Enhanced Cellular Uptake in an Electrostatically Interacting Fucoidan–L-Arginine Fiber Complex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Fucoidan-L-Arginine (Fuc-L-Arg) Fiber Complex
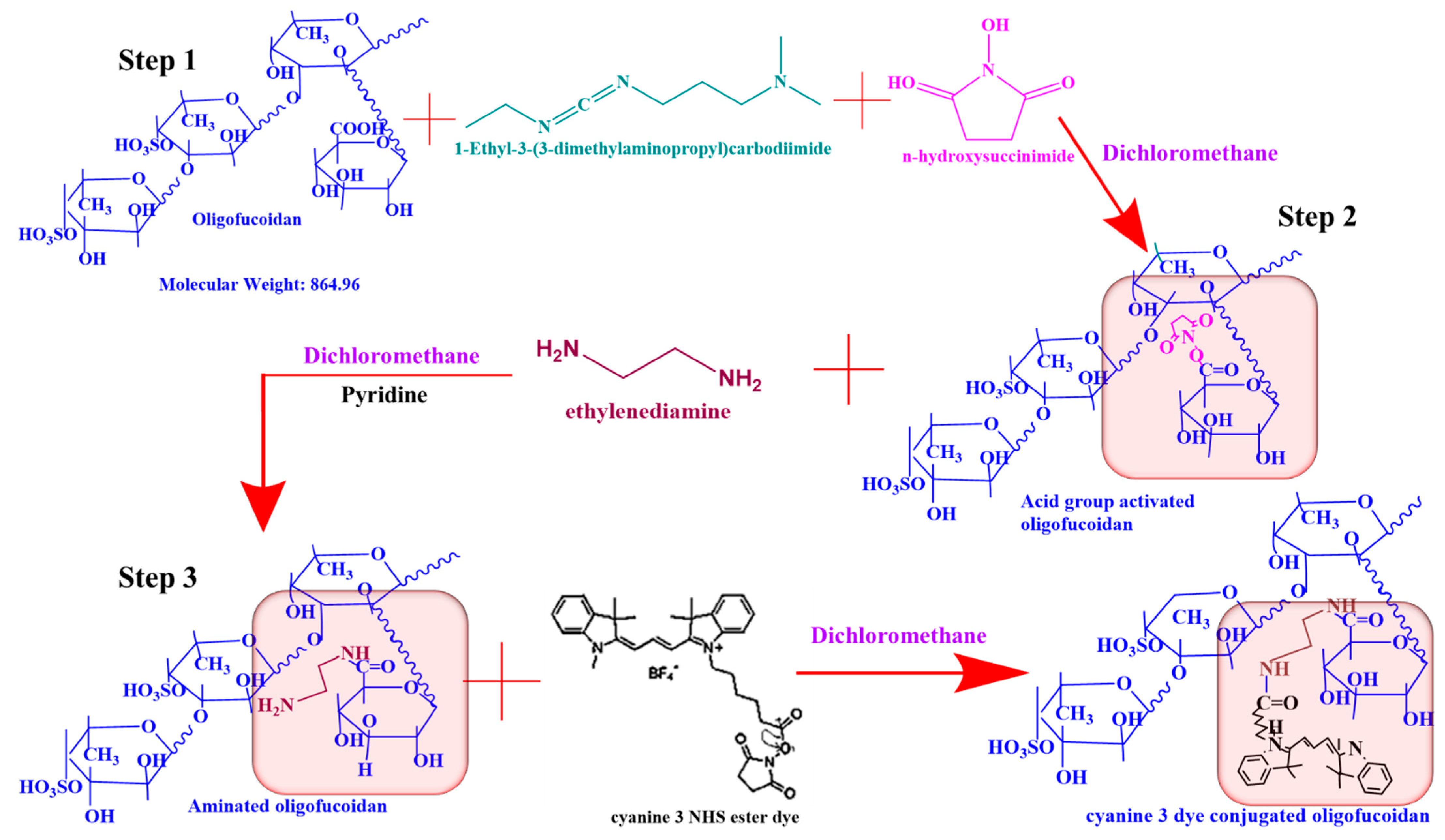
2.3. Conjugation of Oligo-Fucoidan to Cyanine 3 NHS Ester Dye (Fuc-Cy3) as a Biomarker to Track Cellular Uptake
2.4. Characterization of the Fucoidan-L-Arginine Fiber Complex and Fucoidan-Cyanine 3 NHS Ester Dye
2.5. Cell Viability of the Fucoidan-L-Arginine Fiber Complex
2.6. In Vitro Qualitative Cellular Uptake Analysis of the Fuc-L-Arg Fiber Complex
2.7. Quantitative Analysis of the Fuc-L-Arg Fiber Complex (Flow Cytometry)
3. Results and Discussion
3.1. Confirmation of Fuc-L-Arg Fiber Complex Interactions Using Spectroscopy
3.2. Morphology of the Fuc-L-Arg Fiber Complex and Its Impact on Cellular Uptake
3.3. Confirmation of Fucoidan Conjugation to Cy3 Dye Using Fluorescent Spectroscopy Techniques
3.4. UV Spectral Confirmation of the Fuc-Cy3-L-Arg Fiber Complex
3.5. Biocompatibility Test of the Fuc-L-Arg Fiber Complex in HeLa Cells
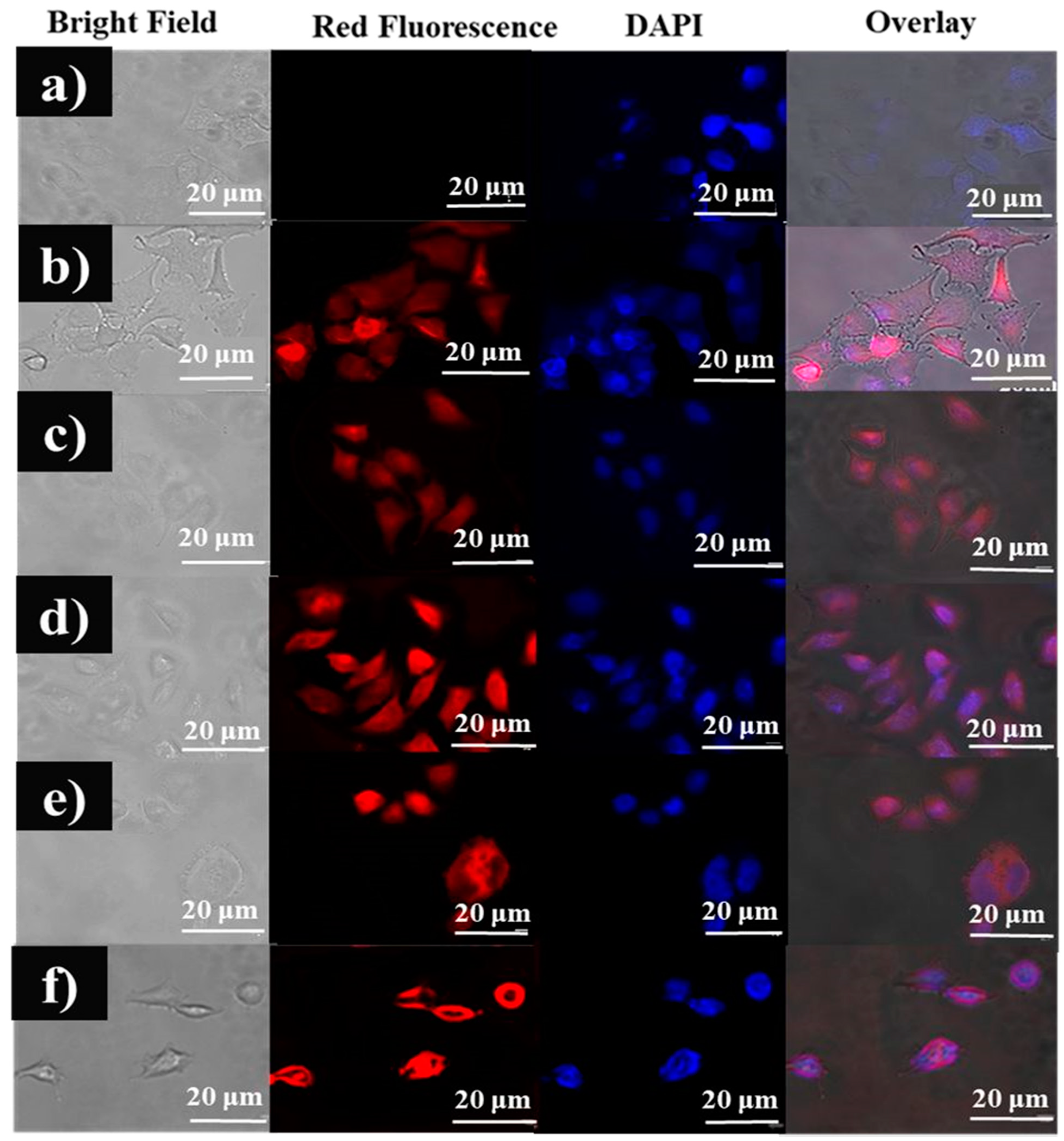
3.6. Qualitative Analysis of the Cellular Uptake of Fuc-Cy3 and the Fuc-Cy3-L-Arg Fiber Complex in HeLa Cells
3.7. Quantitative Flow Cytometry of the Fuc-Cy3 Dye and Fuc-L-Arg Fiber Complex in HeLa Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pacheco, D.; García-Poza, S.; Cotas, J.; Gonçalves, A.M.; Pereira, L. Fucoidan—A valuable source from the ocean to pharmaceutical. Front. Drug Chem. Clin. Res. 2020, 3, 1–4. [Google Scholar]
- Jafari, M.; Sriram, V.; Xu, Z.; Harris, G.M.; Lee, J.-Y. Fucoidan-Doxorubicin Nanoparticles Targeting P-Selectin for Effective Breast Cancer Therapy. Carbohydr. Polym. 2020, 249, 116837. [Google Scholar] [CrossRef]
