Fabrication of Reproducible and Selective Ammonia Vapor Sensor-Pellet of Polypyrrole/Cerium Oxide Nanocomposite for Prompt Detection at Room Temperature
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of PPy and PPy/CeO2
2.3. Characterization and Instrumentation
3. Results and Discussion
3.1. Fourier Transform Infrared Spectroscopic (FT-IR) Studies
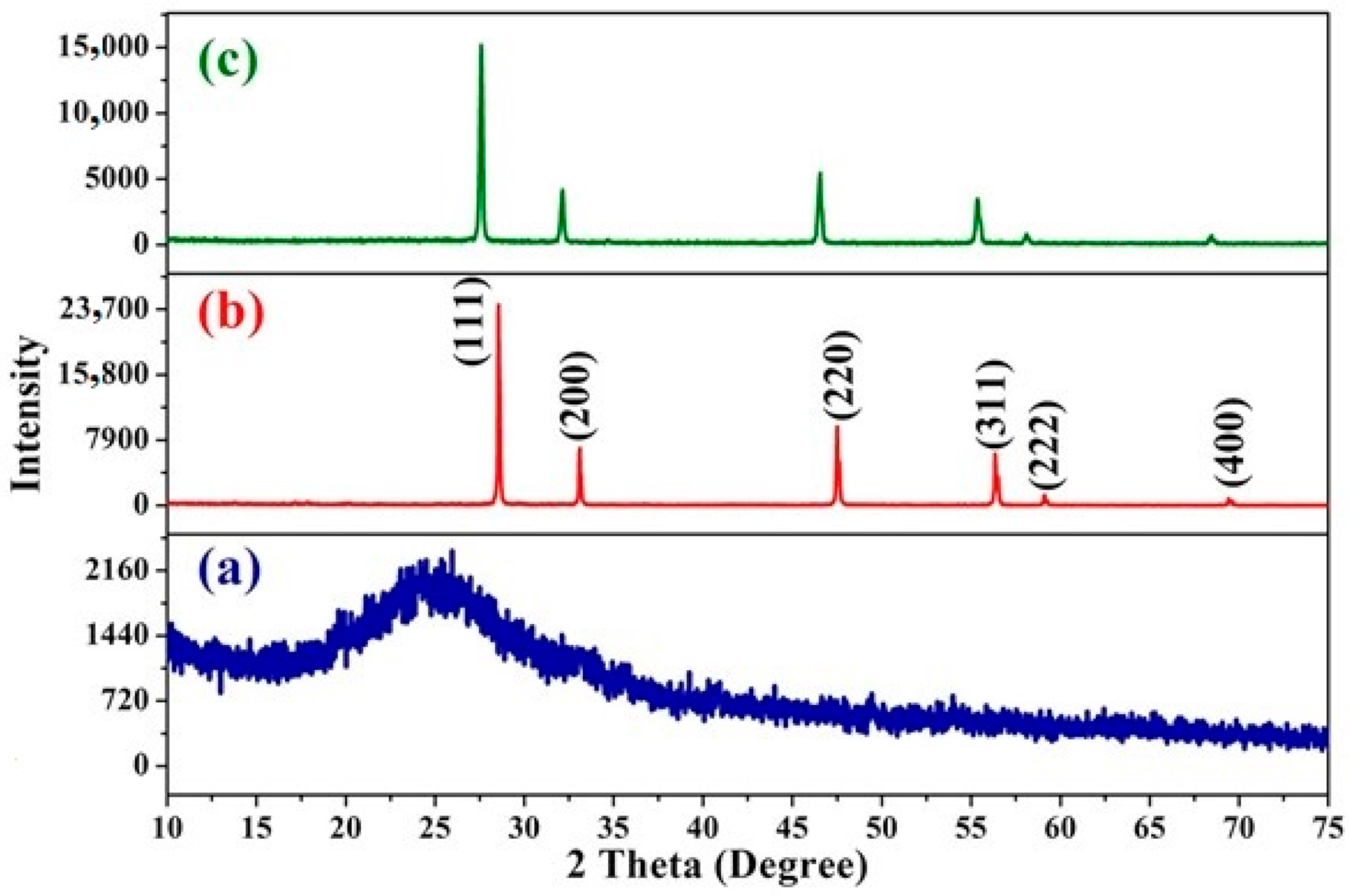
3.2. X-ray Diffraction (XRD) Studies
3.3. Morphological Studies (SEM and TEM)
