRef]
- Hwang, T.; Pu, L.; Kim, S.W.; Oh, Y.-S.; Nam, J.-D. Synthesis and barrier properties of poly(vinylidene chloride-co-acrylonitrile)/SiO2 hybrid composites by sol–gel process. J. Membr. Sci. 2009, 345, 90–96. [Google Scholar] [CrossRef]
- Maes, C.; Luyten, W.; Herremans, G.; Peeters, R.; Carleer, R.; Buntinx, M. Recent updates on the barrier properties of ethylene vinyl alcohol copolymer (EVOH): A review. Polym. Rev. 2018, 58, 209–246. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, C.; Qu, L.; Wu, Y.; Zhang, Y.; Wu, X.; Zou, B.; Chen, W.; Chen, Z.; Chi, Z.; et al. A bulk dielectric polymer film with intrinsic ultralow dielectric constant and outstanding comprehensive properties. Chem. Mater. 2015, 27, 6543–6549. [Google Scholar] [CrossRef]
- Li, T.-L.; Hsu, S.L.-C. Preparation and properties of a high temperature, flexible and colorless ITO coated polyimide substrate. Eur. Polym. J. 2007, 43, 3368–3373. [Google Scholar] [CrossRef]
- Sykes, G.F.; Clair, A.K.S. The effect of molecular structure on the gas transmission rates of aromatic polyimides. J. Appl. Polym. Sci. 1986, 32, 3725–3735. [Google Scholar] [CrossRef]
- Coleman, M.; Koros, W. The transport properties of polyimide isomers containing hexafluoroisopropylidene in the diamine residue. J. Polym. Sci. Part B Polym. Phys. 1994, 32, 1915–1926. [Google Scholar] [CrossRef]
- Madzarevic, Z.P.; Shahid, S.; Nijmeijer, K.; Dingemans, T.J. The role of ortho-, meta- and para-substitutions in the main-chain structure of poly(etherimide)s and the effects on CO2/CH4 gas separation performance. Sep. Purif. Technol. 2019, 210, 242–250. [Google Scholar] [CrossRef]
- Coleman, M.; Koros, W. Isomeric polyimides based on fluorinated dianhydrides and diamines for gas separation applications. J. Membr. Sci. 1990, 50, 285–297. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, L.; Wang, T.; Wang, Q. Influence of monomer conformation on the mechanical and tribological properties of thermosetting polyimides. RSC Adv. 2015, 5, 101533–101543. [Google Scholar] [CrossRef]
- Comesaña-Gándara, B.; Calle, M.; Jo, H.J.; Hernández, A.; de la Campa, J.G.; de Abajo, J.; Lozano, A.E.; Lee, Y.M. Thermally rearranged polybenzoxazoles membranes with biphenyl moieties: Monomer isomeric effect. J. Membr. Sci. 2014, 450, 369–379. [Google Scholar] [CrossRef]
- Song, G.; Zhang, Y.; Wang, D.; Chen, C.; Zhou, H.; Zhao, X.; Dang, G. Intermolecular interactions of polyimides containing benzimidazole and benzoxazole moieties. Polymer 2013, 54, 2335–2340. [Google Scholar] [CrossRef]
- Pei, X.; Chen, G.; Hou, Y.; Fang, X. Comparative study on polyimides derived from isomeric diphenylsulfonetetracarboxylic dianhydrides. High Perform. Polym. 2012, 25, 312–323. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Z.; Gao, L.; Ding, M. Polyimides from isomeric diphenylthioether dianhydrides. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 959–967. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Tan, J.; Zeng, Y.; Liu, J.; Zhang, H.; Pei, Y.; Xiang, X.; Liu, Y. Intrinsic high-barrier polyimide with low free volume derived from a novel diamine monomer containing rigid planar moiety. Polymer 2017, 114, 289–297. [Google Scholar] [CrossRef]
- Chang, K.-S.; Tung, C.-C.; Wang, K.-S.; Tung, K.-L. Free volume analysis and gas transport mechanisms of aromatic polyimide membranes: A molecular simulation study. J. Phys. Chem. B 2009, 113, 9821–9830. [Google Scholar] [CrossRef]