- Jang, B.; Moorthy, M.S.; Manivasagan, P.; Xu, L.; Song, K.; Lee, K.D.; Kwak, M.; Oh, J.; Jin, J.-O. Fucoidan-coated CuS nanoparticles for chemo-and photothermal therapy against cancer. Oncotarget 2018, 9, 12649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, H.Y.; Hwang, P.A. Clinical applications of fucoidan in translational medicine for adjuvant cancer therapy. Clin. Transl. Med. 2019, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Pedersen, L.C. Anticoagulant heparan sulfate: Structural specificity and biosynthesis. Appl. Microbiol. Biotechnol. 2007, 74, 263–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wali, A.F.; Majid, S.; Rasool, S.; Shehada, S.B.; Abdulkareem, S.K.; Firdous, A.; Beigh, S.; Shakeel, S.; Mushtaq, S.; Akbar, I.; et al. Natural products against cancer: Review on phytochemicals from marine sources in preventing cancer. Saudi Pharm. J. Spj. Off. Publ. Saudi Pharm. Soc. 2019, 27, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Raposo, M.F.; de Morais, R.M.; Bernardo de Morais, A.M. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef] [Green Version]
- Tsai, H.L.; Tai, C.J.; Huang, C.W.; Chang, F.R.; Wang, J.Y. Efficacy of Low-Molecular-Weight Fucoidan as a Supplemental Therapy in Metastatic Colorectal Cancer Patients: A Double-Blind Randomized Controlled Trial. Mar. Drugs 2017, 15, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Zhang, Y.; Zhang, W.; Chen, J.; Yang, X.; Ma, P.; Zhang, B.; Liu, B.; Ni, J.; Wang, R. Cell penetrating peptide TAT can kill cancer cells via membrane disruption after attachment of camptothecin. Peptides 2015, 63, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Tokita, Y.; Hirayama, M.; Nakajima, K.; Tamaki, K.; Iha, M.; Nagamine, T. Detection of fucoidan in urine after oral intake of traditional japanese seaweed, Okinawa mozuku (Cladosiphon okamuranus Tokida). J. Nutr. Sci. Vitaminol. 2017, 63, 419–421. [Google Scholar] [CrossRef] [Green Version]
