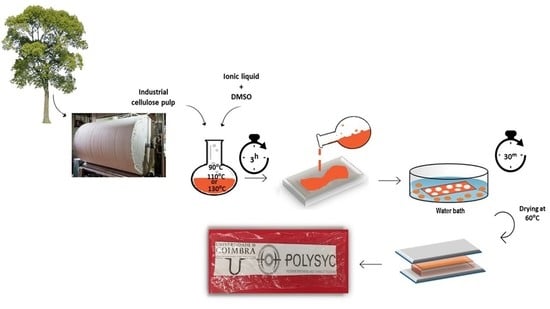
Process Development for Flexible Films of Industrial Cellulose Pulp Using Superbase Ionic Liquids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterizations
2.3. Dissolution of Industrial Pulp
2.4. Preparation of Cellulose/TMG-Based Ionic Liquid Composite Film
2.5. Quantification of Ionic Liquid in Films
3. Results and Discussion
3.1. Cellulose Dissolution
3.2. Cellulose Films Based on TMG Ionic Liquids
3.3. Fourier-Tranform Infrared Spectroscopy
3.4. Scanning Electronic Microscopy
3.5. UV/VIS Spectroscopy
3.6. IL Content in Films
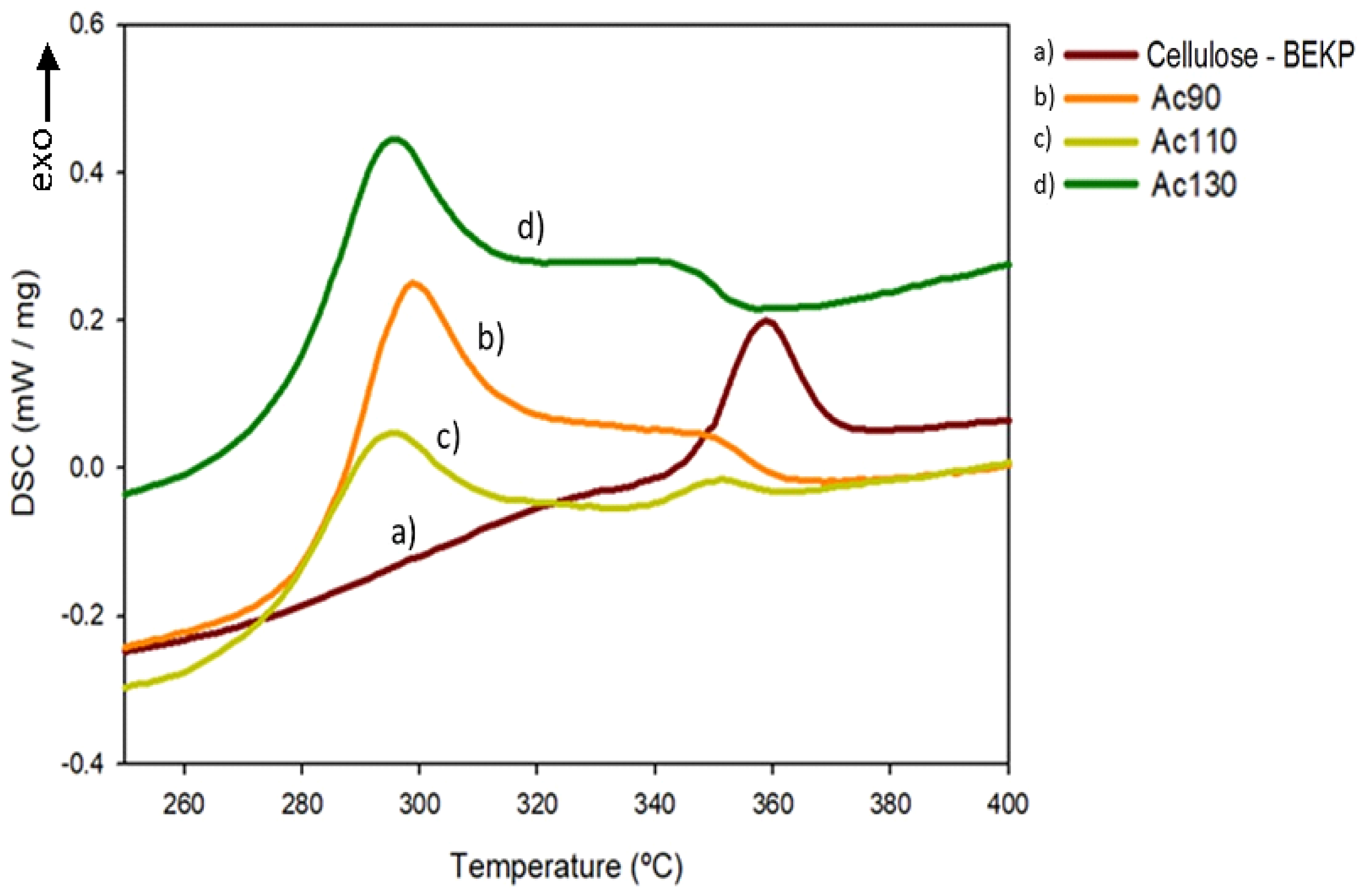
3.7. Thermal Properties
3.8. Crystallinity
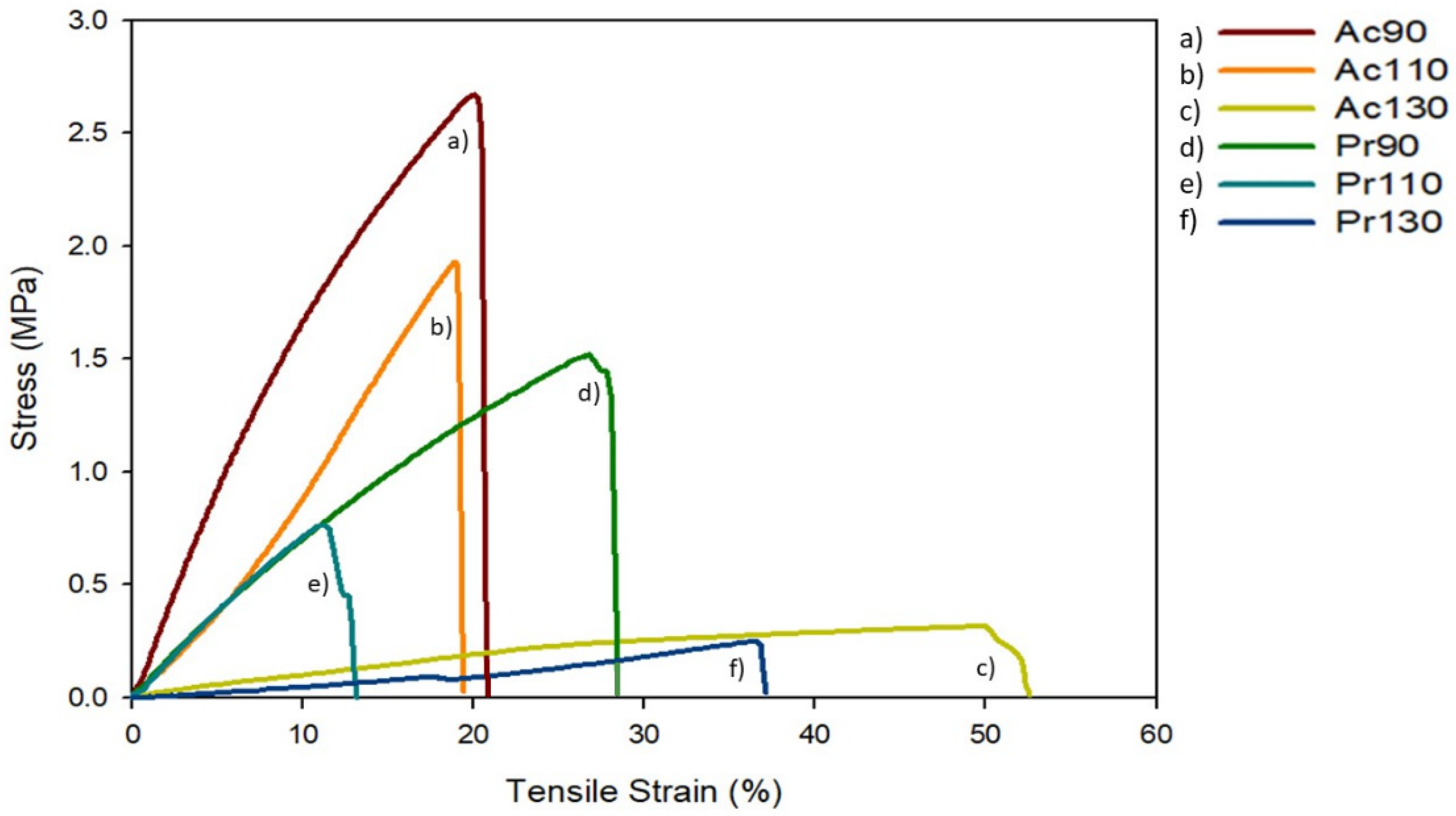
3.9. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gourmelon, G. Global Plastic Production Rises, Recycling Lags; Worldwatch Institute, 2015; pp. 1–7. Available online: http://www.plastic-resource-center.com (accessed on 9 April 2020).
