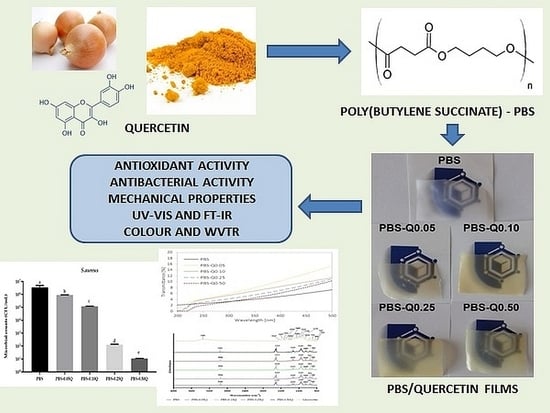
Development and Characterization of Bioactive Poly(butylene-succinate) Films Modified with Quercetin for Food Packaging Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Films
2.3. Thickness and Mechanical Properties of the Films
2.4. The Water Vapor Transmission Rate of the Films
2.5. UV–Vis and FT-IR Spectroscopic Analysis
2.6. Moisture Content, Moisture Sorption and Water Sorption Degrees
2.7. Thermal Characterization
2.8. Color and Opacity Measurements
2.9. Reducing Power and Free Radicals Scavenging Activity
2.10. Antibacterial Activity
2.11. Determination of Quercetin Migration into Model Food Liquid Systems
2.12. Statistical Analysis
3. Results and Discussion
3.1. Free Radicals Scavenging Activity and Reducing Power
3.2. Antibacterial Activity
3.3. Color, Opacity and UV–Vis Blocking Effects
3.4. Thickness, Mechanical Properties and Water Vapor Barrier Properties
3.5. Moisture Content, Moisture Sorption, Water Sorption and Migration of Quercetin into Model Food Liquid Systems
3.6. FT-IR Results
3.7. Results of PBS Films Thermal Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Motelica, L.; Ficai, D.; Ficai, A.; Oprea, O.C.; Kaya, D.A.; Andronescu, E. Biodegradable antimicrobial food packaging: Trends and perspectives. Foods 2020, 9, 1438. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Montes, E.; Castro-Muñoz, R. Edible Films and Coatings as Food-Quality Preservers: An Overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Manepalli, P.H.; Zhu, L.; Narayan-Sarathy, S.; Alavi, S. Morphological, barrier and mechanical properties of films from poly (butylene succinate) reinforced with nanocrystalline cellulose and chitin whiskers using melt extrusion. J. Polym. Res. 2019, 26, 1–10. [Google Scholar] [CrossRef]
- Qi, Z.; Ye, H.; Xu, J.; Peng, J.; Chen, J.; Guo, B. Synthesis and characterizations of attapulgite reinforced branched poly (butylene succinate) nanocomposites. Colloids Surf. A Phys. Eng. Asp. 2013, 436, 26–33. [Google Scholar] [CrossRef]
- Vytejčková, S.; Vápenka, L.; Hradecký, J.; Dobiáš, J.; Hajšlová, J.; Loriot, C.; Vannini, L.; Poustka, J. Testing of polybutylene succinate based films for poultry meat packaging. Polym. Test. 2017, 60, 357–364. [Google Scholar] [CrossRef]
- Wu, C.S.; Liao, H.T.; Jhang, J.J. Palm fibre-reinforced hybrid composites of poly (butylene succinate): Characterisation and assessment of mechanical and thermal properties. Polym. Bull. 2013, 70, 3443–3462. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Fabrication of copper sulfide nanoparticles and limonene incorporated pullulan/carrageenan-based film with improved mechanical and antibacterial properties. Polymers 2020, 12, 2665. [Google Scholar] [CrossRef]
- Zeng, R.T.; Hu, W.; Wang, M.; Zhang, S.D.; Zeng, J.B. Morphology, rheological and crystallization behavior in non-covalently functionalized carbon nanotube reinforced poly (butylene succinate) nanocomposites with low percolation threshold. Polym. Test. 2016, 50, 182–190. [Google Scholar] [CrossRef]