- Gough, C.R.; Rivera-Galletti, A.; Cowan, D.A.; Salas-de la Cruz, D.; Hu, X. Protein and Polysaccharide-Based Fiber Materials Generated from Ionic Liquids: A Review. Molecules 2020, 25, 3362. [Google Scholar] [CrossRef] [PubMed]
- Hiebert, L.; Wice, S.; Mao, W.; Zhang, F.; Daniels, B.; Linhardt, R. In vivo antithrombotic synergy of oral heparin and arginine: Endothelial thromboresistance without changes in coagulation parameters. Thromb. Haemost. 2017, 95, 865–872. [Google Scholar]
- Mogaki, R.; Hashim, P.K.; Okuro, K.; Aida, T. Guanidinium-based “molecular glues” for modulation of biomolecular functions. Chem. Soc. Rev. 2017, 46, 6480–6491. [Google Scholar] [CrossRef]
- Schmitt, C.; Sanchez, C.; Desobry-Banon, S.; Hardy, J. Structure and technofunctional properties of protein-polysaccharide complexes: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 689–753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Qin, X.; Kong, F.; Chen, P.; Pan, G. Improving cellular uptake of therapeutic entities through interaction with components of cell membrane. Drug Deliv. 2019, 26, 328–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azizi, K.; Karimi, M.; Shaterian, H.R.; Heydari, A. Ultrasound irradiation for the green synthesis of chromenes using L-arginine-functionalized magnetic nanoparticles as a recyclable organocatalyst. Rsc. Adv. 2014, 4, 42220–42225. [Google Scholar] [CrossRef]
- Oba, M.; Nagano, Y.; Kato, T.; Tanaka, M. Secondary structures and cell-penetrating abilities of arginine-rich peptide foldamers. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Futaki, S.; Nakase, I. Cell-surface interactions on arginine-rich cell-penetrating peptides allow for multiplex modes of internalization. Acc. Chem. Res. 2017, 50, 2449–2456. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Coutinho, A.J.; Costa Lima, S.A.; Reis, S. Marine Polysaccharides in Pharmaceutical Applications: Fucoidan and Chitosan as Key Players in the Drug Delivery Match Field. Mar. Drugs 2019, 17, 654. [Google Scholar] [CrossRef] [Green Version]
- Laurienzo, P. Marine polysaccharides in pharmaceutical applications: An overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, A.I.; Costa Lima, S.A.; Reis, S. Application of pH-responsive fucoidan/chitosan nanoparticles to improve oral quercetin delivery. Molecules 2019, 24, 346. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.I.; Kim, H.J. Preparation of low molecular weight fucoidan by gamma-irradiation and its anticancer activity. Carbohydr. Polym. 2013, 97, 358–362. [Google Scholar] [CrossRef]
- Deepika, M.S.; Thangam, R.; Sheena, T.S.; Sasirekha, R.; Sivasubramanian, S.; Babu, M.D.; Jeganathan, K.; Thirumurugan, R. A novel rutin-fucoidan complex based phytotherapy for cervical cancer through achieving enhanced bioavailability and cancer cell apoptosis. Biomed. Pharm. 2019, 109, 1181–1195. [Google Scholar] [CrossRef]
- Fang, H.-Y.; Huang, W.-M.; Chen, D.-H. One-step synthesis of positively charged bifunctional carbon dot/silver composite nanoparticles for killing and fluorescence imaging of Gram-negative bacteria. Nanotechnology 2019, 30, 365603. [Google Scholar] [CrossRef]
- Jobin, M.L.; Alves, I.D. On the importance of electrostatic interactions between cell penetrating peptides and membranes: A pathway toward tumor cell selectivity? Biochimie 2014, 107, 154–159. [Google Scholar] [CrossRef]
