Porous Polymers from High Internal Phase Emulsions as Scaffolds for Biological Applications
Abstract
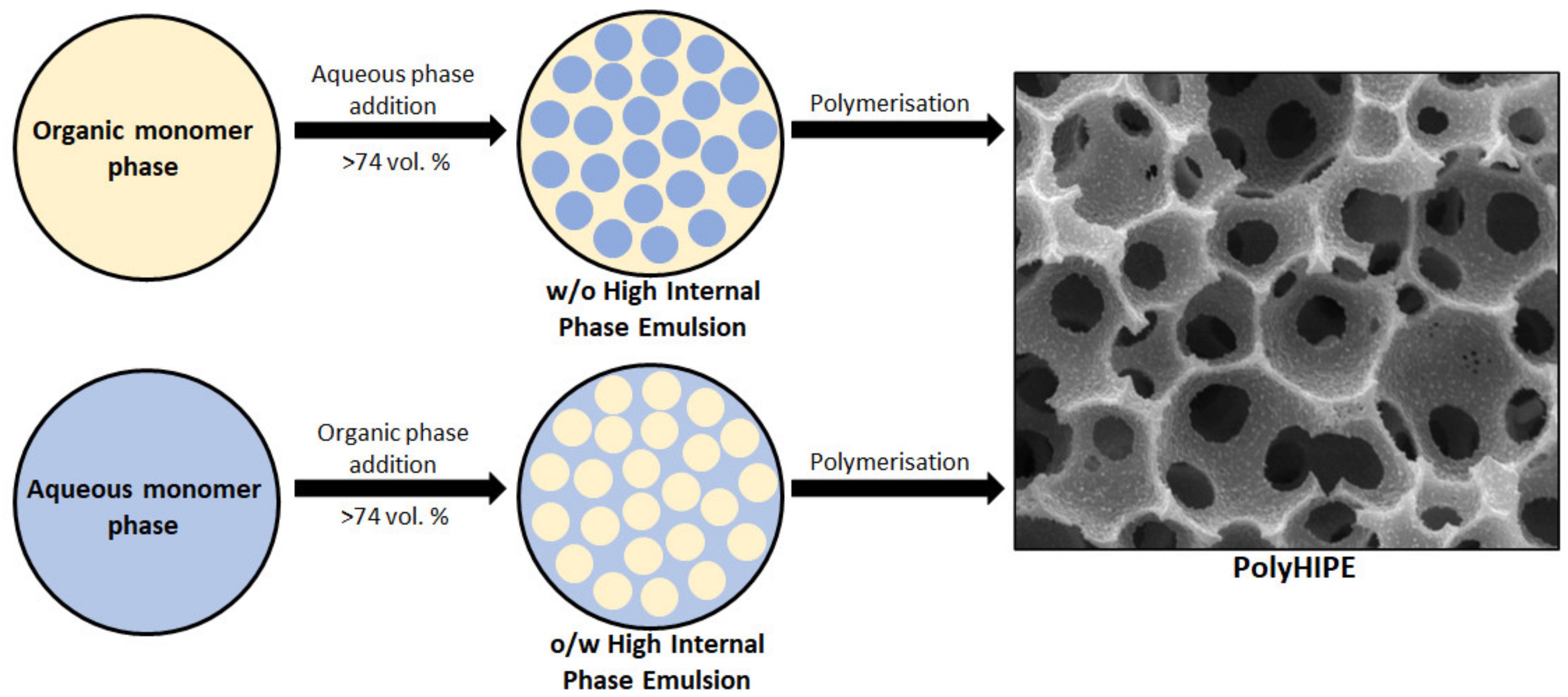
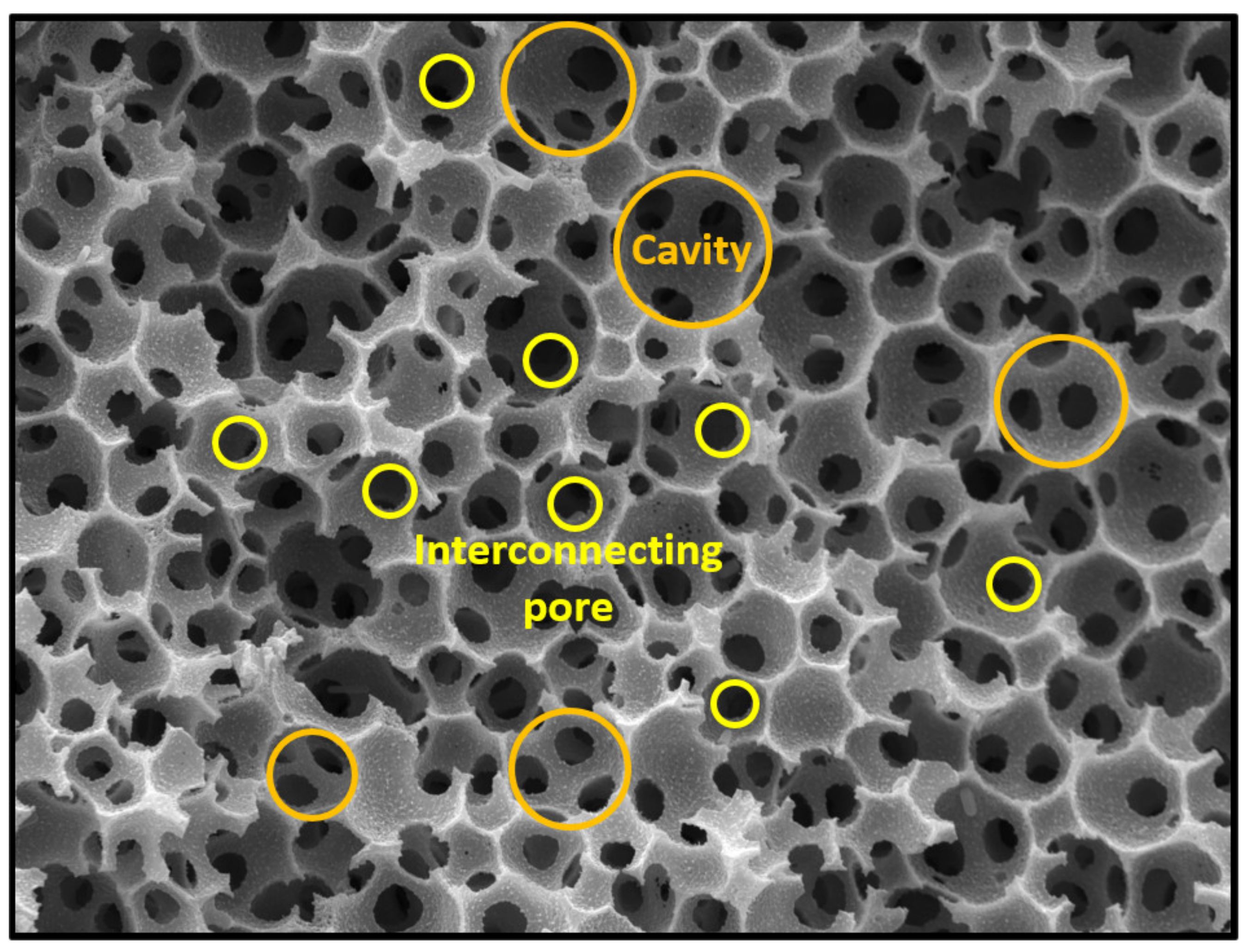
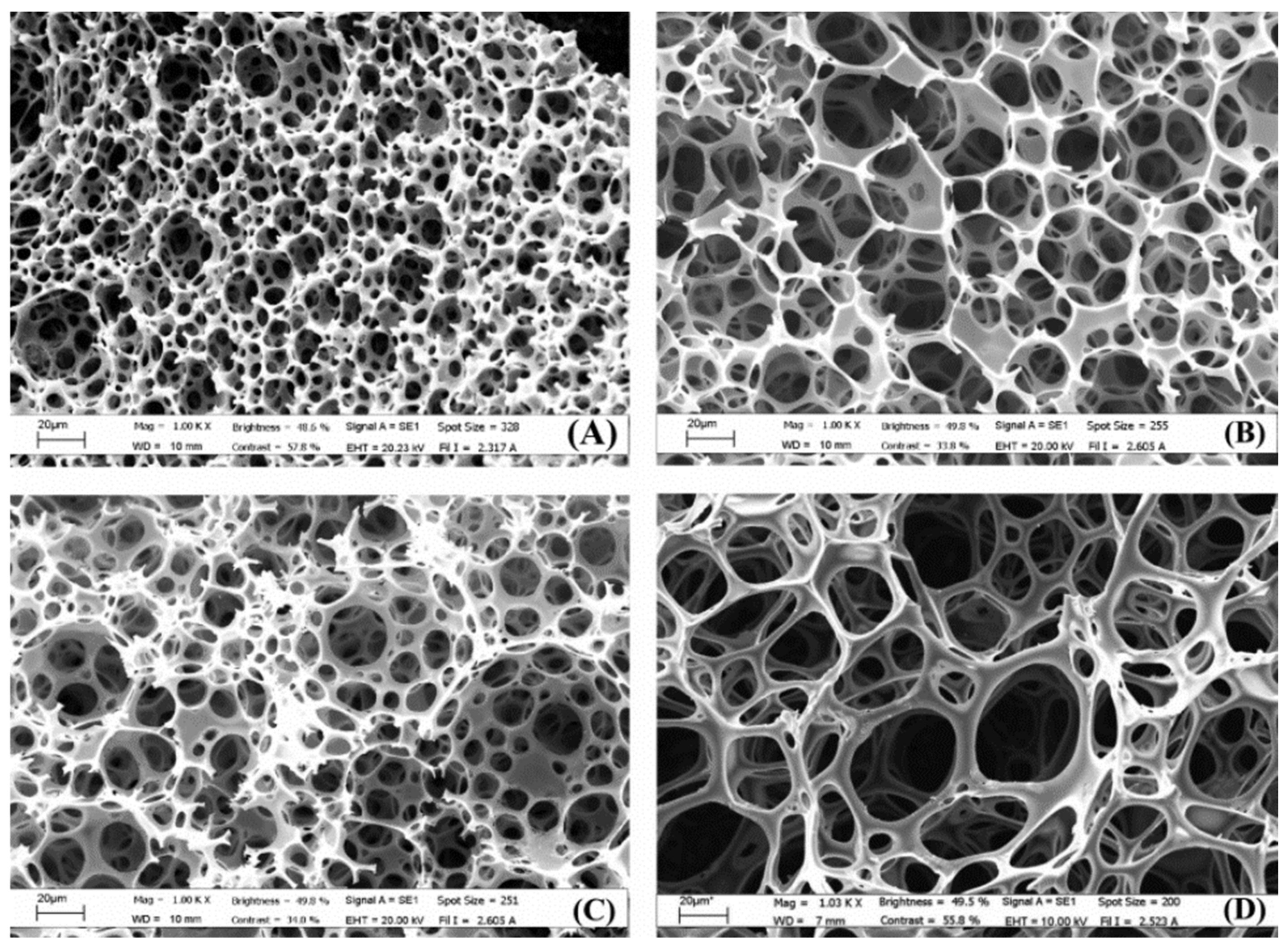
:1. Introduction
2. Cell Culturing and Tissue Generation on PolyHIPEs
2.1. Styrene-Based PolyHIPEs
2.2. Acrylate-Based PolyHIPEs
2.3. Thiol-Ene-Based PolyHIPEs
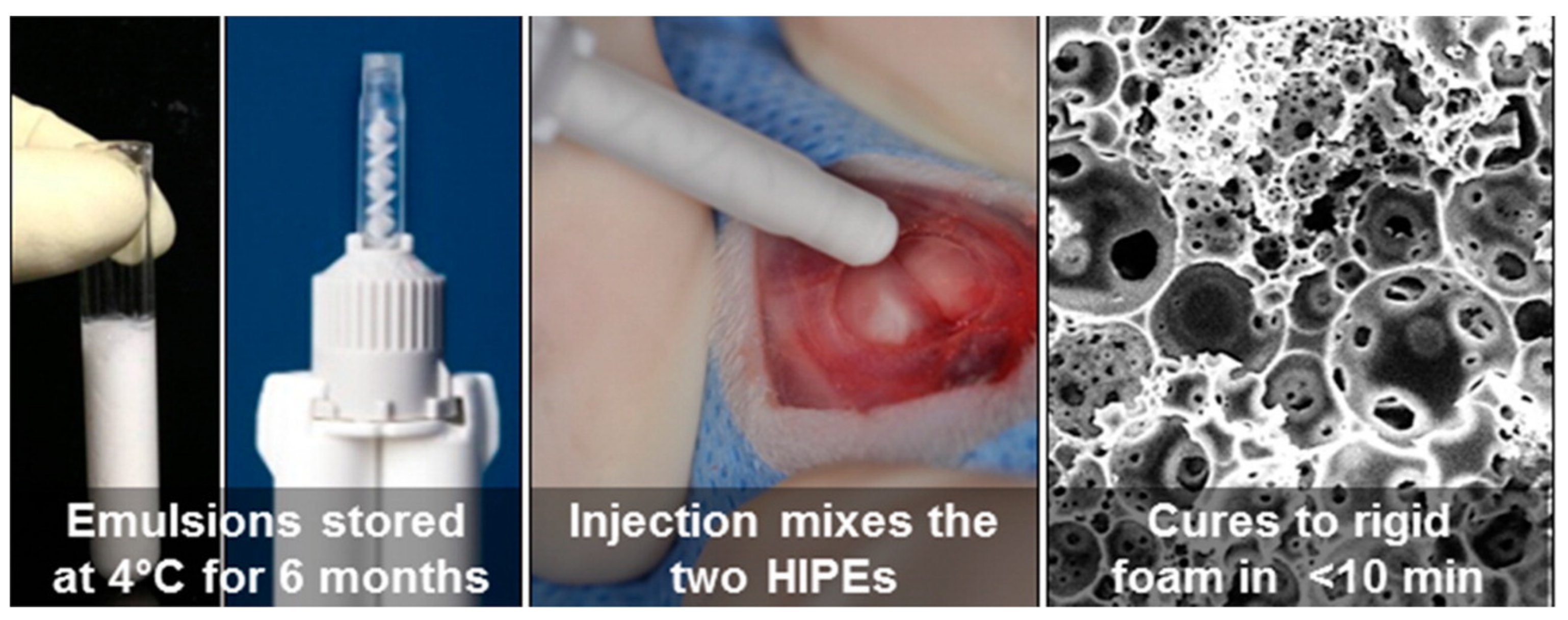
2.4. Polyester-Based PolyHIPEs
2.5. Polysaccharide-Based PolyHIPEs
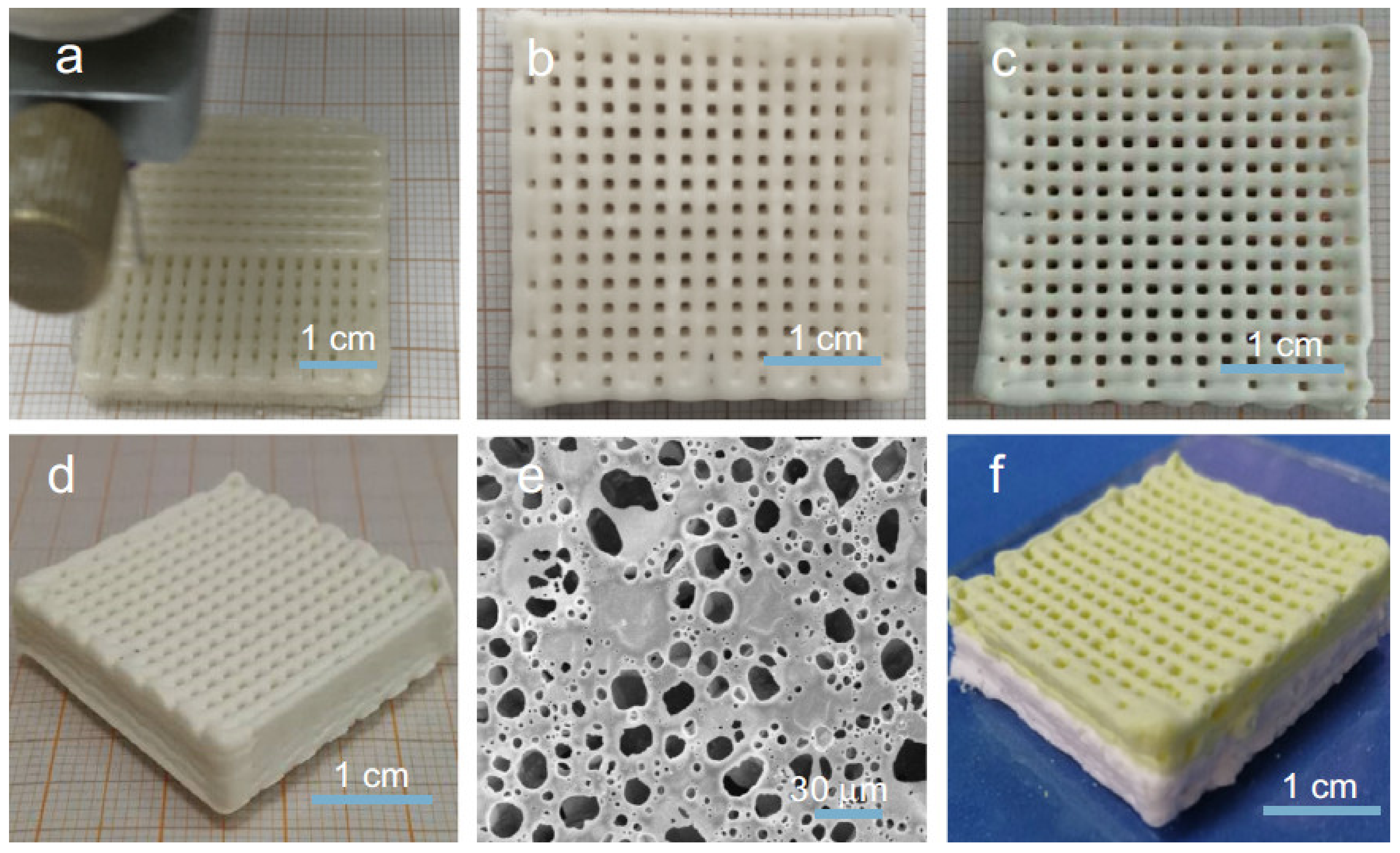
3. Combined and Advanced Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klenk, J.; Keil, U.; Jaensch, A.; Christiansen, M.C.; Nagel, G. Changes in life expectancy 1950–2010: Contributions from age- and disease-specific mortality in selected countries. Popul. Health Metr. 2016, 14, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, S. Does modern medicine increase life-expectancy: Quest for the Moon Rabbit? Indian Heart J. 2016, 68, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, G.C. Living too long. EMBO Rep. 2015, 16, 137–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonelli, M.; Riella, M. Chronic kidney disease and the aging population. Indian J. Nephrol. 2014, 24, 71–74. [Google Scholar] [CrossRef]
- Chiarello, E.; Cadossi, M.; Tedesco, G.; Capra, P.; Calamelli, C.; Shehu, A.; Giannini, S. Autograft, allograft and bone substitutes in reconstructive orthopedic surgery. Aging Clin. Exp. Res. 2013, 25, 101–103. [Google Scholar] [CrossRef]
- Ingulli, E. Mechanism of cellular rejection in transplantation. Pediatr. Nephrol. 2010, 25, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Parveen, S.; Krishnakumar, K.; Sahoo, S. New Era in Health Care: Tissue Engineering. J. Stem Cells Regen. Med. 2006, 1, 8–24. [Google Scholar]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Wu, D.; Xu, F.; Sun, B.; Fu, R.; He, H.; Matyjaszewski, K. Design and Preparation of Porous Polymers. Chem. Rev. 2012, 112, 3959–4015. [Google Scholar] [CrossRef]
- Cameron, N.R. High internal phase emulsion templating as a route to well-defined porous polymers. Polymer 2005, 46, 1439–1449. [Google Scholar] [CrossRef] [Green Version]
- Mert, E.H.; Slugovc, C.; Krajnc, P. Tailoring the mechanical and thermal properties of dicyclopentadiene polyHIPEs with the use of a comonomer. Express Polym. Lett. 2015, 9, 344–353. [Google Scholar] [CrossRef]
- Krajnc, P.; Leber, N.; Štefanec, D.; Kontrec, S.; Podgornik, A. Preparation and characterisation of poly(high internal phase emulsion) methacrylate monoliths and their application as separation media. J. Chromatogr. A 2005, 1065, 69–73. [Google Scholar] [CrossRef]
- Pulko, I.; Krajnc, P. High Internal Phase Emulsion Templating—A Path to Hierarchically Porous Functional Polymers. Macromol. Rapid Commun. 2012, 33, 1731–1746. [Google Scholar] [CrossRef]
- Torquato, S.; Truskett, T.M.; DeBenedetti, P.G. Is Random Close Packing of Spheres Well Defined? Phys. Rev. Lett. 2000, 84, 2064–2067. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Sanguramath, R.A.; Israel, S.; Silverstein, M.S. Emulsion Templating: Porous Polymers and Beyond. Macromolecules 2019, 52, 5445–5479. [Google Scholar] [CrossRef] [Green Version]
- Silverstein, M.S. Emulsion-templated polymers: Contemporary contemplations. Polymer 2017, 126, 261–282. [Google Scholar] [CrossRef]
- Silverstein, M.S. PolyHIPEs: Recent advances in emulsion-templated porous polymers. Prog. Polym. Sci. 2014, 39, 199–234. [Google Scholar] [CrossRef]
- Li, Y.; Gong, C.; Zhang, T.; Feng, X.; Zhou, X.; Li, C. Preparation of PolyHIPE beads and the application in bio-degradation of sulfate containing wastewater. React. Funct. Polym. 2018, 131, 142–149. [Google Scholar] [CrossRef]
- Golub, D.; Krajnc, P. Emulsion templated hydrophilic polymethacrylates. Morphological features, water and dye absorption. React. Funct. Polym. 2020, 149, 104515. [Google Scholar] [CrossRef]
