Designing of Green Plasticizers and Assessment of the Effectiveness of Their Use
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starting Materials
2.2. Synthesis Methods
2.2.1. Synthesis of Ethoxylated Alcohol
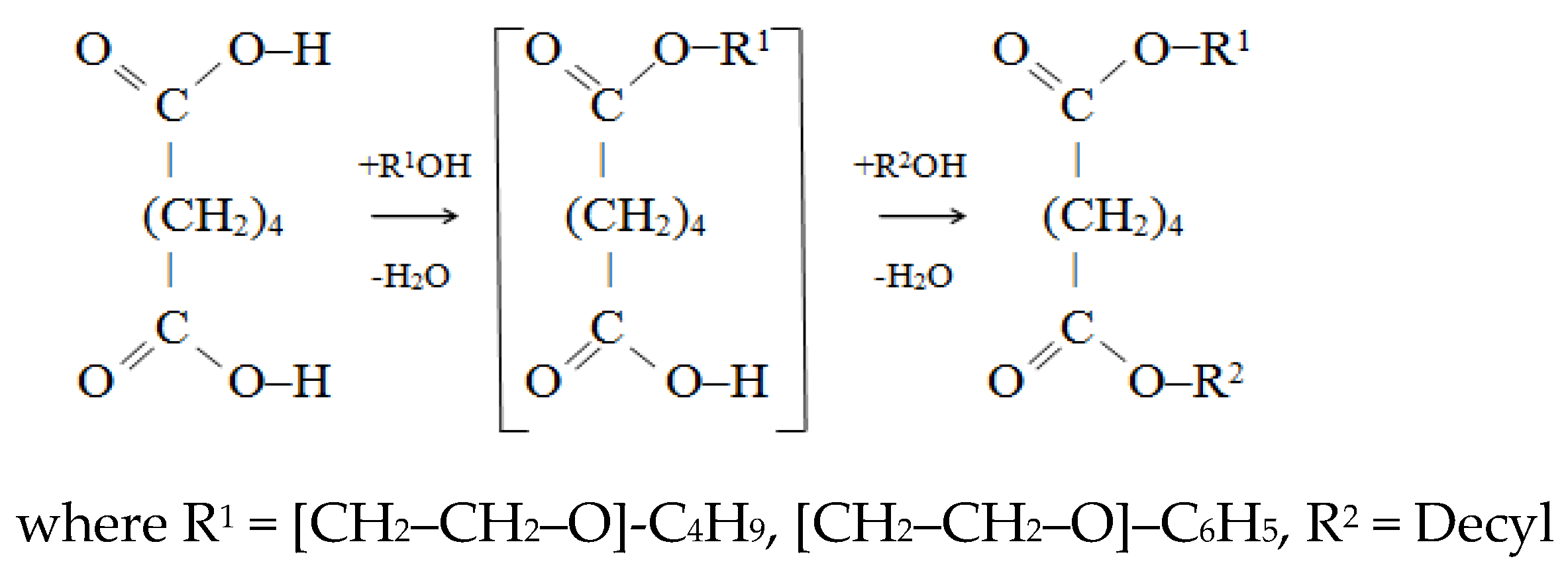
2.2.2. The Synthesis of Unsymmetrical Esters of Adipic Acid
2.3. Methods of Analysis
2.3.1. Analysis of Physicochemical Parameters of Plasticizer
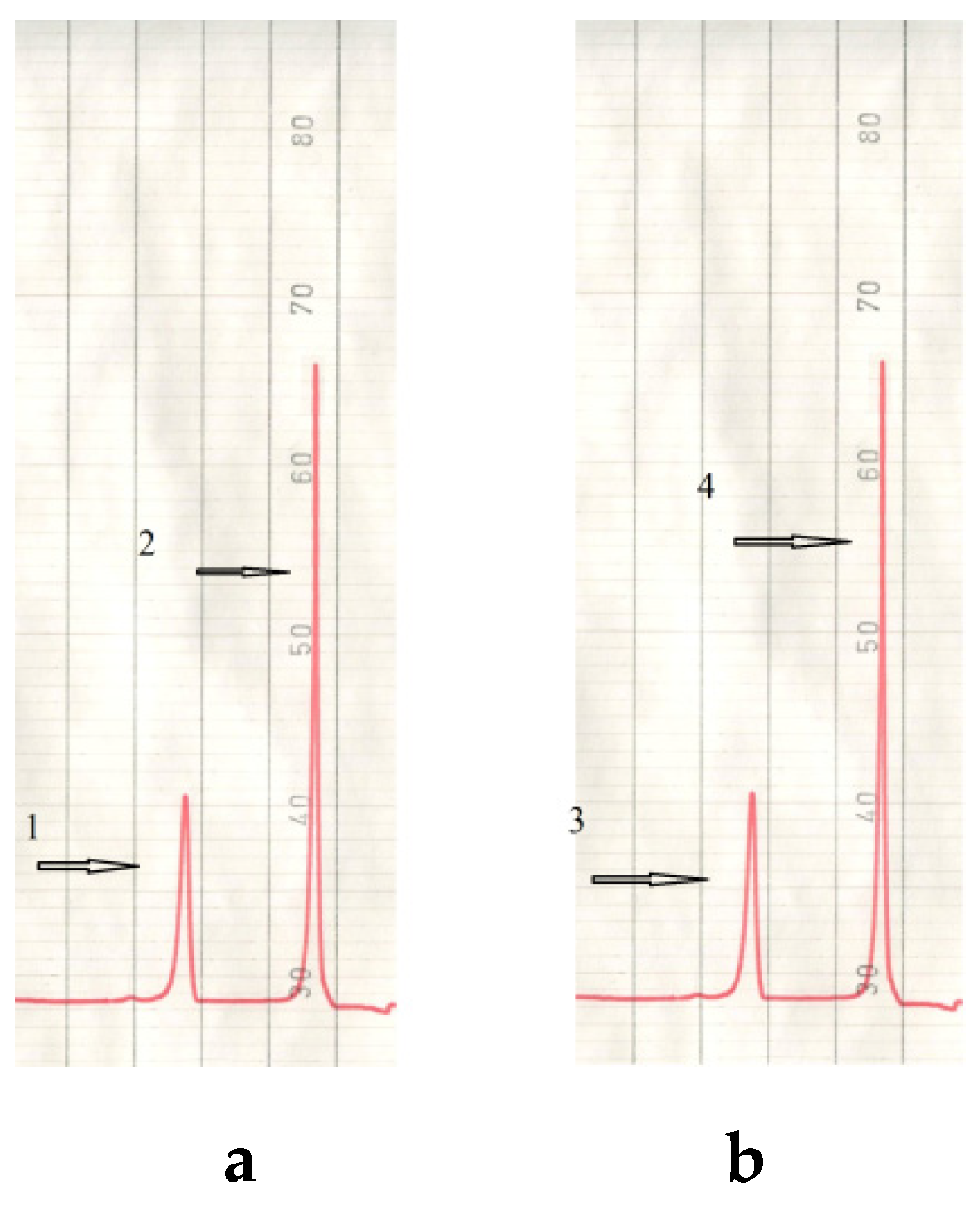
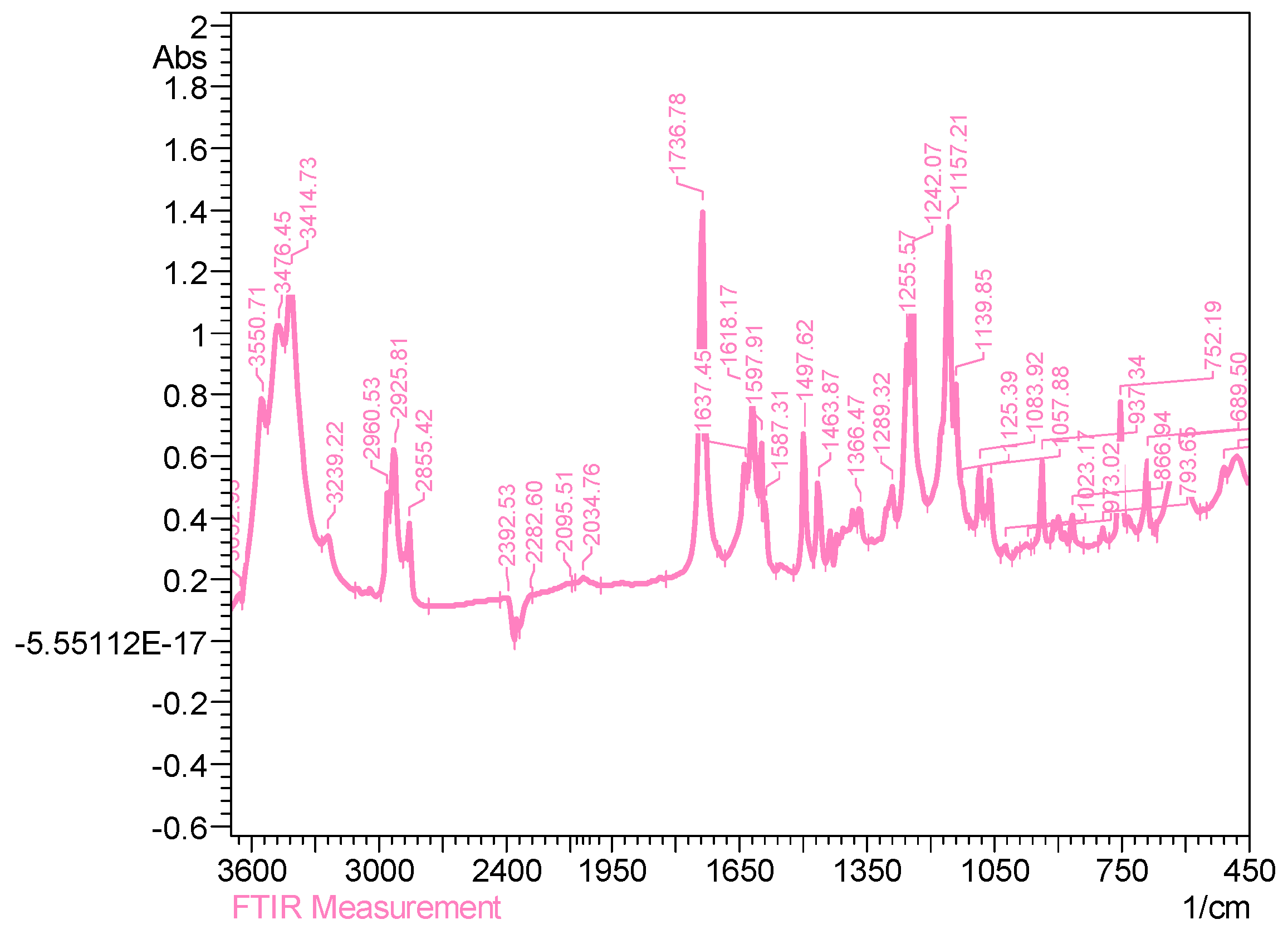
2.3.2. Characterization of Esters of Adipic Acid
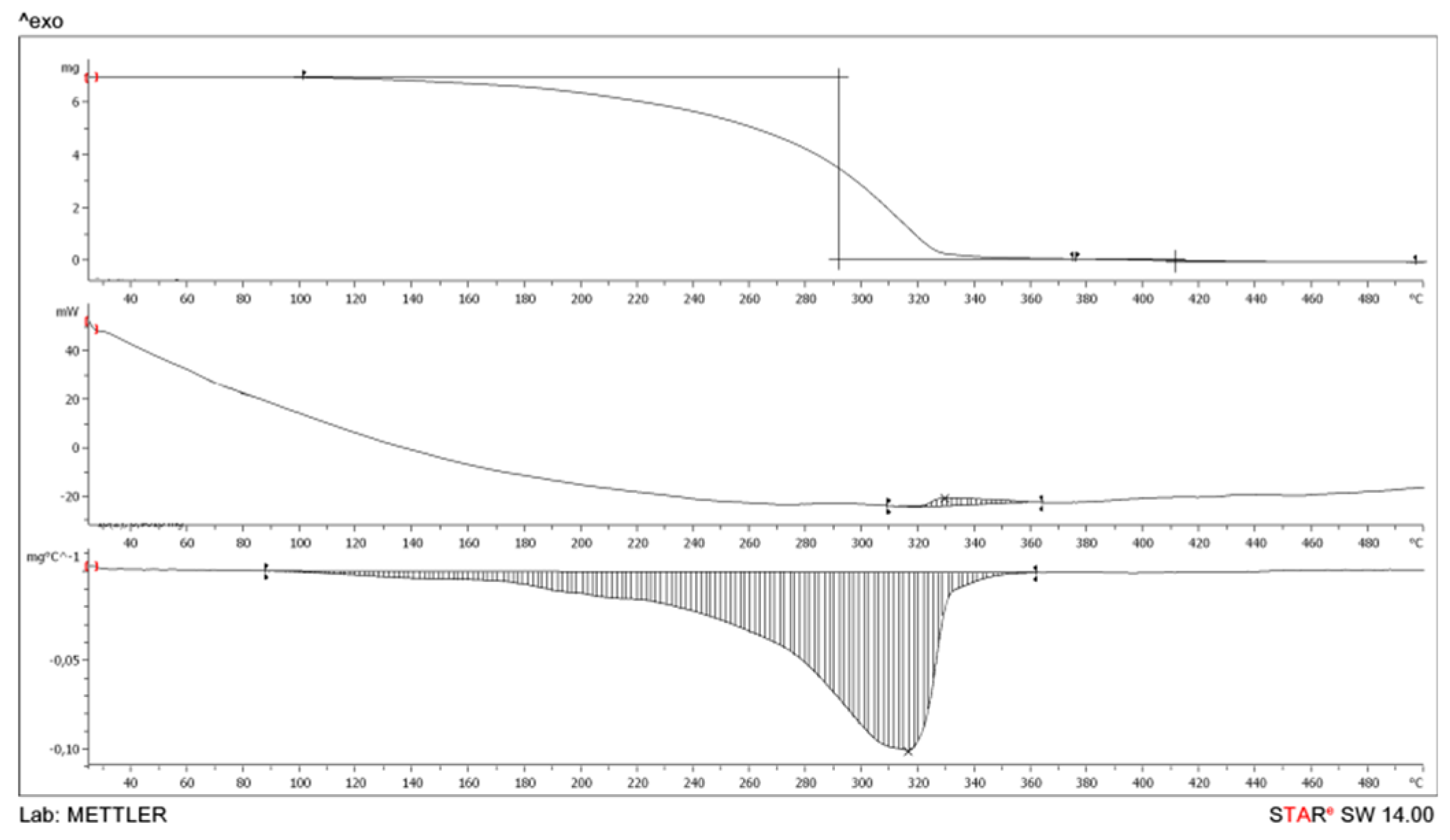
2.3.3. Determination of Thermal Stability of Obtained Plasticizer
- Thermogravimetric curve (TG) characterizes the change in the sample mass during heating (the dependence of the mass on temperature);
- Differential thermogravimetric curve DTG characterizes the rate of change in the sample mass during heating;
- The DSC curve characterizes the thermal effects observed during the heating of the sample.