- McRae, M.P. Therapeutic Benefits of l-Arginine: An Umbrella Review of Meta-analyses. J. Chiropr. Med. 2016, 15, 184–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niyonizigiye, I.; Ngabire, D.; Patil, M.P.; Singh, A.A.; Kim, G.D. In vitro induction of endoplasmic reticulum stress in human cervical adenocarcinoma HeLa cells by fucoidan. Int. J. Biol. Macromol. 2019, 137, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Philip, R.; Campbell, E.; Wheatley, D.N. Arginine deprivation, growth inhibition and tumour cell death: 2. Enzymatic degradation of arginine in normal and malignant cell cultures. Br. J. Cancer 2003, 88, 613–623. [Google Scholar] [CrossRef]
- Talwatkar, S.; Sunatkari, A.; Tamgadge, Y.; Pahurkar, V.; Muley, G. Surface passivation by l-arginine and enhanced optical properties of CdS quantum dots co-doped with Nd 3+–Li+. J. Nanostructure Chem. 2015, 5, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Udayan, A.; Arumugam, M.; Pandey, A. Nutraceuticals From Algae and Cyanobacteria. In Algal Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 65–89. [Google Scholar]
- Wang, L.; Wang, X.; Wu, H.; Liu, R. Overview on biological activities and molecular characteristics of sulfated polysaccharides from marine green algae in recent years. Mar. Drugs 2014, 12, 4984–5020. [Google Scholar] [CrossRef]
- Wang, P.; Kankala, R.K.; Chen, B.; Long, R.; Cai, D.; Liu, Y.; Wang, S. Poly-allylamine hydrochloride and fucoidan-based self-assembled polyelectrolyte complex nanoparticles for cancer therapeutics. J. Biomed. Mater. Res. Part A 2019, 107, 339–347. [Google Scholar] [CrossRef]
- Wu, S.Y.; Parasuraman, V.; Hsieh Chih, T.; Arunagiri, V.; Gunaseelan, S.; Chou, H.Y.; Anbazhagan, R.; Lai, J.Y.; Prasad, N.R. Radioprotective effect of self-assembled low molecular weight Fucoidan-Chitosan nanoparticles. Int. J. Pharm. 2020, 579, 119161. [Google Scholar] [CrossRef] [PubMed]