- Xiong, R.; Grant, A.M.; Ma, R.; Zhang, S.; Tsukruk, V.V. Naturally-derived biopolymer nanocomposites: Interfacial design, properties and emerging applications. Mater. Sci. Eng. R Rep. 2018, 125, 1–41. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Kostag, M.; Jedvert, K.; Achtel, C.; Heinze, T.; El Seoud, O.A. Recent Advances in Solvents for the Dissolution, Shaping and Derivatization of Cellulose: Quaternary Ammonium Electrolytes and their Solutions in Water and Molecular Solvents. Molecules 2018, 23, 511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, Q.; Chen, L.; Huang, L.; Tian, C.; Zheng, L.; Ni, Y. A process for enhancing the accessibility and reactivity of hardwood kraft-based dissolving pulp for viscose rayon production by cellulase treatment. Bioresour. Technol. 2014, 154, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Striegel, A. Theory and applications of DMAC/LICL in the analysis of polysaccharides. Carbohydr. Polym. 1997, 34, 267–274. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L. Rapid Dissolution of Cellulose in LiOH/Urea and NaOH/Urea Aqueous Solutions. Macromol. Biosci. 2005, 5, 539–548. [Google Scholar] [CrossRef]
- Rosenau, T.; Hofinger, A.; Potthast, A.; Kosma, P. On the conformation of the cellulose solvent N-methylmorpholine-N-oxide (NMMO) in solution. Polymer 2003, 44, 6153–6158. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of Cellose with Ionic Liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Verma, C.; Mishra, A.; Chauhan, S.; Verma, P.; Srivastava, V.; Quraishi, M.; Ebenso, E.E. Dissolution of cellulose in ionic liquids and their mixed cosolvents: A review. Sustain. Chem. Pharm. 2019, 13, 100162. [Google Scholar] [CrossRef]
- Haq, M.A.; Habu, Y.; Yamamoto, K.; Takada, A.; Ka-Dokawa, J.-I. Ionic liquid induces flexibility and thermoplasticity in cellulose film. Carbohydr. Polym. 2019, 223, 115058. [Google Scholar] [CrossRef]
- Wilkes, J.S. A short history of ionic liquids—from molten salts to neoteric solvents. Green Chem. 2002, 4, 73–80. [Google Scholar] [CrossRef]
- Sashina, E.S.; Kashirskii, D.A.; Zaborski, M.; Jankowski, S. Synthesis and dissolving power of 1-Alkyl-3-methylpyridinium-based ionic liquids. Russ. J. Gen. Chem. 2012, 82, 1994–1998. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Yu, J.; Zhang, X.; He, J.; Zhang, J. Application of ionic liquids for dissolving cellulose and fabricating cellulose-based materials: State of the art and future trends. Mater. Chem. Front. 2017, 1, 1273–1290. [Google Scholar] [CrossRef]
- Cheng, G.; Zhu, P.; Li, J.; Cheng, F.; Lin, Y.; Zhou, M. All-cellulose films with excellent strength and toughness via a facile approach of dissolution-regeneration. J. Appl. Polym. Sci. 2019, 136, 46925. [Google Scholar] [CrossRef]
- Peng, H.; Wang, S.; Xu, H.; Dai, G. Preparations, properties, and formation mechanism of novel cellulose hydrogel membrane based on ionic liquid. J. Appl. Polym. Sci. 2017, 135, 45488. [Google Scholar] [CrossRef]