2.3.4. Determination of Glass Transition Temperature of Obtained Plasticizers
2.3.5. Determination of Fungal Resistance of Samples of PVC Films
2.3.6. Definition of Indicators of Tensile Stress and Elongation at Break of Samples of PVC Films
2.3.7. Study of the Biodegradation of PVC Films in Compost
2.4. Preparation of Film Samples
3. Results
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pr-2194. Fundamentals of state policy in the field of chemical and biological safety of the Russian Federation for the period up to 2010 and beyond. 04 December 2003. Available online: https://docs.cntd.ru/document/902287625?section=status (accessed on 16 February 2021).
- Voronkova, O.; Yankovskaya, V.; Kovaleva, I.; Epishkin, I.; Iusupova, I.; Berdova, Y. Sustainable territorial development based on the effective use of resource potential. Entrep. Sustain. Issues 2019, 7, 662–673. [Google Scholar] [CrossRef]
- Danilov-Danilian, V.I. Sustainable development. Theoretical and methodological analysis. Econ. Math. Methods 2003, 8, 123–135. [Google Scholar]
- Dzhavatov, D.K.; Sverdlikova, E.A.; Sokolov, M.S.; Avdeeva, O.A.; Yavkin, G.P. The influence of innovation on social and economic development of the Russian regions. Eur. Res. Stud. J. 2018, 21, 767–776. [Google Scholar]
- Akhmadeeva, O.A.; Urusova, A.S. The problem of polymer waste circulation in the Russian Federation. Young Sci. 2016, 8, 486–488. Available online: https://moluch.ru/archive/112/28224 (accessed on 16 February 2021).
- Rakhimov, M.A.; Rakhimova, G.M.; Imanov, E.M. Problems of utilization of polymer waste. Fundam. Res. 2014, 8, 331–332. [Google Scholar]
- Gogol, E.V.; Mingazetdinov, I.K.; Gumerova, G.I. Analysis of existing methods of utilization and processing of polymer waste. Bull. Kazan Technol. Univ. 2013, 10, 163–167. [Google Scholar]
- Gu, J.-D. Biodegradability of plastics: The issues, recent advances, and future perspectives. Environ. Sci. Pollut. Res. 2021, 28, 1278–1282. [Google Scholar] [CrossRef]
- Sazonova, O.V.; Suchkov, V.V.; Isakova, O.N. The influence of anthropogenic load on the conditions of soil self-cleaning in the territory of the sanitary protection zone. Public Health Habitat 2016, 5, 22–25. [Google Scholar]
- Rubin, V.M.; Ilyukova, I.I. Toxic effect of petroleum products upon re-entry. Health Risk Anal. 2015, 1, 69–76. [Google Scholar] [CrossRef]
- Kryatov., I.A.; Tonkopiy, N.I.; Vodianova, M.A. Harmonization of hygienic standards for priority soil contamination with international recommendations. Hyg. Sanit. 2015, 7, 42–48. [Google Scholar]
- Rusakov, N.V.; Merzlaya, G.E.; Afanasyev, R.A. Hygienic assessment of the impact of oil hydrocarbons on agricultural crops. Hyg. Sanit. 2007, 6, 60–62. [Google Scholar]
- Federal Service for Supervision of Consumer Rights Protection and Human Welfare; State report; JSC Codex: Moscow, Russia, 2015; p. 206.