- Motlekar, N.A.; Srivenugopal, K.S.; Wachtel, M.S.; Youan, B.B. Modulation of gastrointestinal permeability of low-molecular-weight heparin by L-arginine: In-vivo and in-vitro evaluation. J. Pharm. Pharmacol. 2006, 58, 591–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, D.B.; Villasenor, R.; Dorr, B.M.; Zerial, M.; Liu, D.R. Cellular uptake mechanisms and endosomal trafficking of supercharged proteins. Chem. Biol. 2012, 19, 831–843. [Google Scholar] [CrossRef] [Green Version]
- Vadivelmurugan, A.; Anbazhagan, R.; Arunagiri, V.; Lai, J.-Y.; Tsai, H.-C. Pluronic F127 self-assembled MoS 2 nanocomposites as an effective glutathione responsive anticancer drug delivery system. Rsc. Adv. 2019, 9, 25592–25601. [Google Scholar] [CrossRef] [Green Version]
- Frohlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [Green Version]
- Hanurry, E.Y.; Hsu, W.H.; Darge, H.F.; Birhan, Y.S.; Mekonnen, T.W.; Andrgie, A.T.; Chou, H.Y.; Cheng, C.C.; Lai, J.Y.; Tsai, H.C. In vitro siRNA delivery via diethylenetriamine- and tetraethylenepentamine-modified carboxyl group-terminated Poly(amido)amine generation 4.5 dendrimers. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110245. [Google Scholar] [CrossRef]
- Lemarchand, C.; Gref, R.; Couvreur, P. Polysaccharide-decorated nanoparticles. Eur. J. Pharm. Biopharm. 2004, 58, 327–341. [Google Scholar] [CrossRef]
- Salatin, S.; Maleki Dizaj, S.; Yari Khosroushahi, A. Effect of the surface modification, size, and shape on cellular uptake of nanoparticles. Cell. Biol. Int. 2015, 39, 881–890. [Google Scholar] [CrossRef]
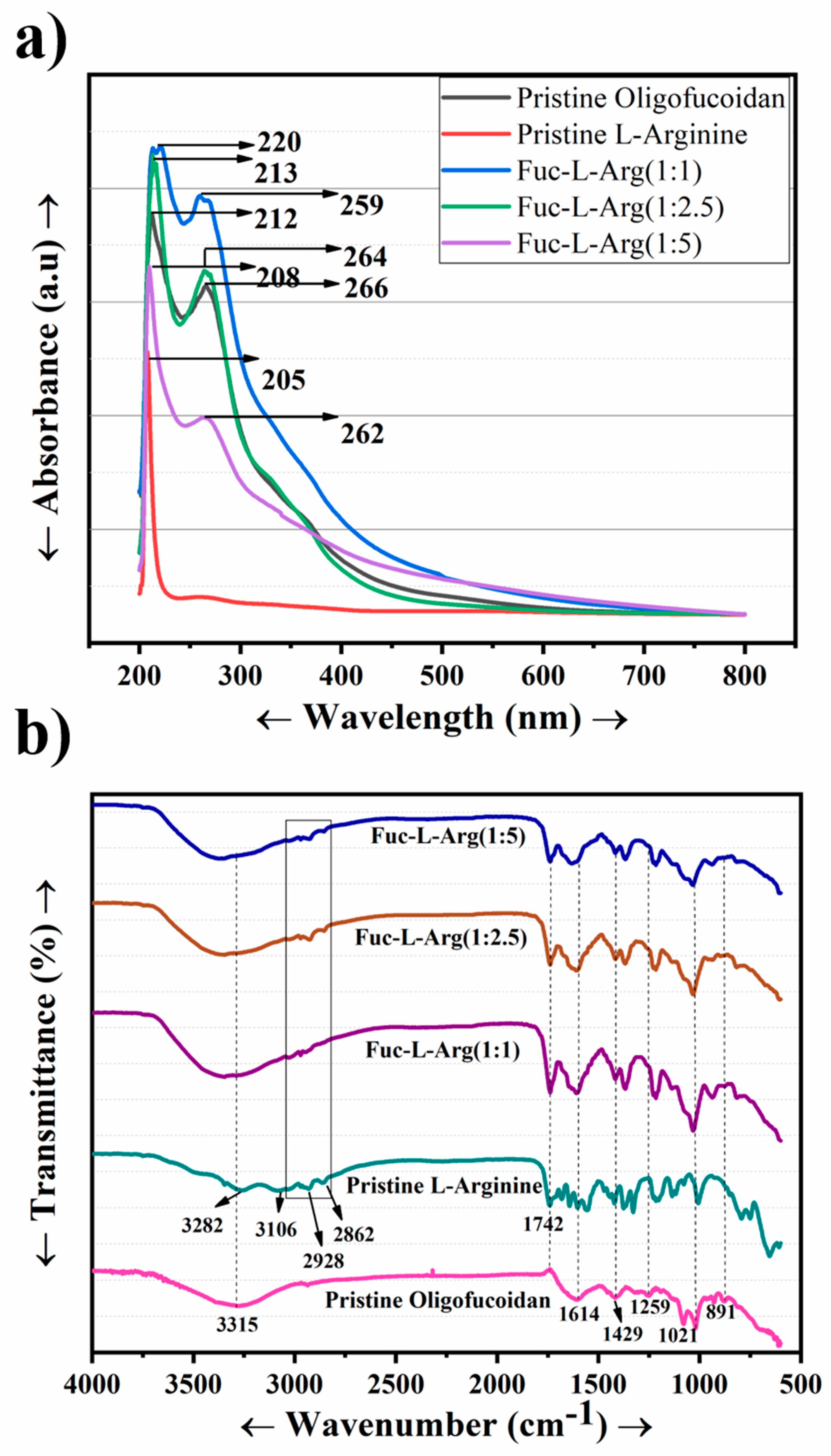






| Compound | UV Absorbance Peaks | FT-IR Characteristic Peaks |
|---|---|---|
| Fucoidan | 212 nm and 266 nm | 3315 (OH) |
| 1614 (C=O) | ||
| 1429 and 891 (C−O−S) | ||
| 1160–1259 (S=O) and 1021 (Saccharide group) | ||
| L-arginine | 205 nm | 3282 and 3106 (NH and OH) |