- Nowicki, J.; Muszyński, M.; Mikkola, J.-P. Ionic liquids derived from organosuperbases: En route to superionic liquids. RSC Adv. 2016, 6, 9194–9208. [Google Scholar] [CrossRef]
- King, A.W.T.; Asikkala, J.; Mutikainen, I.; Järvi, P.; Kilpeläinen, I. Distillable Acid-Base Conjugate Ionic Liquids for Cellulose Dissolution and Processing. Angew. Chem. Int. Ed. 2011, 50, 6301–6305. [Google Scholar] [CrossRef]
- Parviainen, A.; King, A.W.T.; Mutikainen, I.; Hummel, M.; Selg, C.; Hauru, L.K.J.; Sixta, H.; Kilpeläinen, I. Predicting Cellulose Solvating Capabilities of Acid-Base Conjugate Ionic Liquids. ChemSusChem 2013, 6, 2161–2169. [Google Scholar] [CrossRef]
- Lethesh, K.C.; Evjen, S.; Venkatraman, V.; Shah, S.N.; Fiksdahl, A. Highly efficient cellulose dissolution by alkaline ionic liquids. Carbohydr. Polym. 2020, 229, 115594. [Google Scholar] [CrossRef]
- El Seoud, O.A.; Kostag, M.; Jedvert, K.; Malek, N.I. Cellulose in Ionic Liquids and Alkaline Solutions: Advances in the Mechanisms of Biopolymer Dissolution and Regeneration. Polymers 2019, 11, 1917. [Google Scholar] [CrossRef] [Green Version]
- Rinaldi, R. Instantaneous dissolution of cellulose in organic electrolyte solutions. Chem. Commun. 2011, 47, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, R.; King, A.; Parviainen, A.; Kruus, K.; Suurnäkki, A. Cellulose hydrolysis with thermo- and alkali-tolerant cellulases in cellulose-dissolving superbase ionic liquids. RSC Adv. 2013, 3, 20001–20009. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.; Martin, A.; Conrad, C. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Meenatchi, B.; Renuga, V.; Manikandan, A. Cellulose dissolution and regeneration using various imidazolium based protic ionic liquids. J. Mol. Liq. 2017, 238, 582–588. [Google Scholar] [CrossRef]
- King, A.W.T.; Parviainen, A.; Karhunen, P.; Matikainen, J.; Hauru, L.K.J.; Sixta, H.; Kilpeläinen, I. Relative and inherent reactivities of imidazolium-based ionic liquids: The implications for lignocellulose processing applications. RSC Adv. 2012, 2, 8020–8026. [Google Scholar] [CrossRef]
- Kasprzak, D.; Krystkowiak, E.; Stępniak, I.; Galiński, M. Dissolution of cellulose in novel carboxylate-based ionic liquids and dimethyl sulfoxide mixed solvents. Eur. Polym. J. 2019, 113, 89–97. [Google Scholar] [CrossRef]
- Fang, Z.; Li, B.; Liu, Y.; Zhu, J.; Li, G.; Hou, G.; Zhou, J.; Qiu, X. Critical Role of Degree of Polymerization of Cellulose in Super-Strong Nanocellulose Films. Matter 2020, 2, 1000–1014. [Google Scholar] [CrossRef]
- Pang, J.; Liu, X.; Zhang, X.; Wu, Y.; Sun, R. Fabrication of Cellulose Film with Enhanced Mechanical Properties in Ionic Liquid 1-Allyl-3-methylimidaxolium Chloride (AmimCl). Materals 2013, 6, 1270–1284. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Huang, F.; Chen, L.; Huang, L.; Cao, S.; Ma, X. Preparation of transparent film via cellulose regeneration: Correlations between ionic liquid and film properties. Carbohydr. Polym. 2019, 203, 214–218. [Google Scholar] [CrossRef]