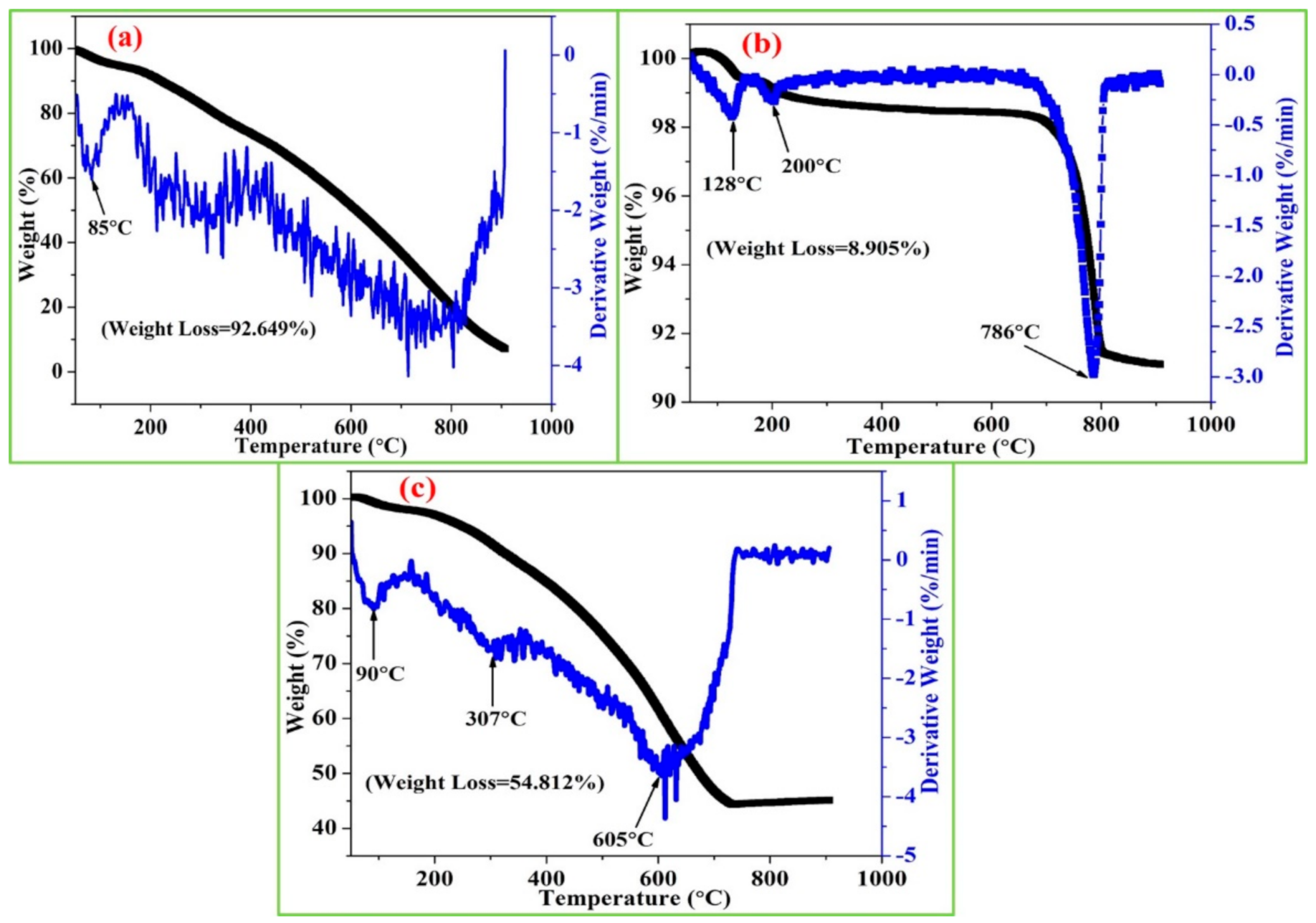
3.4. Thermogravimetric Analysis (TGA)
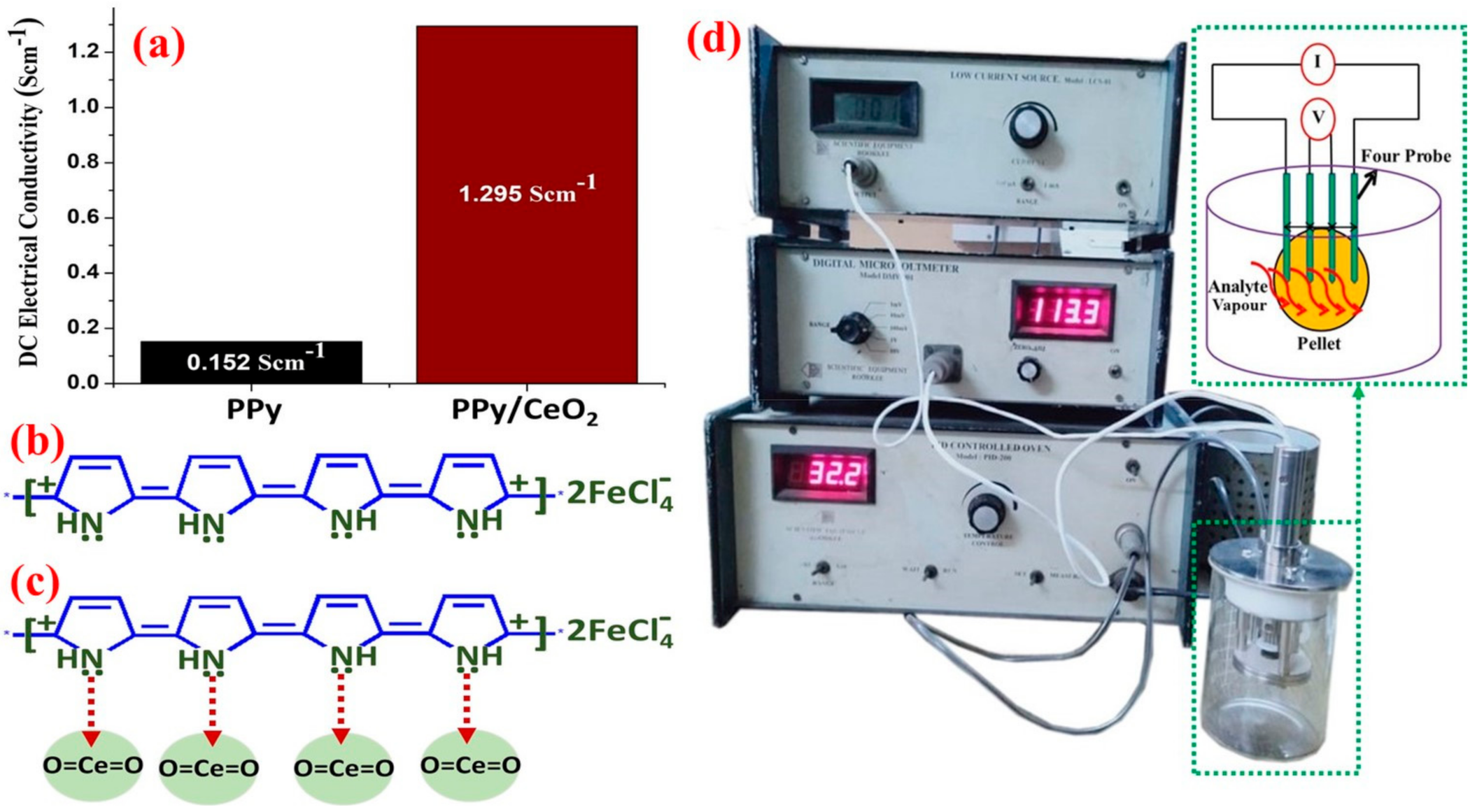
3.5. DC Electrical Conductivity Studies