| 2928 and 2862 (CH) | ||
| 1742 (COO−) | ||
| Fuc-L-Arg fiber complex | 220 nm and 266 nm (1:1) | 3325 (OH) |
| 213 nm and 264 nm (1:2.5) | 2928 and 2862 (CH) | |
| 208 nm and 262 nm (1:5) | 1742 (COO−) |
| S. No. | Oligo-Fucoidan | L-Arginine | Fuc-L-Arg (1:1) | Fuc-L-Arg (1:2.5) | Fuc-L-Arg (1:5) |
|---|---|---|---|---|---|
| Without Cy3 dye (pH 7.4) | −139.6 | 1.7 | −4.4 | −2.7 | 6.1 |
| With Cy3 dye (pH 7.4) | −76.6 | 0.7 | −7.1 | −1.6 | 5.5 |
| Groups | Control | Cy3 Dye | Fuc-Cy3 | Fuc-L-Arg (1:1) | Fuc-L-Arg (1:2.5) | Fuc-L-Arg (1:5) |
|---|---|---|---|---|---|---|
| Cy3 Dye Intensity | 109 | 10,412 | 16,955.33333 | 18,617.66667 | 18,961 | 19,142 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arunagiri, V.; Tsai, H.-C.; Darge, H.F.; Hanurry, E.Y.; Lee, C.Y.; Lai, J.-Y.; Wu, S.-Y. Enhanced Cellular Uptake in an Electrostatically Interacting Fucoidan–L-Arginine Fiber Complex. Polymers 2021, 13, 1795. https://doi.org/10.3390/polym13111795
Arunagiri V, Tsai H-C, Darge HF, Hanurry EY, Lee CY, Lai J-Y, Wu S-Y. Enhanced Cellular Uptake in an Electrostatically Interacting Fucoidan–L-Arginine Fiber Complex. Polymers. 2021; 13(11):1795. https://doi.org/10.3390/polym13111795
Chicago/Turabian StyleArunagiri, Vinothini, Hsieh-Chih Tsai, Haile Fentahun Darge, Endiries Yibru Hanurry, Chang Yi Lee, Juin-Yih Lai, and Szu-Yuan Wu. 2021. "Enhanced Cellular Uptake in an Electrostatically Interacting Fucoidan–L-Arginine Fiber Complex" Polymers 13, no. 11: 1795. https://doi.org/10.3390/polym13111795
APA StyleArunagiri, V., Tsai, H.-C., Darge, H. F., Hanurry, E. Y., Lee, C. Y., Lai, J.-Y., & Wu, S.-Y. (2021). Enhanced Cellular Uptake in an Electrostatically Interacting Fucoidan–L-Arginine Fiber Complex. Polymers, 13(11), 1795. https://doi.org/10.3390/polym13111795