- Wang, R.; Li, W.; Lu, R.; Peng, J.; Liu, X.; Liu, K.; Peng, H. A facile synthesis of cationic and super-hydrophobic polyHIPEs as precursors to carbon foam and adsorbents for removal of non-aqueous-phase dye. Colloids Surf. A 2020, 605, 125334. [Google Scholar] [CrossRef]
- Vásquez, L.; Davis, A.; Gatto, F.; An, M.N.; Drago, F.; Pompa, P.P.; Athanassiou, A.; Fragouli, D. Multifunctional PDMS polyHIPE filters for oil-water separation and antibacterial activity. Sep. Purif. Technol. 2021, 255, 117748. [Google Scholar] [CrossRef]
- Luo, J.; Huang, Z.; Liu, L.; Wang, H.; Ruan, G.; Zhao, C.; Du, F. Recent advances in separation applications of polymerized high internal phase emulsions. J. Sep. Sci. 2021, 44, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Gitli, T.; Silverstein, M.S. Emulsion templated bicontinuous hydrophobic-hydrophilic polymers: Loading and release. Polymer 2011, 52, 107–115. [Google Scholar] [CrossRef]
- Dikici, B.A.; Claeyssens, F. Basic Principles of Emulsion Templating and Its Use as an Emerging Manufacturing Method of Tissue Engineering Scaffolds. Front. Bioeng. Biotechnol. 2020, 8, 875. [Google Scholar] [CrossRef]
- Gurevitch, I.; Silverstein, M.S. Nanoparticle-Based and Organic-Phase-Based AGET ATRP PolyHIPE Synthesis within Pickering HIPEs and Surfactant-Stabilized HIPEs. Macromolecules 2011, 44, 3398–3409. [Google Scholar] [CrossRef]
- Beuermann, S.; Buback, M. Rate coefficients of free-radical polymerization deduced from pulsed laser experiments. Prog. Polym. Sci. 2002, 27, 191–254. [Google Scholar] [CrossRef]
- Benaddi, A.O.; Cohen, O.; Matyjaszewski, K.; Silverstein, M.S. RAFT polymerization within high internal phase emulsions: Porous structures, mechanical behaviors, and uptakes. Polymer 2021, 213, 123327. [Google Scholar] [CrossRef]
- Kovačič, S.; Jeřábek, K.; Krajnc, P.; Slugovc, C. Ring opening metathesispolymerisation of emulsion templated dicyclopentadiene giving open porous materials with excellent mechanical properties. Polym. Chem. 2012, 3, 325–328. [Google Scholar] [CrossRef]
- Sergent, B.; Birot, M.; Deleuze, H. Preparation of thiol—Ene porous polymers by emulsion templating. React. Funct. Polym. 2012, 72, 962–966. [Google Scholar] [CrossRef]
- Mert, H.H.; Mert, M.S.; Mert, E.H. A statistical approach for tailoring the morphological and mechanical properties of polystyrene PolyHIPEs: Looking through experimental design. Mater. Res. Express 2019, 6, 115306. [Google Scholar] [CrossRef]
- Barbetta, A.; Dentini, M.; Zannoni, E.M.; De Stefano, M.E. Tailoring the Porosity and Morphology of Gelatin-Methacrylate PolyHIPE Scaffolds for Tissue Engineering Applications. Langmuir 2005, 21, 12333–12341. [Google Scholar] [CrossRef]
- Huš, S.; Kolar, M.; Krajnc, P. Tailoring morphological features of cross-linked emulsion-templated poly(glycidyl methacrylate). Des. Monomers Polym. 2015, 18, 698–703. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, S.; Hagiwara, K.; Hasebe, T.; Hotta, A. Surface modification of polymers by plasma treatments for the enhancement of biocompatibility and controlled drug release. Surf. Coat. Technol. 2013, 233, 99–107. [Google Scholar] [CrossRef]
- Koler, A.; Krajnc, P. Surface Modification of Hypercrosslinked Vinylbenzyl Chloride PolyHIPEs by Grafting via RAFT. Macromol. Chem. Phys. 2021, 222, 2000381. [Google Scholar] [CrossRef]
- Sevšek, U.; Krajnc, P. Methacrylic acid microcellular highly porous monoliths: Preparation and functionalisation. React. Funct. Polym. 2012, 72, 221–226. [Google Scholar] [CrossRef]
- Bokhari, M.; Carnachan, R.; Cameron, N.R.; Przyborski, S.A. Novel cell culture device enabling three-dimensional cell growth and improved cell function. Biochem. Biophys. Res. Commun. 2007, 354, 1095–1100. [Google Scholar] [CrossRef]
- Bokhari, M.; Carnachan, R.J.; Cameron, N.R.; Przyborski, S.A. Culture of HepG2 liver cells on three dimensional polystyrene scaffolds enhances cell structure and function during toxicological challenge. J. Anat. 2007, 211, 567–576. [Google Scholar] [CrossRef]