- Okada, T.; Ishige, R.; Ando, S. Effects of chain packing and structural isomerism on the anisotropic linear and volumetric thermal expansion behaviors of polyimide films. Polymer 2018, 146, 386–395. [Google Scholar] [CrossRef]
- Ando, S.; Sekiguchi, K.; Mizoroki, M.; Okada, T.; Ishige, R. Anisotropic linear and volumetric thermal-expansion behaviors of self-standing polyimide films analyzed by thermomechanical analysis (TMA) and optical interferometry. Macromol. Chem. Phys. 2018, 219, 1700354. [Google Scholar] [CrossRef]
- Kochi, M.; Chen, C.; Yokota, R.; Hasegawa, M.; Hergenrother, P. Isomeric biphenyl polyimides. (II) glass transitions and secondary relaxation processes. High Perform. Polym. 2016, 17, 335–347. [Google Scholar] [CrossRef]
- Ishii, J.; Takata, A.; Oami, Y.; Yokota, R.; Vladimirov, L.; Hasegawa, M. Spontaneous molecular orientation of polyimides induced by thermal imidization (6). Mechanism of negative in-plane CTE generation in non-stretched polyimide films. Eur. Polym. J. 2010, 46, 681–693. [Google Scholar] [CrossRef]
- Inagaki, N.; Cech, V.; Narushima, K.; Takechi, Y. Oxygen and water vapor gas barrier poly(ethylene naphthalate) films by deposition of SiOx plasma polymers from mixture of tetramethoxysilane and oxygen. J. Appl. Polym. Sci. 2007, 104, 915–925. [Google Scholar] [CrossRef]
- Gargalaka, J.; Couto, R.A.A.; Constantino, V.R.L.; Toma, H.E.; Araki, K. Influence of the relative amounts of crystalline and amorphous phases on the mechanical properties of polyamide-6 nanocomposites. J. Appl. Polym. Sci. 2012, 125, 3239–3249. [Google Scholar] [CrossRef]
- Park, C.H.; Tocci, E.; Lee, Y.M.; Drioli, E. Thermal treatment effect on the structure and property change between hydroxy-containing polyimides (HPIs) and thermally rearranged polybenzoxazole (TR-PBO). J. Phys. Chem. B 2012, 116, 12864–12877. [Google Scholar] [CrossRef] [PubMed]
- Mattozzi, A.; Hedenqvist, M.S.; Gedde, U.W. Diffusivity of n-hexane in poly(ethylenestat-octene)s assessed by molecular dynamics simulation. Polymer 2007, 48, 5174–5180. [Google Scholar] [CrossRef]
- Miyata, S.; Sato, S.; Nagai, K.; Nakagawa, T.; Kudo, K. Relationship between gas transport properties and fractional free volume determined from dielectric constant in polyimide films containing the hexafluoroisopropylidene group. J. Appl. Polym. Sci. 2008, 107, 3933–3944. [Google Scholar] [CrossRef]
- Low, B.T.; Chung, T.S.; Chen, H.; Jean, Y.-C.; Pramoda, K.P. Tuning the free volume cavities of polyimide membranes via the construction of pseudo-interpenetrating networks for enhanced gas separation performance. Macromolecules 2009, 42, 7042–7054. [Google Scholar] [CrossRef]
- Belov, N.A.; Zharov, A.A.; Shashkin, A.V.; Shaikh, M.Q.; Raetzke, K.; Yampolskii, Y.P. Gas transport and free volume in hexafluoropropylene polymers. J. Membr. Sci. 2011, 383, 70–77. [Google Scholar] [CrossRef]
- Hu, C.-C.; Chang, C.-S.; Ruaan, R.-C.; Lai, J.-Y. Effect of free volume and sorption on membrane gas transport. J. Membr. Sci. 2003, 226, 51–61. [Google Scholar] [CrossRef]
- Neyertz, S.; Brown, D.; Pandiyan, S.; van der Vegt, N.F.A. Carbon dioxide diffusion and plasticization in fluorinated polyimides. Macromolecules 2010, 43, 7813–7827. [Google Scholar] [CrossRef]