- Roy, S.; Kim, H.C.; Panicker, P.S.; Rhim, J.W.; Kim, J. Cellulose nanofiber-based nanocomposite films reinforced with zinc oxide nanorods and grapefruit seed extract. Nanomaterials 2021, 11, 877. [Google Scholar] [CrossRef]
- Joy, J.; Jose, C.; Yu, X.; Mathew, L.; Thomas, S.; Pilla, S. The influence of nanocellulosic fiber, extracted from Helicteres isora, on thermal, wetting and viscoelastic properties of poly (butylene succinate) composites. Cellulose 2017, 24, 4313–4323. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, A.M.; Zuccari, G. Biodegradable and compostable shopping bags under investigation by FTIR spectroscopy. Appl. Sci. 2021, 11, 621. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Jędra, F.; Mizielińska, M. New Poly (lactic acid) Active Packaging Composite Films Incorporated with Fungal Melanin. Polymers 2018, 10, 386. [Google Scholar] [CrossRef] [Green Version]
- Stȩpień, K.; Miles, C.; McClain, A.; Wiśniewska, E.; Sobolewski, P.; Kohn, J.; Puskas, J.; Wagner, H.D.; El Fray, M. Biocopolyesters of Poly (butylene succinate) Containing Long-Chain Biobased Glycol Synthesized with Heterogeneous Titanium Dioxide Catalyst. ACS Sustain. Chem. Eng. 2019, 7, 10623–10632. [Google Scholar] [CrossRef]
- Sonseca, A.; Sahay, R.; Stepien, K.; Bukala, J.; Wcislek, A.; McClain, A.; Sobolewski, P.; Sui, X.M.; Puskas, J.E.; Kohn, J.; et al. Architectured helically coiled scaffolds from elastomeric poly (butylene succinate) (PBS) copolyester via wet electrospinning. Mater. Sci. Eng. C 2020, 108, 110505. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, W.; Tian, B.; Li, D.; Liu, C.; Jiang, B.; Feng, Z. Preparation and characterization of coating based on protein nanofibers and polyphenol and application for salted duck egg yolks. Foods 2020, 9, 449. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liang, S.; Zhang, J.; Chi, Y.; Tian, B.; Li, L.; Jiang, B.; Li, D.; Feng, Z.; Liu, C. Preparation of whey protein isolate nanofibrils by microwave heating and its application as carriers of lipophilic bioactive substances. LWT 2020, 125, 109213. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Guo, B. Photodegradation behavior of poly (butylene succinate-co-butylene adipate)/ZnO nanocomposites. Colloids Surf. A Phys. Eng. Asp. 2016, 489, 173–181. [Google Scholar] [CrossRef]
- Ayu, R.S.; Khalina, A.; Harmaen, A.S.; Zaman, K.; Nurrazi, N.M.; Isma, T.; Lee, C.H. Effect of Empty Fruit Brunch reinforcement in PolyButylene-Succinate/Modified Tapioca Starch blend for Agricultural Mulch Films. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, S.; Misra, M.; Mohanty, A.K. Enhanced properties of lignin-based biodegradable polymer composites using injection moulding process. Compos. Part A Appl. Sci. Manuf. 2011, 42, 1710–1718. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Zuccari, G. Nanotechnology application in food packaging: A plethora of opportunities versus pending risks assessment and public concerns. Food Res. Int. 2020, 137, 109664. [Google Scholar] [CrossRef]