- Bokhari, M.; Carnachan, R.J.; Przyborski, S.A.; Cameron, N.R. Emulsion-templated porous polymers as scaffolds for three dimensional cell culture: Effect of synthesis parameters on scaffold formation and homogeneity. J. Mater. Chem. 2007, 17, 4088–4094. [Google Scholar] [CrossRef]
- Sun, P.; Yang, S.; Sun, X.; Wang, Y.; Jia, Y.; Shang, P.; Tian, H.; Li, G.; Li, R.; Zhang, X.; et al. Preparation of PolyHIPE Scaffolds for 3D Cell Culture and the Application in Cytotoxicity Evaluation of Cigarette Smoke. Polymers 2019, 11, 959. [Google Scholar] [CrossRef] [Green Version]
- Pakeyangkoon, P.; Magaraphan, R.; Malakul, P.; Nithitanakul, M. Surface Modification of High Internal Phase Emulsion Foam as a Scaffold for Tissue Engineering Application via Atmospheric Pressure Plasma Treatment. Adv. Sci. Technol. 2012, 77, 172–177. [Google Scholar] [CrossRef]
- Hayward, A.S.; Sano, N.; Przyborski, S.A.; Cameron, N.R. Acrylic-Acid-Functionalized PolyHIPE Scaffolds for Use in 3D Cell Culture. Macromol. Rapid Commun. 2013, 34, 1844–1849. [Google Scholar] [CrossRef]
- Hayward, A.S.; Eissa, A.M.; Maltman, D.J.; Sano, N.; Przyborski, S.A.; Cameron, N.R. Galactose-Functionalized PolyHIPE Scaffolds for Use in Routine Three Dimensional Culture of Mammalian Hepatocytes. Biomacromolecules 2013, 14, 4271–4277. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-Z. Biomimetic Synthesis of Collagen/Nano-Hydroxyapitate Scaffold for Tissue Engineering. J. Bionic Eng. 2008, 5, 1–8. [Google Scholar] [CrossRef]
- Akay, G.; Birch, M.; Bokhari, M. Microcellular polyHIPE polymer supports osteoblast growth and bone formation in vitro. Biomaterials 2004, 25, 3991–4000. [Google Scholar] [CrossRef]
- Preechawong, J.; Noulta, K.; Dubas, S.; Nithitanakul, M.; Sapsrithong, P. Nanolayer Film on Poly(Styrene/Ethylene Glycol Dimethacrylate) High Internal Phase Emulsion Porous Polymer Surface as a Scaffold for Tissue Engineering Application. J. Nanomater. 2019, 2019, 7268192. [Google Scholar] [CrossRef] [Green Version]
- Viswanathan, P.; Chirasatitsin, S.; Ngamkham, K.; Engler, A.J.; Battaglia, G. Cell Instructive Microporous Scaffolds through Interface Engineering. J. Am. Chem. Soc. 2012, 134, 20103–20109. [Google Scholar] [CrossRef] [Green Version]
- Viswanathan, P.; Ondeck, M.G.; Chirasatitsin, S.; Ngamkham, K.; Reilly, G.C.; Engler, A.J.; Battaglia, G. 3D surface topology guides stem cell adhesion and differentiation. Biomaterials 2015, 52, 140–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokhari, M.; Birch, M.; Akay, G. Polyhipe polymer: A novel scaffold for in vitro bone tissue engineering. Adv. Exp. Med. Biol. 2003, 534, 247–254. [Google Scholar] [PubMed]
- Hayman, M.; Smith, K.; Cameron, N.; Przyborski, S. Enhanced neurite outgrowth by human neurons grown on solid three-dimensional scaffolds. Biochem. Biophys. Res. Commun. 2004, 314, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Hayman, M.; Smith, K.; Cameron, N.; Przyborski, S. Growth of human stem cell-derived neurons on solid three-dimensional polymers. J. Biochem. Biophys. Methods 2005, 62, 231–240. [Google Scholar] [CrossRef]
- Busby, W.; Cameron, N.R.; Jahoda, C. Tissue engineering matrixes by emulsion templating. Polym. Int. 2002, 51, 871–881. [Google Scholar] [CrossRef]
- Busby, W.; Cameron, N.R.; Jahoda, C. Emulsion-Derived Foams (PolyHIPEs) Containing Poly(ε-caprolactone) as Matrixes for Tissue Engineering. Biomacromolecules 2001, 2, 154–164. [Google Scholar] [CrossRef]
- Bokhari, M.A.; Akay, G.; Zhang, S.; Birch, M.A. The enhancement of osteoblast growth and differentiation in vitro on a peptide hydrogel-polyHIPE polymer hybrid material. Biomaterials 2005, 26, 5198–5208. [Google Scholar] [CrossRef]
- Lumelsky, Y.; Zoldan, J.; Levenberg, A.S.; Silverstein, M.S. Porous Polycaprolactone-Polystyrene Semi-interpenetrating Polymer Networks Synthesized within High Internal Phase Emulsions. Macromolecules 2008, 41, 1469–1474. [Google Scholar] [CrossRef] [Green Version]
- Whitely, M.; Rodriguez-Rivera, G.; Waldron, C.; Mohiuddin, S.; Cereceres, S.; Sears, N.; Ray, N.; Cosgriff-Hernandez, E. Porous PolyHIPE microspheres for protein delivery from an injectable bone graft. Acta Biomater. 2019, 93, 169–179. [Google Scholar] [CrossRef]