- Ivanov, A.V.; Vasiliev, V.V. The state of health of the population in the territories of intensive use of pesticides. Hyg. Sanit. 2005, 2, 24–27. [Google Scholar]
- Ivshina, I.B.; Krivoruchko, A.V.; Kuyukina, M.S. Bioremediation of soils disturbed by hydrocarbons and heavy metals using Rhodococcus-biosurfactants and immobilized rhodococci. Agrar. Bull. Ural. 2012, 8, 65–68. [Google Scholar]
- Budarina, O.V.; Molkov, Y.N.; Ponomareva, O.Y.; Ulyanova, A.V. Immunological methods for assessing health when exposed to air pollution. Hyg. Sanit. 2014, 93, 31–33. [Google Scholar]
- Kryatov, I.A.; Rusakov, N.V. Ecological and hygienic problem of soil pollution. Bull. Russ. Acad. Med Sci. 2006, 5, 18–21. [Google Scholar]
- Kryatov, I.A.; Tonkopiy, N.I.; Pirtahia, N.V. Hygienic rationing for the purpose of soil protection. Conform. Assess. methods 2009, 11, 18–19. [Google Scholar]
- Rusakov, N.V. Waste, Environment, People; Publishing House Medicine: Moscow, Russia, 2004; p. 231. [Google Scholar]
- Yakovlev, A.S.; Nikulina, Y.G. Environmental regulation of the permissible residual oil content in the soils of lands for various economic purposes. Soil Sci. 2013, 2, 234. [Google Scholar]
- Amaral-Zettler, L.A.; Zettler, E.R.; Miner, T.J. Ecology of the plasticsphere. Nat. Rev. Microbiol. 2020, 18, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.-D. Microbiological deterioration and degradation of synthetic polymeric materials: Recent research advances. Int. Biodeterior. Biodegrad. 2003, 52, 69–91. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Vikhareva, I.; Zaripov, I.; Kinzyabulatova, D.; Minigazimov, N.; Aminova, G. Biodegradable polymer materials and modifying additives: State of the art. Part I. Nanotechnol. Constr. A Sci. Internet-J. 2020, 12, 320–325. [Google Scholar] [CrossRef]
- Vikhareva, I.N.; Aminova, G.K.; Builova, E.A.; Mazitova, A.K. Synthesis and study of the properties of a plasticizer based on petrochemical raw materials. Oil Gas Bus. 2020, 4, 57–73. [Google Scholar] [CrossRef]
- Ahmed, T.; Shahid, M.; Azeem, F.; Rasul, I.; Shah, A.A.; Noman, M.; Hameed, A.; Manzoor, N.; Manzoor, I.; Muhammad, S. Biodegradation of plastics: Current scenario and future prospects for environmental safety. Environ. Sci. Pollut. Res. 2018, 25, 7287–7298. [Google Scholar] [CrossRef]
- Haines, A.; Amann, M.; Borgford-Parnell, N.; Leonard, S.; Kuylenstierna, J.; Shindell, D. Short-lived climate pollutant mitigation and the Sustainable Development Goals. Nat. Clim. Chang. 2017, 7, 863–869. [Google Scholar] [CrossRef]
- Nilsson, M.; Griggs, D.; Visbeck, M. Policy: Map the interactions between Sustainable Development Goals. Nat. Cell Biol. 2016, 534, 320–322. [Google Scholar] [CrossRef]