- Bai, R.; Zhang, X.; Yong, H.; Wang, X.; Liu, Y.; Liu, J. Development and characterization of antioxidant active packaging and intelligent Al 3+-sensing films based on carboxymethyl chitosan and quercetin. Int. J. Biol. Macromol. 2019, 126, 1074–1084. [Google Scholar] [CrossRef]
- Yadav, S.; Mehrotra, G.K.; Bhartiya, P.; Singh, A.; Dutta, P.K. Preparation, physicochemical and biological evaluation of quercetin based chitosan-gelatin film for food packaging. Carbohydr. Polym. 2020, 227, 115348. [Google Scholar] [CrossRef]
- Braga, L.R.; Pérez, L.M.; Soazo, M.d.V.; Machado, F. Evaluation of the antimicrobial, antioxidant and physicochemical properties of Poly(Vinyl chloride) films containing quercetin and silver nanoparticles. LWT 2019, 101, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Tongdeesoontorn, W.; Mauer, L.J.; Wongruong, S.; Sriburi, P.; Rachtanapun, P. Physical and antioxidant properties of cassava starch-carboxymethyl cellulose incorporated with quercetin and TBHQ as active food packaging. Polymers 2020, 12, 366. [Google Scholar] [CrossRef] [Green Version]
- Łopusiewicz, Ł.; Kwiatkowski, P.; Drozłowska, E.; Trocer, P.; Kostek, M.; Śliwiński, M.; Polak-Śliwińska, M.; Kowalczyk, E.; Sienkiewicz, M. Preparation and characterization of carboxymethyl cellulose-based bioactive composite films modified with fungal melanin and carvacrol. Polymers 2021, 13, 499. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozlowska, E.; Trocer, P.; Kostek, M.; Śliwiński, M.; Henriques, M.H.F.; Bartkowiak, A.; Sobolewski, P. Whey protein concentrate/isolate biofunctional films modified with melanin from watermelon (Citrullus lanatus) seeds. Materials 2020, 13, 3876. [Google Scholar] [CrossRef]
- Luzi, F.; Pannucci, E.; Santi, L.; Kenny, J.M.; Torre, L.; Bernini, R.; Puglia, D. Gallic acid and quercetin as intelligent and active ingredients in poly (vinyl alcohol) films for food packaging. Polymers 2019, 11, 1999. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Wang, X.; Wang, L.; Wu, S.; Li, D.; Liu, C.; Feng, Z. Fabrication and characterization of a microemulsion stabilized by integrated phosvitin and gallic acid. J. Agric. Food Chem. 2020, 68, 5437–5447. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Wu, Q.; Gu, Y.; Kan, J.; Jin, C. Effect of protocatechuic acid incorporation on the physical, mechanical, structural and antioxidant properties of chitosan film. Food Hydrocoll. 2017, 73, 90–100. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, Y.; Bai, R.; Zhang, X.; Yuan, L.; Liu, J. Preparation of pH-sensitive and antioxidant packaging films based on κ-carrageenan and mulberry polyphenolic extract. Int. J. Biol. Macromol. 2019, 134, 993–1001. [Google Scholar] [CrossRef]
- Talón, E.; Trifkovic, K.T.; Nedovic, V.A.; Bugarski, B.M.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant edible films based on chitosan and starch containing polyphenols from thyme extracts. Carbohydr. Polym. 2017, 157, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.; Liu, J. Active packaging films and edible coatings based on polyphenol-rich propolis extract: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2106–2145. [Google Scholar] [CrossRef] [PubMed]