- Moglia, R.S.; Whitely, M.; Dhavalikar, P.; Robinson, J.; Pearce, H.; Brooks, M.; Stuebben, M.; Cordner, N.; Cosgriff-Hernandez, E. Injectable Polymerized High Internal Phase Emulsions with Rapid in Situ Curing. Biomacromolecules 2014, 15, 2870–2878. [Google Scholar] [CrossRef] [Green Version]
- Nalawade, A.C.; Ghorpade, R.V.; Shadbar, S.; Qureshi, M.S.; Chavan, N.N.; Khan, A.A.; Ponrathnam, S. Inverse high internal phase emulsion polymerization (i-HIPE) of GMMA, HEMA and GDMA for the preparation of superporous hydrogels as a tissue engineering scaffold. J. Mater. Chem. B 2016, 4, 450–460. [Google Scholar] [CrossRef]
- Owen, R.; Sherborne, C.; Evans, R.; Reilly, G.C.; Claeyssens, F. Combined Porogen Leaching and Emulsion Templating to Produce Bone Tissue Engineering Scaffolds. Int. J. Bioprint. 2020, 6, 265. [Google Scholar] [CrossRef]
- McGann, C.L.; Streifel, B.C.; Lundin, J.G.; Wynne, J.H. Multifunctional polyHIPE wound dressings for the treatment of severe limb trauma. Polymer 2017, 126, 408–418. [Google Scholar] [CrossRef]
- Owen, R.; Sherborne, C.; Paterson, T.; Green, N.H.; Reilly, G.C.; Claeyssens, F. Emulsion templated scaffolds with tunable mechanical properties for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2016, 54, 159–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paterson, T.E.; Gigliobianco, G.; Sherborne, C.; Green, N.H.; Dugan, J.M.; MacNeil, S.; Reilly, G.C.; Claeyssens, F. Porous microspheres support mesenchymal progenitor cell ingrowth and stimulate angiogenesis. APL Bioeng. 2018, 2, 026103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, J.L.; McEnery, M.A.; Pearce, M.H.; Whitely, M.E.; Munoz-Pinto, D.J.; Hahn, M.S.; Li, H.; Sears, N.A.; Cosgriffhernandez, E.M. Osteoinductive PolyHIPE Foams as Injectable Bone Grafts. Tissue Eng. Part A 2016, 22, 403–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Streifel, B.C.; Lundin, J.G.; Sanders, A.M.; Gold, K.A.; Wilems, T.S.; Williams, S.J.; Cosgriff-Hernandez, E.; Wynne, J.H. Hemostatic and Absorbent PolyHIPE-Kaolin Composites for 3D Printable Wound Dressing Materials. Macromol. Biosci. 2018, 18, e1700414. [Google Scholar] [CrossRef]
- Sherborne, C.; Owen, R.; Reilly, G.C.; Claeyssens, F. Light-based additive manufacturing of PolyHIPEs: Controlling the surface porosity for 3D cell culture applications. Mater. Des. 2018, 156, 494–503. [Google Scholar] [CrossRef]
- Malayeri, A.; Sherborne, C.; Paterson, T.; Mittar, S.; Asencio, I.O.; Hatton, P.; Claeyssens, F. Osteosarcoma growth on trabecular bone mimicking structures manufactured via laser direct write. Int. J. Bioprint. 2016, 2, 67–77. [Google Scholar] [CrossRef]
- Moglia, R.S.; Holm, J.L.; Sears, N.A.; Wilson, C.J.; Harrison, D.M.; Cosgriff-Hernandez, E. Injectable PolyHIPEs as High-Porosity Bone Grafts. Biomacromolecules 2011, 12, 3621–3628. [Google Scholar] [CrossRef] [Green Version]
- Robinson, J.L.; Moglia, R.S.; Stuebben, M.C.; McEnery, M.A.; Cosgriff-Hernandez, E. Achieving Interconnected Pore Architecture in Injectable PolyHIPEs for Bone Tissue Engineering. Tissue Eng. Part A 2014, 20, 1103–1112. [Google Scholar] [CrossRef] [Green Version]
- Corti, M.; Calleri, E.; Perteghella, S.; Ferrara, A.; Tamma, R.; Milanese, C.; Mandracchia, D.; Brusotti, G.; Torre, M.L.; Ribatti, D.; et al. Polyacrylate/polyacrylate-PEG biomaterials obtained by high internal phase emulsions (HIPEs) with tailorable drug release and effective mechanical and biological properties. Mater. Sci. Eng. C 2019, 105, 110060. [Google Scholar] [CrossRef]
- Bahmaee, H.; Owen, R.; Boyle, L.; Perrault, C.M.; Garcia-Granada, A.A.; Reilly, G.C.; Claeyssens, F. Design and Evaluation of an Osteogenesis-on-a-Chip Microfluidic Device Incorporating 3D Cell Culture. Front. Bioeng. Biotechnol. 2020, 8, 557111. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol-Ene Click Chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef]