- Mazitova, A.; Vikhareva, I.; Maskova, A.; Gareeva, N.; Shaikhullin, I. Study of the effect of additives on biodegradation of PVC materials. Nanotechnologies Constr. A Sci. Inter. -J. 2020, 12, 94–99. [Google Scholar] [CrossRef]
- Mazitova, A.K.; Vikhareva, I.N.; Aminova, G.K.; Savicheva, J.N. Application of Zinc Oxide to Obtain and Modify Properties of Adipate Plasticizer of Polyvinyl Chloride. Polymers 2020, 12, 1728. [Google Scholar] [CrossRef] [PubMed]
- James, C.; Duncan, C. Handbook of Green Chemistry and Technology; Blackwell Sci. Ltd.: Oxford/London, UK, 2002; p. 560. [Google Scholar]
- Baltacioğlu, H.; Balköse, D. Effect of zinc stearate and/or epoxidized soybean oil on gelation and thermal stability of PVC-DOP plastigels. J. Appl. Polym. Sci. 1999, 74, 2488–2498. [Google Scholar] [CrossRef]
- Choi, J.S.; Park, W.H. Effect of biodegradable plasticizers on thermal and mechanical properties of poly(3-hydroxybutyrate). Polym. Test. 2004, 23, 455–460. [Google Scholar] [CrossRef]
- Interstate Standard 8728-88. Plasticizers. Specifications; IPK Publishing House’s Standards of Quality: Moscow, Russia, 2003; p. 11. [Google Scholar]
- Interstate Standard 18329-2014. Liquid Resins and Plasticizers. Methods for Determination of Density; FSA STANDARTINFORM: Moscow, Russia, 2015; p. 8. [Google Scholar]
- Interstate Standard 9998–86. Polyvinylchloride Films for Household Use. General Specifications; IPK Publishing House’s Standards of Quality: Moscow, Russia, 2002; p. 11. [Google Scholar]
- Interstate Standard 9. 060. Fabrics. Method of Laboratory Tests for Microbiological Destruction Stability; FSA STANDARTINFORM: Moscow, Russia, 1994; p. 10. [Google Scholar]
- Miller, S.A.; Bann, B.; Thrower, R.D. 716. The reaction between phenol and ethylene oxide. J. Chem. Soc. 1950, 1, 3623–3628. [Google Scholar] [CrossRef]
- Staude, F. The lithium phenoxide catalized edition of propylene oxide to phenol. Polym. J. 1971, 2, 468–478. [Google Scholar] [CrossRef] [Green Version]
- Eidus, Y.T. On the synthesis of carboxylic acids under acid catalysis from carbon monoxide, olefins and acylating compounds. J. Org. Chem. 1968, 4, 1214–1219. [Google Scholar]
- Grossman, F. Guidance on the Development of PVC-Based Compositions; Scientific Bases and Technologies: Moscow, Russia, 2009; p. 550. [Google Scholar]
- Pekhtasheva, E.L. Bio-Damage and Protection of Non-Food Goods; Skill: Moscow, Russia, 2002; p. 224. [Google Scholar]
- Gerasimenko, A.A. Protection Against Corrosion, Aging and Bio-Damage of Machinery, Equipment and Structures; Machine Engineering: Moscow, Russia, 1987; p. 688. [Google Scholar]
- Orekhov, D.A.; Vlasova, G.M.; Makarevich, A.V.; Pinchuk, L.S. Biodegradable films based on thermoplastics. Rep. Natl. Acad. Sci. Belarus 2000, 44, 100–103. [Google Scholar]