- Condat, M.; Babinot, J.; Tomane, S.; Malval, J.P.; Kang, I.K.; Spillebout, F.; Mazeran, P.E.; Lalevée, J.; Andalloussi, S.A.; Versace, D.L. Development of photoactivable glycerol-based coatings containing quercetin for antibacterial applications. RSC Adv. 2016, 6, 18235–18245. [Google Scholar] [CrossRef]
- Souza, M.P.; Vaz, A.F.M.; Silva, H.D.; Cerqueira, M.A.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Development and Characterization of an Active Chitosan-Based Film Containing Quercetin. Food Bioprocess Technol. 2015, 8, 2183–2191. [Google Scholar] [CrossRef] [Green Version]
- Giteru, S.G.; Coorey, R.; Bertolatti, D.; Watkin, E.; Johnson, S.; Fang, Z. Physicochemical and antimicrobial properties of citral and quercetin incorporated kafirin-based bioactive films. Food Chem. 2015, 168, 341–347. [Google Scholar] [CrossRef]
- Giteru, S.G.; Oey, I.; Ali, M.A.; Johnson, S.K.; Fang, Z. Effect of kafirin-based films incorporating citral and quercetin on storage of fresh chicken fillets. Food Control 2017, 80, 37–44. [Google Scholar] [CrossRef]
- Chen, X.; Lee, D.S.; Zhu, X.; Yam, K.L. Release kinetics of tocopherol and quercetin from binary antioxidant controlled-release packaging films. J. Agric. Food Chem. 2012, 60, 3492–3497. [Google Scholar] [CrossRef]
- Jin, T.; Yan, L.; Liu, W.; Liu, S.; Liu, C.; Zheng, L. Preparation and physicochemical/antimicrobial characteristics of asparagus cellulose films containing quercetin. Food Sci. Hum. Wellness 2021, 10, 251–257. [Google Scholar] [CrossRef]
- Han, T.; Lu, L.; Ge, C. Development and Properties of High Density Polyethylene (HDPE) and Ethylene-Vinyl Acetate Copolymer (EVA) Blend Antioxidant Active Packaging Films Containing Quercetin. Packag. Technol. Sci. 2015, 28, 415–423. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Jędrzejewski, R.; Pilawka, R. Deep eutectic solvents as simultaneous plasticizing and crosslinking agents for starch. Int. J. Biol. Macromol. 2019, 129, 1040–1046. [Google Scholar] [CrossRef]
- Łupina, K.; Kowalczyk, D.; Kazimierczak, W. Gum Arabic/Gelatin and Water-Soluble Soy Polysaccharides/Gelatin Blend Films as Carriers of Astaxanthin—A Comparative Study of the Kinetics of Release and Antioxidant Properties. Polymers 2021, 13, 1062. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Larrañeta, E.; Fong, M.L.; Martin, N.K.; Irwin, N.J.; Mutjé, P.; Tarrés, Q.; Delgado-Aguilar, M. Lignin/poly (butylene succinate) composites with antioxidant and antibacterial properties for potential biomedical applications. Int. J. Biol. Macromol. 2020, 145, 92–99. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Jędra, F.; Bartkowiak, A. The application of melanin modified gelatin coatings for packaging and the oxidative stability of pork lard. World Sci. News 2018, 101, 108–119. [Google Scholar]