- Sušec, M.; Liska, R.; Russmüller, G.; Kotek, J.; Krajnc, P. Microcellular Open Porous Monoliths for Cell Growth by Thiol-Ene Polymerization of Low-Toxicity Monomers in High Internal Phase Emulsions. Macromol. Biosci. 2014, 15, 253–261. [Google Scholar] [CrossRef]
- Naranda, J.; Sušec, M.; Maver, U.; Gradišnik, L.; Gorenjak, M.; Vukasović, A.; Ivkovic, A.; Rupnik, M.S.; Vogrin, M.; Krajnc, P. Polyester type polyHIPE scaffolds with an interconnected porous structure for cartilage regeneration. Sci. Rep. 2016, 6, 28695. [Google Scholar] [CrossRef]
- Caldwell, S.; Johnson, D.W.; Didsbury, M.P.; Murray, B.A.; Wu, J.J.; Przyborski, S.A.; Cameron, N.R. Degradable emulsion-templated scaffolds for tissue engineering from thiol-ene photopolymerisation. Soft Matter 2012, 8, 10344–10351. [Google Scholar] [CrossRef] [Green Version]
- Murphy, A.R.; Ghobrial, I.; Jamshidi, P.; Laslett, A.; O’Brien, C.M.; Cameron, N.R. Tailored emulsion-templated porous polymer scaffolds for iPSC-derived human neural precursor cell culture. Polym. Chem. 2017, 8, 6617–6627. [Google Scholar] [CrossRef] [Green Version]
- Richardson, S.A.; Rawlings, T.M.; Muter, J.; Walker, M.; Brosens, J.J.; Cameron, N.R.; Eissa, A.M. Covalent Attachment of Fibronectin onto Emulsion-Templated Porous Polymer Scaffolds Enhances Human Endometrial Stromal Cell Adhesion, Infiltration, and Function. Macromol. Biosci. 2019, 19, e1800351. [Google Scholar] [CrossRef]
- Ratcliffe, J.L.; Walker, M.; Eissa, A.M.; Du, S.; Przyborski, S.A.; Laslett, A.; Cameron, N.R. Optimized peptide functionalization of thiol-acrylate emulsion-templated porous polymers leads to expansion of human pluripotent stem cells in 3D culture. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1974–1981. [Google Scholar] [CrossRef]
- Severn, C.; Eissa, A.; Langford, C.; Parker, A.; Walker, M.; Dobbe, J.; Streekstra, G.; Cameron, N.; Toye, A. Ex vivo culture of adult CD34+ stem cells using functional highly porous polymer scaffolds to establish biomimicry of the bone marrow niche. Biomaterials 2019, 225, 119533. [Google Scholar] [CrossRef]
- Lee, A.; Langford, C.R.; Rodriguez-Lorenzo, L.M.; Thissen, H.; Cameron, N.R. Bioceramic nanocomposite thiol-acrylate polyHIPE scaffolds for enhanced osteoblastic cell culture in 3D. Biomater. Sci. 2017, 5, 2035–2047. [Google Scholar] [CrossRef] [Green Version]
- Eissa, A.M.; Barros, F.S.V.; Vrljicak, P.; Brosens, J.J.; Cameron, N.R. Enhanced Differentiation Potential of Primary Human Endometrial Cells Cultured on 3D Scaffolds. Biomacromolecules 2018, 19, 3343–3350. [Google Scholar] [CrossRef]
- Murphy, A.R.; Haynes, J.M.; Laslett, A.L.; Cameron, N.R.; O’Brien, C.M. Three-dimensional differentiation of human pluripotent stem cell-derived neural precursor cells using tailored porous polymer scaffolds. Acta Biomater. 2020, 101, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.W.; Langford, C.R.; Didsbury, M.P.; Lipp, B.; Przyborski, S.; Cameron, N.R. Fully biodegradable and biocompatible emulsion templated polymer scaffolds by thiol-acrylate polymerization of polycaprolactone macromonomers. Polym. Chem. 2015, 6, 7256–7263. [Google Scholar] [CrossRef] [Green Version]
- Paljevac, M.; Gradišnik, L.; Lipovšek, S.; Maver, U.; Kotek, J.; Krajnc, P. Multiple-Level Porous Polymer Monoliths with Interconnected Cellular Topology Prepared by Combining Hard Sphere and Emulsion Templating for Use in Bone Tissue Engineering. Macromol. Biosci. 2018, 18, 1700306. [Google Scholar] [CrossRef] [PubMed]
- Whitely, M.; Cereceres, S.; Dhavalikar, P.; Salhadar, K.; Wilems, T.; Smith, B.; Mikos, A.; Cosgriff-Hernandez, E. Improved in situ seeding of 3D printed scaffolds using cell-releasing hydrogels. Biomaterials 2018, 185, 194–204. [Google Scholar] [CrossRef]
- Whitely, M.E.; Robinson, J.L.; Stuebben, M.C.; Pearce, H.A.; McEnery, M.A.; Cosgriff-Hernandez, E. Prevention of Oxygen Inhibition of PolyHIPE Radical Polymerization Using a Thiol-Based Cross-Linker. ACS Biomater. Sci. Eng. 2017, 3, 409–419. [Google Scholar] [CrossRef] [Green Version]