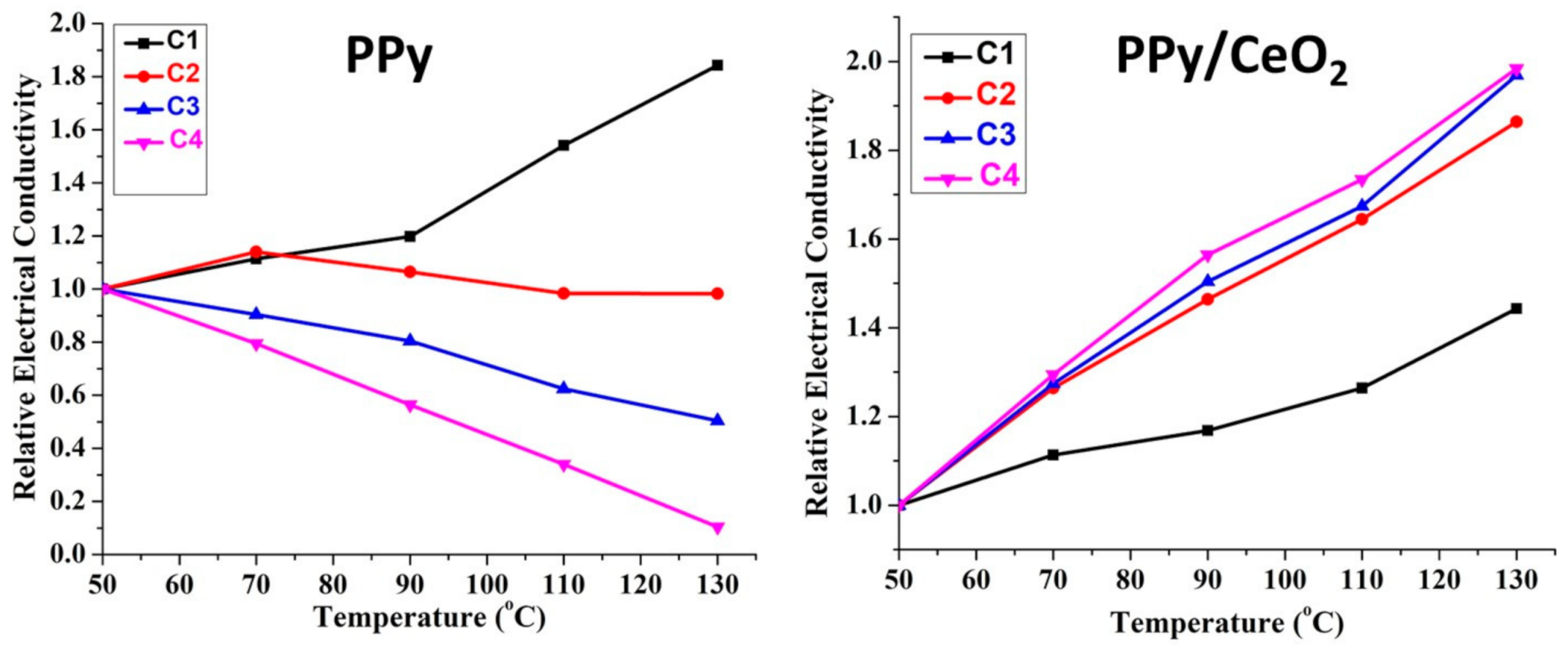
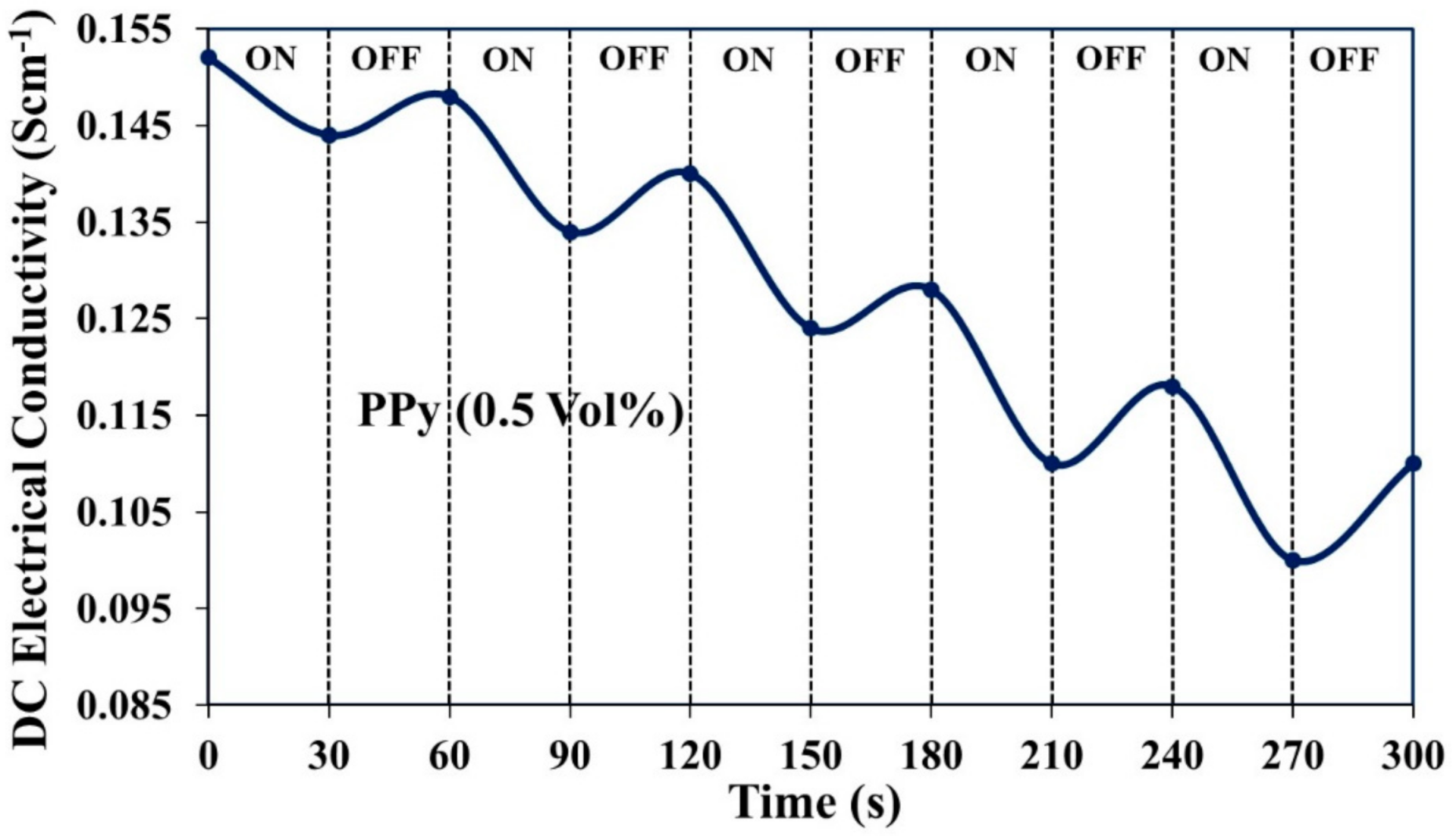
3.5.1. DC Electrical Conductivity Retention under Isothermal Ageing Condition