- Madigan, J.M.; Martinko, J.M.; Parker, J. Biology of Microorganisms, 8th ed.; Brock, T., Ed.; Simon and Viacom Company: New York, NY, USA; Prentice Hall Inc: Englewood Cliffs, NJ, USA.
- Brandl, M.; Gross, R.A.; Lenz, R.W.; Fuller, G. Plastics from bacteria and for bacteria: Poly(b-Hydroxyalkanoates) as natural biocompatible. Biodegrad. Polyest. Adv. Biochem. Eng. I Biotechnol. 1990, 41, 78–93. [Google Scholar]
- Lee, B.; Pometto, A.L.; Fratzke, A.; Bailey, T.B. Biodegradation of degradable plastic polyethylene by Phanerochaete and Streptomyces species. Appl. Environ. Microbiol. 1991, 57, 678–685. [Google Scholar] [CrossRef] [Green Version]
- Seppala, J.; Linko, Y.Y.; Su, T. Photo and biodegradation of high volume thermoplastics. Acta Polytech. Scand. Chem. Technol. Mettalurgy Ser. 1991, 198, 33. [Google Scholar]
- Albertsson, A.-C.; Andersson, S.O.; Karlsson, S. The mechanism of biodegradation of polyethylene. Polym. Degrad. Stab. 1987, 18, 73–87. [Google Scholar] [CrossRef]
- Klemchuck, P.P. Chemistry of plastics casts a negative vote. Modem Plast. 1989, 66, 48–53. [Google Scholar]
- Kırbaş, Z.; Keskin, N.E.V.İ.N.; Güner, A. Biodegradation of Polyvinylchloride (PVC) by White Rot Fungi. Bull. Environ. Contam. Toxicol. 1999, 63, 335–342. [Google Scholar] [CrossRef]
- Gu, J.D.; Ford, T.E.; Mitchel, R. Microbial Corrosion of Metals; Review; The Uhlig Corrosion Handbook–Wiley: New York, NY, USA, 2000; pp. 915–927. [Google Scholar]
- Gu, J.D.; Ford, T.E.; Mitton, D.B.; Mitchel, R. Microbial Degradation and Deterioration of Polymeric Materials; Review; The Uhlig Corrosion Handbook–Wiley: New York, NY, USA, 2000; pp. 439–460. [Google Scholar]
- Gu, J.D.; Mitchel, R. Biodeterioration. In The Prokaryotes: An Evolving Electronic Resource for the Microbiological Community, 3rd ed.; Dworkin, M., Falkow, S., Rosenberg, E., Shleifer, K.-H., Stackebrandt, E., Eds.; Springer-Werlag: New York, NY, USA, 2001. [Google Scholar]
- Glass, J.E.; Swift, G. Agricultural and Synthetic Polymers, Biodegradation and Utilization, ACS Symposium Series 433; American Chemical Society: Washington, DC, USA, 1989; pp. 9–64. [Google Scholar]
- Danilov-Danilyan, V.I. Temporary Methodology for Determining Prevented Environmental Damage; State Committee for Ecology: Moscow, Russia, 1999; p. 41. [Google Scholar]
- Mazitova, A.K.; Vikhareva, I.N.; Aminova, G.K.; Timofeev, A.A.; Buylova, E.A.; Distanov, R.S. Investigation of the effect of the amount of additives on the properties of adipic acid esters. Nanotechnol. Constr. 2019, 11, 94–99. [Google Scholar] [CrossRef]
- Plakunov, V.K.; Gannesen, A.V.; Mart’Yanov, S.V.; Zhurina, M.V. Biocorrosion of Synthetic Plastics: Degradation Mechanisms and Methods of Protection. Microbiology 2020, 89, 647–659. [Google Scholar] [CrossRef]