- Mansour, H.M.; Sohn, M.; Al-Ghananeem, A.; DeLuca, P.P. Materials for Pharmaceutical Dosage Forms: Molecular Pharmaceutics and Controlled Release Drug Delivery Aspects. Int. J. Mol. Sci. 2010, 11, 3298–3322. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Tan, B.; Luo, W.; Xu, R.; Liu, Y.; Hussain, I. Emulsion-templated poly(acrylamide)s by using polyvinyl alcohol (PVA) stabilized CO2-in-water emulsions and their applications in tissue engineering scaffolds. RSC Adv. 2015, 5, 92017–92024. [Google Scholar] [CrossRef]
- Lumelsky, Y.; Lalush-Michael, I.; Levenberg, S.; Silverstein, M.S. A degradable, porous, emulsion-templated polyacrylate. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 7043–7053. [Google Scholar] [CrossRef]
- Changotade, S.; Bostan, G.R.; Consalus, A.; Poirier, F.; Peltzer, J.; Lataillade, J.-J.; Lutomski, D.; Rohman, G. Preliminary In Vitro Assessment of Stem Cell Compatibility with Cross-Linked Poly(ε-caprolactone urethane) Scaffolds Designed through High Internal Phase Emulsions. Stem Cells Int. 2015, 2015, 283796. [Google Scholar] [CrossRef] [Green Version]
- Nair, P.A.; Ramesh, P. Electrospun biodegradable calcium containing poly(ester-urethane)urea: Synthesis, fabrication, in vitro degradation, and biocompatibility evaluation. J. Biomed. Mater. Res. Part A 2013, 101, 1876–1887. [Google Scholar] [CrossRef]
- Dikici, B.A.; Sherborne, C.; Reilly, G.C.; Claeyssens, F. Emulsion templated scaffolds manufactured from photocurable polycaprolactone. Polymer 2019, 175, 243–254. [Google Scholar] [CrossRef]
- Hu, Y.; Han, W.; Chen, Y.; Zou, R.; Ouyang, Y.; Zhou, W.; Yang, Z.; Wang, C. One-Pot Fabrication of Poly(ε-Caprolactone)-Incorporated Bovine Serum Albumin/Calcium Alginate/Hydroxyapatite Nanocomposite Scaffolds by High Internal Phase Emulsion Templates. Macromol. Mater. Eng. 2017, 302, 1600367. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, H.; Du, Z.; Liu, Y.; Yang, Y.; Wang, C. Pickering high internal phase emulsion-based hydroxyapatite–poly(ε-caprolactone) nanocomposite scaffolds. J. Mater. Chem. B 2015, 3, 3848–3857. [Google Scholar] [CrossRef]
- Hu, Y.; Gu, X.; Yang, Y.; Huang, J.; Hu, M.; Chen, W.; Tong, Z.; Wang, C. Facile Fabrication of Poly(l-lactic Acid)-Grafted Hydroxyapatite/Poly(lactic-co-glycolic Acid) Scaffolds by Pickering High Internal Phase Emulsion Templates. ACS Appl. Mater. Interfaces 2014, 6, 17166–17175. [Google Scholar] [CrossRef]
- Planell, J.A.; Best, S.M.; Lacroix, D.; Merolli, A. Bone Repair Biomaterials; Woodhead Publishing: Cambridge, UK, 2009. [Google Scholar]
- Luo, W.; Zhang, S.; Li, P.; Xu, R.; Zhang, Y.; Liang, L.; Wood, C.D.; Lu, Q.; Tan, B. Surfactant-free CO2-in-water emulsion-templated poly (vinyl alcohol) (PVA) hydrogels. Polymer 2015, 61, 183–191. [Google Scholar] [CrossRef]
- Dikici, B.A.; Dikici, S.; Reilly, G.C.; MacNeil, S.; Claeyssens, F. A Novel Bilayer Polycaprolactone Membrane for Guided Bone Regeneration: Combining Electrospinning and Emulsion Templating. Materials 2019, 12, 2643. [Google Scholar] [CrossRef] [Green Version]
- Dikici, S.; Dikici, B.A.; Bhaloo, S.I.; Balcells, M.; Edelman, E.; MacNeil, S.; Reilly, G.C.; Sherborne, C.; Claeyssens, F. Assessment of the Angiogenic Potential of 2-Deoxy-D-Ribose Using a Novel in vitro 3D Dynamic Model in Comparison with Established in vitro Assays. Front. Bioeng. Biotechnol. 2020, 7, 2643. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, J.; Li, X.; Hu, X.; Zhou, W.; Dong, X.; Wang, C.; Yang, Z.; Binks, B.P. Facile preparation of bioactive nanoparticle/poly(ε-caprolactone) hierarchical porous scaffolds via 3D printing of high internal phase Pickering emulsions. J. Colloid Interface Sci. 2019, 545, 104–115. [Google Scholar] [CrossRef]