- Gatto, M.T.; Falcocchio, S.; Grippa, E.; Mazzanti, G.; Battinelli, L.; Nicolosi, G.; Lambusta, D.; Saso, L. Antimicrobial and anti-lipase activity of quercetin and its C2-C16 3-O-acyl-esters. Bioorg. Med. Chem. 2002, 10, 269–272. [Google Scholar] [CrossRef]
- Bukhari, S.B.; Memon, S.; Mahroof-Tahir, M.; Bhanger, M.I. Synthesis, characterization and antioxidant activity copper-quercetin complex. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 71, 1901–1906. [Google Scholar] [CrossRef]
- Lee, S.H.; Wang, S. Biodegradable polymers/bamboo fiber biocomposite with bio-based coupling agent. Compos. Part A Appl. Sci. Manuf. 2006, 37, 80–91. [Google Scholar] [CrossRef]
- Wan, C.; Chen, B. Reinforcement of biodegradable poly (butylene succinate) with low loadings of graphene oxide. J. Appl. Polym. Sci. 2013, 127, 5094–5099. [Google Scholar] [CrossRef]
- Jurasekova, Z.; Domingo, C.; Garcia-Ramos, J.V.; Sanchez-Cortes, S. Effect of pH on the chemical modification of quercetin and structurally related flavonoids characterized by optical (UV-visible and Raman) spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 12802–12811. [Google Scholar] [CrossRef] [Green Version]
- Srinivas, K.; King, J.W.; Howard, L.R.; Monrad, J.K. Solubility and solution thermodynamic properties of quercetin and quercetin dihydrate in subcritical water. J. Food Eng. 2010, 100, 208–218. [Google Scholar] [CrossRef]
- Kuley, E.; Durmus, M.; Balikci, E.; Ucar, Y.; Regenstein, J.M.; Özoğul, F. Fish spoilage bacterial growth and their biogenic amine accumulation: Inhibitory effects of olive by-products. Int. J. Food Prop. 2017, 20, 1029–1043. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.J.; Shin, H.S. Development of a freshness indicator for monitoring the quality of beef during storage. Food Sci. Biotechnol. 2019, 28, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Comi, G. Chapter 8—Spoilage of Meat and Fish. In The Microbiological Quality of Food; Bevilacqua, A., Corbo, M.R., Sinigaglia, M., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2017; pp. 179–210. ISBN 978-0-08-100502-6. [Google Scholar]
- Berlier, G.; Gastaldi, L.; Ugazio, E.; Miletto, I.; Iliade, P.; Sapino, S. Stabilization of quercetin flavonoid in MCM-41 mesoporous silica: Positive effect of surface functionalization. J. Colloid Interface Sci. 2013, 393, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.B.; Mohan, R. Thermal, mechanical, and rheological properties of biodegradable polybutylene succinate/carbon nanotubes nanocomposites. Polym. Compos. 2010, 31, 1309–1314. [Google Scholar] [CrossRef]







| Sample | RP (700 nm) | DPPH (%) | ABTS (%) | O2− (%) |
|---|---|---|---|---|
| PBS | 0.41 ± 0.28 a | 0.21 ± 0.00 a | 1.61 ± 0.31 a | 0.21 ± 0.01 a |
| PBS-Q0.05 | 0.88 ± 0.03 b | 38.79 ± 0.07 b | 25.51 ± 0.82 b | 15.21 ± 0.12 b |
| PBS-Q0.10 | 0.96 ± 0.05 b | 42.77 ± 0.07 c | 48.84 ± 3.43 c | 16.69 ± 0.23 c |
| PBS-Q0.25 | 1.68 ± 0.13 c | 80.12 ± 0.00 d | 79.16 ± 5.74 d | 29.23 ± 0.35 d |
| PBS-Q0.50 | 2.08 ± 0.01 d | 80.90 ± 0.07 e | 99.07 ± 0.28 e | 38.06 ± 0.11 e |