- Giacomucci, L.; Raddadi, N.; Soccio, M.; Lotti, N.; Fava, F. Polyvinyl chloride biodegradation by Pseudomonas citronellolis and Bacillus flexus. New Biotechnol. 2019, 52, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Giacomucci, L.; Raddadi, N.; Soccio, M.; Lotti, N.; Fava, F. Biodegradation of polyvinyl chloride plastic films by enriched anaerobic marine consortia. Mar. Environ. Res. 2020, 158, 104949. [Google Scholar] [CrossRef] [PubMed]





| Ester | Indicators | |||
|---|---|---|---|---|
| Molecular Weight | Acid Number, mg KOH/g | Ester Number, mg KOH/g | d204 | |
| Decyl butoxyethyl adipate (DBEA) | 388 | 0.1 | 288 | 1.1003 |
| Decyl phenoxyethyl adipate (DPEA) | 406 | 0.1 | 273 | 0.9957 |
| Sample | Characteristics | |||
|---|---|---|---|---|
| Temperature, °C | Δm, % | |||
| Beginning | Maximal Value | End | at 180 °C | |
| DBEA | 153 | 278 | 498 | 0.8 |
| DPEA | 101 | 316 | 499 | 0.9 |
| DOP | 132 | 284 | 497 | 1.0 |
| Sample | Endotherm Characteristics | Exotherm Characteristics | ||
|---|---|---|---|---|
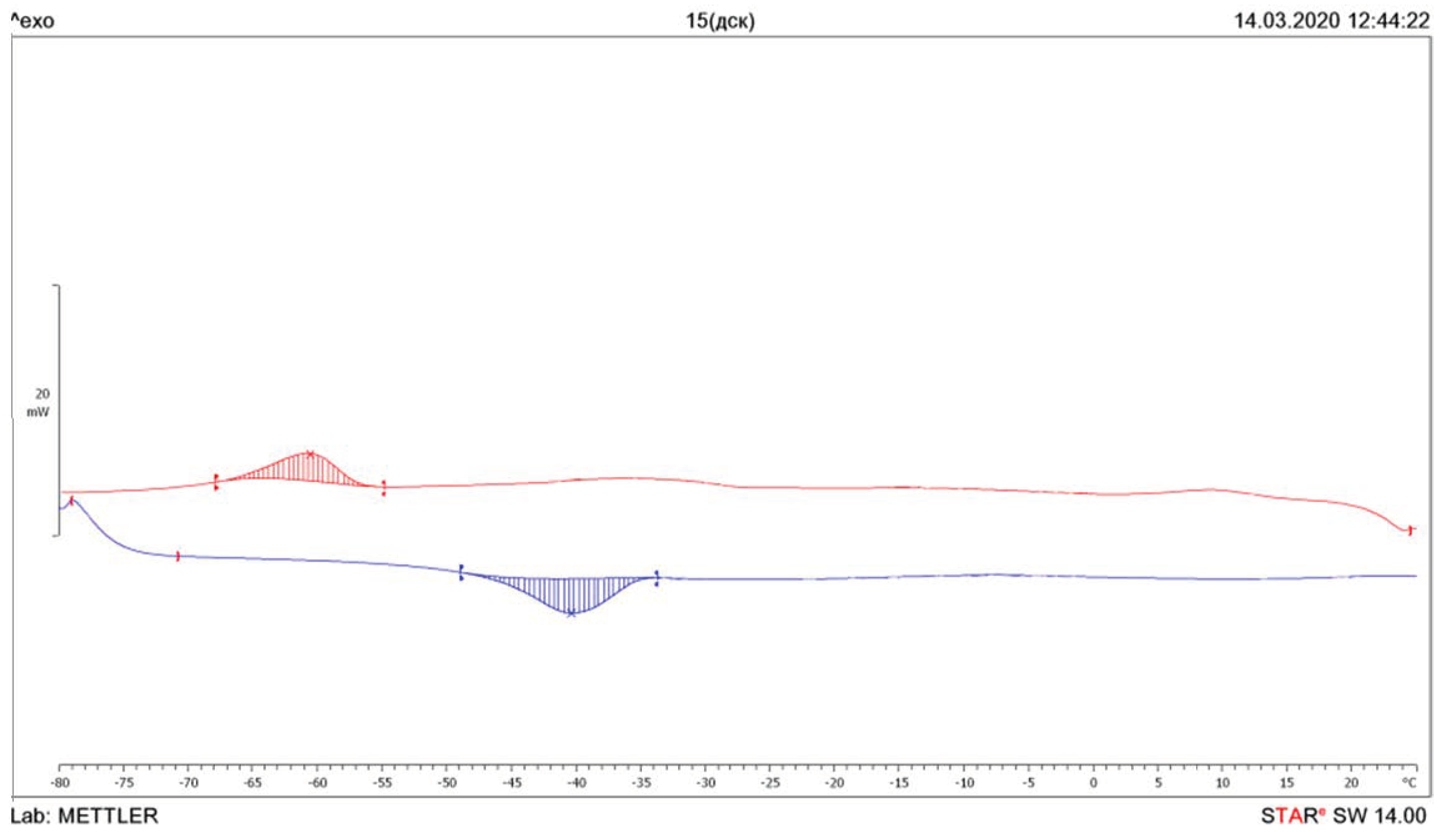
| Temperature, °C | ΔHm, J/g | Temperature, °C | ΔHcr, J/g | |
| DBEA | −40.3 | −14.1 | −60.5 | 9.4 |
| DPEA | 84.0 | −67.8 | 8.0 | 131.2 |
| Plasticizer | Sample Number | Tc, °C |
|---|---|---|
| DOP | 113 | |
| DBEA | 1 | 135 |
| DPEA | 2 | 169 |
| Composition Number | Micromycete | ||
|---|---|---|---|
| Aspergillus niger | Pénicillium funiculosum | Trichodermalignorum | |
| 1 | 4 | 4 | 4 |
| 2 | 3 | 3 | 3 |
| Composition Number | Tensile Breaking Stress, MPa | Elongation at Break, % |
|---|---|---|
| 1 | 16,5 | 227 |
| 2 | 17,0 | 221 |
| Exposure Time, Days | Composition 1 | Composition 2 | ||
|---|---|---|---|---|
| Change in Tensile Stress, % | Change in Elongation at Break, % | Change in Tensile Stress, % | Change in Elongation at Break, % | |
| 10 | −7.1 | 1.5 | −4.2 | 1.0 |
| 30 | −21.4 | 3.4 | −18.9 | 2.7 |
| 45 | −43.3 | 7.4 | −37.4 | 6.8 |
| 90 | −64.9 | 15.7 | −60.3 | 14.9 |
| 120 | −95.7 | 31.2 | −91.3 | 29.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazitova, A.K.; Aminova, G.K.; Vikhareva, I.N. Designing of Green Plasticizers and Assessment of the Effectiveness of Their Use. Polymers 2021, 13, 1761. https://doi.org/10.3390/polym13111761
Mazitova AK, Aminova GK, Vikhareva IN. Designing of Green Plasticizers and Assessment of the Effectiveness of Their Use. Polymers. 2021; 13(11):1761. https://doi.org/10.3390/polym13111761
Chicago/Turabian StyleMazitova, Aliya K., Guliya K. Aminova, and Irina N. Vikhareva. 2021. "Designing of Green Plasticizers and Assessment of the Effectiveness of Their Use" Polymers 13, no. 11: 1761. https://doi.org/10.3390/polym13111761
APA StyleMazitova, A. K., Aminova, G. K., & Vikhareva, I. N. (2021). Designing of Green Plasticizers and Assessment of the Effectiveness of Their Use. Polymers, 13(11), 1761. https://doi.org/10.3390/polym13111761