- Dikici, B.A.; Reilly, G.C.; Claeyssens, F. Boosting the Osteogenic and Angiogenic Performance of Multiscale Porous Polycaprolactone Scaffolds by In Vitro Generated Extracellular Matrix Decoration. ACS Appl. Mater. Interfaces 2020, 12, 12510–12524. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Hu, Y.; Wang, C.; Binks, B.P. Fabrication of Hierarchical Macroporous Biocompatible Scaffolds by Combining Pickering High Internal Phase Emulsion Templates with Three-Dimensional Printing. ACS Appl. Mater. Interfaces 2017, 9, 22950–22958. [Google Scholar] [CrossRef]
- Ng, J.Y.; Obuobi, S.; Chua, M.L.; Zhang, C.; Hong, S.; Kumar, Y.; Gokhale, R.; Ee, P.L.R. Biomimicry of microbial polysaccharide hydrogels for tissue engineering and regenerative medicine—A review. Carbohydr. Polym. 2020, 241, 116345. [Google Scholar] [CrossRef]
- Diekjürgen, D.; Grainger, D.W. Polysaccharide matrices used in 3D in vitro cell culture systems. Biomaterials 2017, 141, 96–115. [Google Scholar] [CrossRef]
- Barbetta, A.; Massimi, M.; Di Rosario, B.; Nardecchia, S.; De Colli, M.; Devirgiliis, L.C.; Dentini, M. Emulsion Templated Scaffolds that Include Gelatin and Glycosaminoglycans. Biomacromolecules 2008, 9, 2844–2856. [Google Scholar] [CrossRef]
- Barbetta, A.; Dentini, M.; De Vecchis, M.S.; Filippini, P.; Formisano, G.; Caiazza, S. Scaffolds Based on Biopolymeric Foams. Adv. Funct. Mater. 2005, 15, 118–124. [Google Scholar] [CrossRef]
- Zhou, S.; Bismarck, A.; Steinke, J.H.G. Ion-responsive alginate based macroporous injectable hydrogel scaffolds prepared by emulsion templating. J. Mater. Chem. B 2013, 1, 4736–4745. [Google Scholar] [CrossRef]
- Tan, H.; Wei, J.; Sun, G.; Mu, C.; Lin, W.; Ngai, T. Interconnected macroporous 3D scaffolds templated from gelatin nanoparticle-stabilized high internal phase emulsions for biomedical applications. Soft Matter 2017, 13, 3871–3878. [Google Scholar] [CrossRef]
- Tan, H.; Tu, Z.; Jia, H.; Gou, X.; Ngai, T. Hierarchical Porous Protein Scaffold Templated from High Internal Phase Emulsion Costabilized by Gelatin and Gelatin Nanoparticles. Langmuir 2018, 34, 4820–4829. [Google Scholar] [CrossRef]
- Liu, S.; Jin, M.; Chen, Y.; Gao, H.; Shi, X.; Cheng, W.; Ren, L.; Wang, Y. High internal phase emulsions stabilised by supramolecular cellulose nanocrystals and their application as cell-adhesive macroporous hydrogel monoliths. J. Mater. Chem. B 2017, 5, 2671–2678. [Google Scholar] [CrossRef]
- De Colli, M.; Massimi, M.; Barbetta, A.; Di Rosario, B.L.; Nardecchia, S.; Devirgiliis, L.C.; Dentini, M. A biomimetic porous hydrogel of gelatin and glycosaminoglycans cross-linked with transglutaminase and its application in the culture of hepatocytes. Biomed. Mater. 2012, 7, 055005. [Google Scholar] [CrossRef]
- Barbetta, A.; Massimi, M.; Devirgiliis, L.C.; Dentini, M. Enzymatic Cross-Linking versus Radical Polymerization in the Preparation of Gelatin PolyHIPEs and Their Performance as Scaffolds in the Culture of Hepatocytes. Biomacromolecules 2006, 7, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.H.L.; Bismarck, A.; Chan-Park, M.B. Injectable, Interconnected, High-Porosity Macroporous Biocompatible Gelatin Scaffolds Made by Surfactant-Free Emulsion Templating. Macromol. Rapid Commun. 2015, 36, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Teng, W.; Long, T.J.; Zhang, Q.; Yao, K.; Shen, T.T.; Ratner, B.D. A tough, precision-porous hydrogel scaffold: Ophthalmologic applications. Biomaterials 2014, 35, 8916–8926. [Google Scholar] [CrossRef] [PubMed]

| Monomers | Porosity (%) | Pore Size (μm) | Cells | Reference |
|---|---|---|---|---|
| STY; DVB; EHA | 90 | N/A | HepG2 | [37] |
| STY; DVB | 95 | N/A | HepG2 | [38] |
| STY; DVB; EHA | 90 | ≈90 | MG63 | [39] |
| STY; DVB | 95 * | ≈12 | A549 | [40] |
| STY; EGDMA | 90 | N/A | L929 | [41] |
| STY; DVB; EHA | 89–92 * | ≈19; ≈60 | Hepatocytes | [42] |
| STY; DVB; EHA; PFPA | 90 | ≈69 | HepG2 | [43] |
| STY; DVB | 95 | ≈100; ≈60; ≈40 | Primary rat osteoblasts | [45] |
| STY; EGDMA | 90 | 30–40 | L929 | [46] |
| PS-PAA; PS-PEO | 80 | 40–120 | hES-MP | [47] |
| PS-PAA; PBD-PEO | 90 | 40–80 | hES-MP | [48] |
| STY | 95 | ≈100; ≈40 | Rat osteoblasts | [49] |
| STY; DVB | 90 | 5–20 | TERA2.cl.SP12 | [50] |
| STY; DVB | 90 | 5–20 | TERA2.cl.SP12 | [51] |