| Sample | L* | a* | b* | ΔE | YI | Opacity | C |
|---|---|---|---|---|---|---|---|
| PBS | 85.34 ± 1.28 a | −0.33 ± 0.19 a | 2.86 ± 0.51 a | used as standard | 4.78 ± 0.84 a | 11.94 ± 1.07 a | 2.89 ± 0.48 a |
| PBS-Q0.05 | 80.58 ± 3.69 ab | −0.19 ± 0.47 a | 18.38 ± 1.86 b | 15.69 ± 1.77 a | 31.40 ± 3.13 b | 12.40 ± 2.04 a | 18.39 ± 1.85 b |
| PBS-Q0.10 | 83.61 ± 1.38 a | −0.80 ± 0.17 a | 19.53 ± 4.70 b | 17.92 ± 3.60 b | 34.44 ± 7.43 b | 12.72 ± 0.10 b | 19.55 ± 4.69 b |
| PBS-Q0.25 | 79.59 ± 4.63 b | −1.48 ± 0.21 b | 21.89 ± 2.28 bc | 20.46 ± 1.47 c | 39.24 ± 2.80 bc | 14.48 ± 0.14 c | 21.93 ± 2.28 bc |
| PBS-Q0.50 | 76.35 ± 4.97 b | −2.66 ± 0.37 c | 26.34 ±5.49 c | 25.90 ± 4.27 d | 49.19 ± 9.52 c | 14.96 ± 0.25 d | 26.45 ± 5.47 c |
| Sample | TS (MPa) | EB (%) | YM (MPa) | Thickness (mm) | WVTR (g/m2 × Day) |
|---|---|---|---|---|---|
| PBS | 11.80 ± 2.20 a | 155.00 ± 32.10 a | 144.00 ± 19.00 a | 0.18 ± 0.02 a | 60.27 ± 5.08 a |
| PBS-Q0.05 | 11.90 ± 2.90 a | 140.00 ± 48.40 ab | 147.00 ± 17.00 a | 0.18 ± 0.04 a | 63.02 ± 3.44 a |
| PBS-Q0.10 | 12.10 ± 1.10 a | 114.00 ± 14.21 abc | 119.00 ± 14.30 b | 0.17 ± 0.05 a | 62.79 ± 4.09 a |
| PBS-Q0.25 | 11.90 ± 0.70 a | 94.00 ± 30.50 cb | 128.00 ± 13.50 b | 0.16 ± 0.01 a | 59.21 ± 7.13 a |
| PBS-Q0.50 | 8.40 ± 2.50 a | 81.00 ± 9.20 c | 100.00 ± 16.50 b | 0.19 ± 0.04 a | 59.41 ± 8.29 a |
| Sample | MC (%) | MS (50% RH) (%) | MS (80%) (%) | WS (%) |
|---|---|---|---|---|
| PBS | 1.58 ± 0.11 a | 1.21 ± 0.12 Aa | 1.26 ± 0.12 Ba | 1.61 ± 0.13 a |
| PBS-Q0.05 | 1.21 ± 0.07 b | 1.17 ± 0.06 Aa | 1.20 ± 0.15 Ba | 1.17 ± 0.15 b |
| PBS-Q0.10 | 0.77 ± 0.03 c | 0.68 ± 0.13 Ab | 0.84 ± 0.11 Bb | 0.77 ± 0.09 c |
| PBS-Q0.25 | 0.76 ± 0.07 c | 0.61 ± 0.13 Ab | 0.82 ± 0.17 Bb | 0.77 ± 0.06 c |
| PBS-Q0.50 | 0.64 ± 0.10 c | 0.51 ± 0.08 Ab | 0.74 ± 0.09 Bb | 0.64 ± 0.13 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łopusiewicz, Ł.; Zdanowicz, M.; Macieja, S.; Kowalczyk, K.; Bartkowiak, A. Development and Characterization of Bioactive Poly(butylene-succinate) Films Modified with Quercetin for Food Packaging Applications. Polymers 2021, 13, 1798. https://doi.org/10.3390/polym13111798
Łopusiewicz Ł, Zdanowicz M, Macieja S, Kowalczyk K, Bartkowiak A. Development and Characterization of Bioactive Poly(butylene-succinate) Films Modified with Quercetin for Food Packaging Applications. Polymers. 2021; 13(11):1798. https://doi.org/10.3390/polym13111798
Chicago/Turabian StyleŁopusiewicz, Łukasz, Magdalena Zdanowicz, Szymon Macieja, Krzysztof Kowalczyk, and Artur Bartkowiak. 2021. "Development and Characterization of Bioactive Poly(butylene-succinate) Films Modified with Quercetin for Food Packaging Applications" Polymers 13, no. 11: 1798. https://doi.org/10.3390/polym13111798
APA StyleŁopusiewicz, Ł., Zdanowicz, M., Macieja, S., Kowalczyk, K., & Bartkowiak, A. (2021). Development and Characterization of Bioactive Poly(butylene-succinate) Films Modified with Quercetin for Food Packaging Applications. Polymers, 13(11), 1798. https://doi.org/10.3390/polym13111798