- Hauru, L.K.J.; Hummel, M.; Nieminen, K.; Michud, A.; Sixta, H. Cellulose regeneration and spinnability from ionic liquids. Soft Matter 2015, 12, 1487–1495. [Google Scholar] [CrossRef] [Green Version]
- Sundberg, J.; Toriz, G.; Gatenholm, P. Moisture induced plasticity of amorphous cellulose films from ionic liquid. Polymer 2013, 54, 6555–6560. [Google Scholar] [CrossRef]
- Elhi, F.; Aid, T.; Koel, M. Ionic liquids as solvents for making composite materials from cellulose. Proc. Estonian Acad. Sci. 2016, 65, 255. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Y.; Li, J.; Wang, F.; Chang, G.; Fu, T.; Zhou, W. Effects of temperature on cellulose hydrogen bonds during dissolution in ionic liquid. Carbohydr. Polym. 2018, 201, 387–391. [Google Scholar] [CrossRef]
- Pang, J.-H.; Liu, X.; Wu, M.; Wu, Y.-Y.; Zhang, X.-M.; Sun, R.-C. Fabrication and Characterization of Regenerated Cellulose Films Using Different Ionic Liquids. J. Spectrosc. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.Y.; Marchessault, R.H. Infrared spectra of crystalline polysaccharides. II. Native celluloses in the region from 640 to 1700 cm.1. J. Polym. Sci. 1959, 39, 269–278. [Google Scholar] [CrossRef]
- Bian, H.; Chen, L.; Dong, M.; Wang, L.; Wang, R.; Zhou, X.; Wu, C.; Wang, X.; Ji, X.; Dai, H. Natural lignocellulosic nanofibril film with excellent ultraviolet blocking performance and robust environment resistance. Int. J. Biol. Macromol. 2021, 166, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Kilzer, F.J.; Broido, A. Speculations on the nature of cellulose pyrolysis. Pyrodinamics 1965, 2, 151–163. [Google Scholar]
- Conesa, J.A.; Caballero, J.; Marcilla, A.; Font, R. Analysis of different kinetic models in the dynamic pyrolysis of cellulose. Thermochim. Acta 1995, 254, 175–192. [Google Scholar] [CrossRef]
- Nada, A.; Hassan, M.L. Thermal behavior of cellulose and some cellulose derivatives. Polym. Degrad. Stab. 2000, 67, 111–115. [Google Scholar] [CrossRef]
- Szcześniak, L.; Rachocki, A.; Tritt-Goc, J. Glass transition temperature and thermal decomposition of cellulose powder. Cellul. 2007, 15, 445–451. [Google Scholar] [CrossRef]
- Chen, F.; Kan, Z.; Hua, S.; Liu, Z.; Yang, M. A new understanding concerning the influence of structural changes on the thermal behavior of cellulose. J. Polym. Res. 2015, 22, 1–8. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Kim, H.C.; Kim, H.Y.; Chung, Y.S.; Park, W.H.; Youk, J.H. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef] [PubMed]
- Berga, L.; Bruce, I.; Nicol, T.W.J.; Holding, A.J.; Isobe, N.; Shimizu, S.; Walker, A.J.; Reid, J.E.S.J. Cellulose dissolution and regeneration using a non-aqueous, non-stoichiometric protic ionic liquid system. Cellulose 2020, 27, 1–11. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, X.; Hao, M.; Huang, C.; Xue, Z.; Mu, T. Preparation and characterization of regenerated cellulose from ionic liquid using different methods. Carbohydr. Polym. 2015, 117, 99–105. [Google Scholar] [CrossRef]






| Sample | Solubility in TMG Based Ionic Liquids | ||