| STY; PCL/PLA | 90 | N/A | Several | [52] |
| STY; PCL; MMA | 75–90 | 5–100 | fibroblasts | [53] |
| STY; DVB | 95 | ≈100 | Primary rat osteoblasts | [54] |
| STY; PCL | 88 | 18–30 | Mouse skeletal cells (C2) | [55] |
| Monomers | Porosity (%) | Pore Size (μm) | Cells | Reference |
|---|---|---|---|---|
| EGDMA | 75 | ≈12, ≈14, ≈29 | hMSC | [56] |
| PFDMA; EGDMA; BDMA | 75 | ≈5 | hMSC | [57] |
| GMMA; GDMA; HEMA | 92–97 * | 20–30 | NIH/3T3 | [58] |
| EHA; TMPTA | 90 | ≈780; 10–50 | MLO-A5 | [59] |
| PEGDA; Na/Ca Acrylate; PNIPAM | N/A | 32–44 | HeLa cells | [60] |
| EHA; TMPTA; IBOA | 75–90 | 20–30 | hES-MP | [61] |
| EHA; IBOA; TMPTA | 80 | ≈25 | hES-MP | [62] |
| PFDMA; BDMA | 75 | 7–9 | hMSC | [63] |
| PEGMA; PEGDA; Na Acrylate | 83 | ≈3 | HDFs | [64] |
| IBOA; TMPTA | 80 | ≈40 | MLO-A5 | [65] |
| EHA; IBOA; TMPTA | 80 | ≈33 | MG63 | [66] |
| PFDMA | 75 | 4–29 | NIH/3T3 | [67] |
| PFDMA | 75 | ≈12 | hMSC | [68] |
| GMA; BA; TMPTA; HDDA; PEGMA | 80 | N/A | Human fibroblasts | [69] |
| EHA; IBOA; TMPTA | 80 | ≈16 | hES-MP | [70] |
| Monomers | Porosity (%) | Pore Size (μm) | Cells | Reference |
|---|---|---|---|---|
| PETMP; DVA | 85 | ≈13 | MC3T3-E1 | [72] |
| PETMP; DVA | 85 | ≈82 | Chondrocyte | [73] |
| TMPTMP; TMPTA; DPEHA | 90 | ≈108 | HaCaTs | [74] |
| TMPTMP; TMPTA; HDDA; PEGDA | 80/85 | ≈30; ≈44; ≈45; ≈63 | iPSC-hNPCs | [75] |
| TMPTMP; TMPTA | 80 | 20–30 | HESCs | [76] |
| TMPTMP; TMPTA | 80 | ≈38 | WA09 (H9) | [77] |
| TMPTMP; DPEHA | 90 | ≈37 | (CD34+) HSPC | [78] |
| TMPTMP; DPEHA | 90 | ≈58, ≈57, ≈99 | MG63 | [79] |
| TMPTMP; DPEHA | 80 | ≈25 | HEECs, HESCs | [80] |
| TMPTMP; PEGDA; TMPTA | 80 | N/A | hPSC-NPCs | [81] |
| TMPTMP; PCL; TMPTA; DPEHA | 90/95 | ≈60 | L929 | [82] |
| PETMP; DVA | 90 | ≈70 | Osteoblasts | [83] |
| DTT; PEGDA | 75 | N/A | hMSCs | [84] |
| PETMP; PFDMA | 75 | ≈6 | hMSCs | [85] |
| Monomers | Porosity (%) | Pore Size (μm) | Cells | Reference |
|---|---|---|---|---|
| AAm | ≈90 * | ≈10; ≈17; ≈19 | H9c2 | [87] |
| PCL; tBA; EHA | 88 | 1000–3000 | Mouse skeletal cells (C2) | [88] |
| PCL; HMDI | 75 | 150–1800 | hMSCs | [89] |
| PCL | 82 | ≈15; ≈20; ≈69 | HDFs | [91] |
| PCL | 81–91 * | Tens of micrometres | mBMSCs | [92] |
| PCL | 90 * | Few μm to a few 100 μm | mBMSCs | [93] |
| PLLA; PLGA | 86 * | N/A | mBMSCs | [94] |
| PVA; Glutaraldehyde | 80 | ≈10; ≈20 | Fibroblasts/H9c2 | [96] |
| PCL | 85 | ≈34 | MLO-A5 | [97] |
| PCL | 80 | ≈30 | HAECs | [98] |
| PCL; PLLA | 96* | N/A | mBMSCs | [99] |
| PCL | 89 | ≈8 | MLO-A5 | [100] |
| PCL; PLLA | 75 | 10–30 | mBMSCs | [101] |
| Monomers | Porosity (%) | Pore Size (μm) | Cells | Reference |
|---|---|---|---|---|
| Gelatine; HA; CS | 90 | ≈28 | C3A/HepG2 | [104] |
| Dextran; Pullulan | 90/95 | ≈20 | Mouse neural cells | [105] |
| Alginate | N/A | 7–12 | A549 | [106] |
| Gelatine; AAm; MBAA | 80 | ≈104 | HepG2 | [107] |
| Gelatin | 80 | ≈25 | L929 | [108] |
| Gelatine; AAm; MBAA | 80 | 30–62 | mBMSCs | [109] |
| Gelatine; HA; CS | 90 | 10–20; 10–50 | C3A | [110] |
| Gelatine | 90/92 | ≈60; ≈84 | Rat hepatocytes/HepG2 | [111] |
| Gelatine; PNIPAM | 95/96 | ≈70; ≈80 | Foreskin fibroblasts | [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kramer, S.; Cameron, N.R.; Krajnc, P. Porous Polymers from High Internal Phase Emulsions as Scaffolds for Biological Applications. Polymers 2021, 13, 1786. https://doi.org/10.3390/polym13111786
Kramer S, Cameron NR, Krajnc P. Porous Polymers from High Internal Phase Emulsions as Scaffolds for Biological Applications. Polymers. 2021; 13(11):1786. https://doi.org/10.3390/polym13111786
Chicago/Turabian StyleKramer, Stanko, Neil R. Cameron, and Peter Krajnc. 2021. "Porous Polymers from High Internal Phase Emulsions as Scaffolds for Biological Applications" Polymers 13, no. 11: 1786. https://doi.org/10.3390/polym13111786
APA StyleKramer, S., Cameron, N. R., & Krajnc, P. (2021). Porous Polymers from High Internal Phase Emulsions as Scaffolds for Biological Applications. Polymers, 13(11), 1786. https://doi.org/10.3390/polym13111786