- Musto, P.; Ragosta, G.; Mensitieri, G.; Lavorgna, M. On the molecular mechanism of H2O diffusion into polyimides: A vibrational spectroscopy investigation. Macromolecules 2007, 40, 9614–9627. [Google Scholar] [CrossRef]
- Zhang, Y.; Lee, W.H.; Seong, J.G.; Bae, J.Y.; Zhuang, Y.; Feng, S.; Wan, Y.; Lee, Y.M. Alicyclic segments upgrade hydrogen separation performance of intrinsically microporous polyimide membranes. J. Membr. Sci. 2020, 611, 118363. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, Q.; Zhu, L.; Liu, S.; Chi, Z.; Chen, X.; Zhang, Y.; Xu, J. The preparations and water vapor barrier properties of polyimide films containing amide moieties. Polymers 2017, 9, 677. [Google Scholar] [CrossRef] [PubMed]
- Wijmans, J.G.; Baker, R. The solution-diffusion model: A review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
















| PI | Tg1 (°C) | Tg2 (°C) | Td5% (°C) | Td10% (°C) | CTE 3 (ppm∙K−1) | Tensile Strength (MPa) | Tensile Modulus (GPa) | Elongation at Break (%) |
|---|---|---|---|---|---|---|---|---|
| 2,7-CPPI 4 | 413 | 437 | 556 | 580 | 2.89 | 143.8 ± 3.5 | 4.5 ± 0.2 | 9.3 ± 0.4 |
| M-2,7-CPPI | 395 | 437 | 563 | 601 | 6.39, 49.52 | 120.0 ± 3.1 | 2.0 ± 0.2 | 7.6 ± 0.3 |
| PIs | WVP (g·mil·m−2·day−1) | OP (cm3·mil·m−2·day−1) | WVTR (g·m−2·day−1) | OTR (cm3·m−2·day−1) |
|---|---|---|---|---|
| 2,7-CPPI 1 | 0.3 ± 0.02 | 0.5 ± 0.03 | 0.1 ± 0.01 | 0.2 ± 0.01 |
| M-2,7-CPPI | 30.3 ± 0.6 | 35.8 ± 0.6 | 10.2 ± 0.2 | 12.1 ± 0.2 |
| PI | Density (g∙cm−3) | 2θ (°) | d-Spacing (Å) | Rg (Å) | N 1 (H-Bonds) | CED (J∙cm−3) |
|---|---|---|---|---|---|---|
| 2,7-CPPI | 1.57 | 21.91 | 4.05 | 54.3 | 49 | 570 |
| M-2,7-CPPI | 1.56 | 15.10 | 5.86 | 47.5 | 38 | 456 |
| PI | τ1 (ns) | I1 (%) | τ2 (ns) | I2 (%) | R (Å) | Vf2 (Å3) | FFV1 (%) | FFV2 (O2,%) | FFV2 (H2O,%) | FFV0 2 (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2,7-CPPI 3 | 0.17 | 7.4 | 0.34 | 92.4 | 2.14 | 41.03 | 6.82 | 6.29 | 11.51 | 35.33 |
| M-2,7-CPPI | 0.16 | 12.6 | 0.35 | 85.7 | 2.26 | 48.33 | 7.46 | 7.05 | 12.32 | 37.90 |
| PIs | D 1 | S 2 | P 3 | |||
|---|---|---|---|---|---|---|
| H2O | O2 | H2O | O2 | H2O | O2 | |
| 2,7-CPPI | 3.24 | 6.55 | 0.64 | 0.013 | 2.07 | 0.09 |
| M-2,7-CPPI | 7.84 | 14.20 | 2.39 | 0.044 | 18.74 | 0.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Xie, F.; Huang, J.; Tan, J.; Chen, C.; Jiang, L.; Sun, W.; Zhang, H. The Effect of Molecular Isomerism on the Barrier Properties of Polyimides: Perspectives from Experiments and Simulations. Polymers 2021, 13, 1749. https://doi.org/10.3390/polym13111749
Liu Y, Xie F, Huang J, Tan J, Chen C, Jiang L, Sun W, Zhang H. The Effect of Molecular Isomerism on the Barrier Properties of Polyimides: Perspectives from Experiments and Simulations. Polymers. 2021; 13(11):1749. https://doi.org/10.3390/polym13111749
Chicago/Turabian StyleLiu, Yiwu, Fengyun Xie, Jie Huang, Jinghua Tan, Chengliang Chen, Linbing Jiang, Wei Sun, and Hailiang Zhang. 2021. "The Effect of Molecular Isomerism on the Barrier Properties of Polyimides: Perspectives from Experiments and Simulations" Polymers 13, no. 11: 1749. https://doi.org/10.3390/polym13111749
APA StyleLiu, Y., Xie, F., Huang, J., Tan, J., Chen, C., Jiang, L., Sun, W., & Zhang, H. (2021). The Effect of Molecular Isomerism on the Barrier Properties of Polyimides: Perspectives from Experiments and Simulations. Polymers, 13(11), 1749. https://doi.org/10.3390/polym13111749