|---|---|---|---|
| [TMG][OAc] | [TMG][Pr] | [TMG][Fo] | |
| MCC a | 5 wt.% | 5 wt.% | Not soluble |
| BEKP b | 2 wt.% | 2 wt.% | Not soluble |
| Film | IL (wt.%) |
|---|---|
| Ac90 | 45.1 |
| Ac110 | 63.1 |
| Ac130 | 88.6 |
| Pr90 | 48.4 |
| Pr110 | 61.7 |
| Pr130 | 95.0 |
| Sample | DSC | TGA | DTG | ||||
|---|---|---|---|---|---|---|---|
| Ts a (°C) | Tc b (°C) | Tonset c (°C) | Tend d (°C) | W500 e (%) | Solvpeak f (°C) | Celpeak g (°C) | |
| BEKP | - | 358.9 | - | 355.1 | 3.0 | - | 373.3 |
| [TMG][OAc] | 205.9 | - | 140.8 | 193.9 | 1.7 | 189.2 | - |
| [TMG][Pr] | 213.6 | - | 159.1 | 222.7 | 2.3 | 215.4 | - |
| Ac90 | 198.4 | 299.1 | 187.2 | 343.0 | 25.3 | 254.4 | 353.7 |
| Ac110 | 212.6 | 295.6 | 157.3 | 339.3 | 19.5 | 251.9 | 357.3 |
| Ac130 | 213.5 | 295.4 | 144.1 | 349.5 | 4.0 | 256.5 | 360.4 |
| Pr90 | 203.4 | 338.4 | 146.5 | 343.5 | 22.6 | 256.4 | 357.7 |
| Pr110 | 203.5 | 300.5 | 173.3 | 352.1 | 6.9 | 254.8 | 357.0 |
| Pr130 | 224.4 | 302.9 | 204.6 | 354.0 | 2.4 | 255.0 | 355.2 |
| Film | IL Content: Extraction Test (wt.%) | IL Content a (wt.%) | IL + Residual Mass b (wt.%) | Cellulose Content c (wt.%) |
|---|---|---|---|---|
| Ac90 | 45.1 | 27.5 | 49.8 | 34.6 |
| Ac110 | 63.1 | 37.7 | 54.2 | 26.4 |
| Ac130 | 88.6 | 58.9 | 65.5 | 12.9 |
| Pr90 | 48.4 | 29.5 | 49.1 | 34.3 |
| Pr110 | 61.7 | 54.7 | 61.6 | 20.3 |
| Pr130 | 95.0 | 81.3 | 83.8 | 7.1 |
| Sample | Crystallinity Index (%) |
|---|---|
| Cellulose—BEKP | 52.8 |
| Ac90 | 9.2 |
| Ac110 | 16.1 |
| Ac130 | 27.1 |
| Pr90 | 25.1 |
| Pr110 | 21.1 |
| Pr130 | 24.5 |
| Film | Maximum Stress ± SD (MPa) | Elongation at Break ± SD (%) | Young’s Modulus ± SD (MPa) |
|---|---|---|---|
| Ac90 | 3.0 ± 0.5 | 28.7 ± 8.8 | 1.00 ± 0.10 |
| Ac110 | 1.6 ± 0.3 | 18.9 ± 2.5 | 0.12 ± 0.02 |
| Ac130 | 0.30 ± 0.03 | 46.9 ± 5.5 | 0.015 ± 0.002 |
| Pr90 | 1.3 ± 0.4 | 19.5 ± 3.9 | 0.79 ± 0.09 |
| Pr110 | 0.9 ± 0.2 | 14.7 ± 2.0 | 0.09 ± 0.01 |
| Pr130 | 0.17 ± 0.05 | 33.9 ± 3.2 | 0.006 ± 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, D.C.M.; Rebelo, R.C.; De Bon, F.; Coelho, J.F.J.; Serra, A.C. Process Development for Flexible Films of Industrial Cellulose Pulp Using Superbase Ionic Liquids. Polymers 2021, 13, 1767. https://doi.org/10.3390/polym13111767
Ribeiro DCM, Rebelo RC, De Bon F, Coelho JFJ, Serra AC. Process Development for Flexible Films of Industrial Cellulose Pulp Using Superbase Ionic Liquids. Polymers. 2021; 13(11):1767. https://doi.org/10.3390/polym13111767
Chicago/Turabian StyleRibeiro, Diana C. M., Rafael C. Rebelo, Francesco De Bon, Jorge F. J. Coelho, and Arménio C. Serra. 2021. "Process Development for Flexible Films of Industrial Cellulose Pulp Using Superbase Ionic Liquids" Polymers 13, no. 11: 1767. https://doi.org/10.3390/polym13111767
APA StyleRibeiro, D. C. M., Rebelo, R. C., De Bon, F., Coelho, J. F. J., & Serra, A. C. (2021). Process Development for Flexible Films of Industrial Cellulose Pulp Using Superbase Ionic Liquids. Polymers, 13(11), 1767. https://doi.org/10.3390/polym13111767