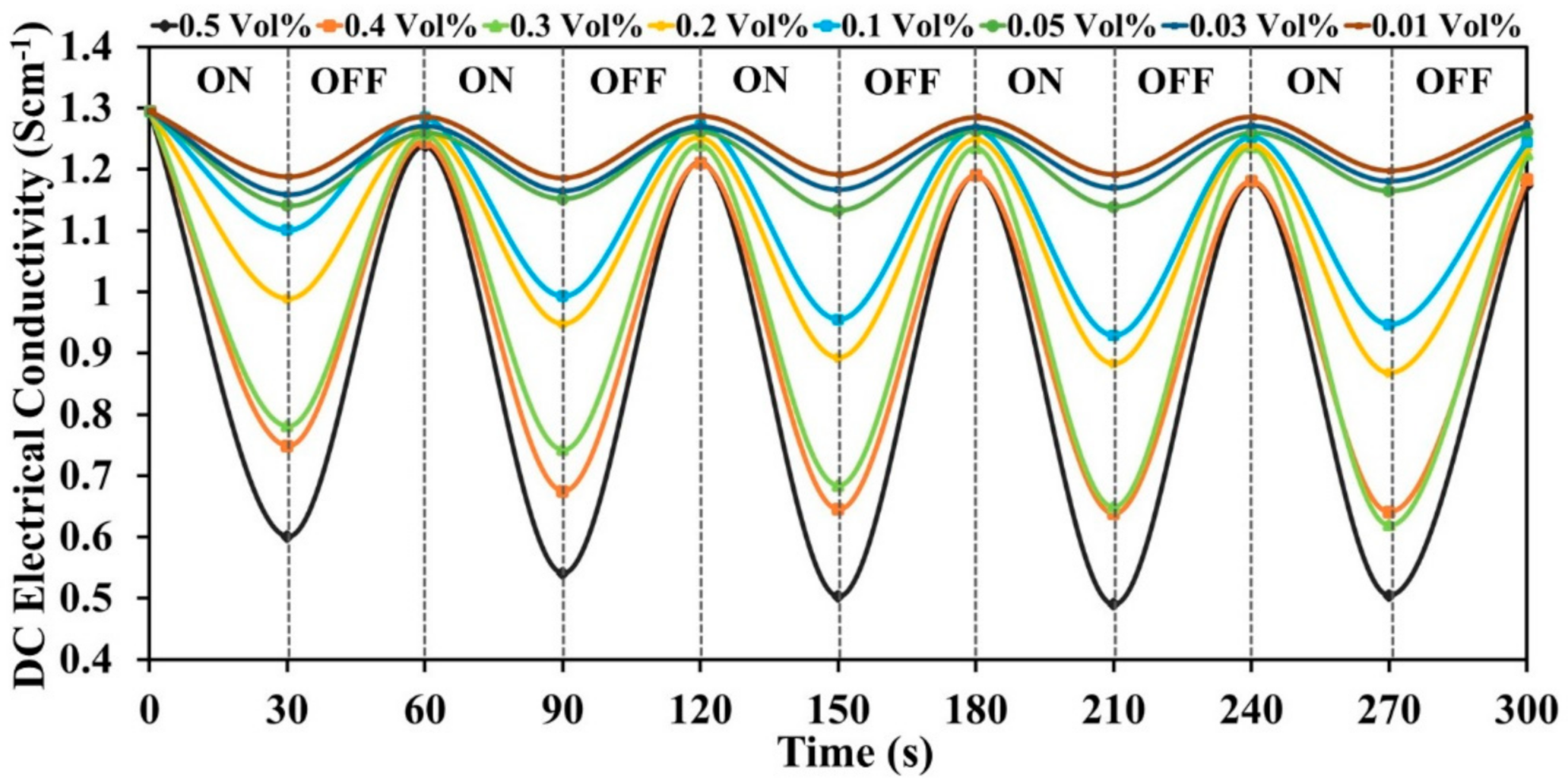
3.5.2. DC Electrical Conductivity Retention under Cyclic Ageing Condition
3.6. Ammonia Vapor Sensing Studies
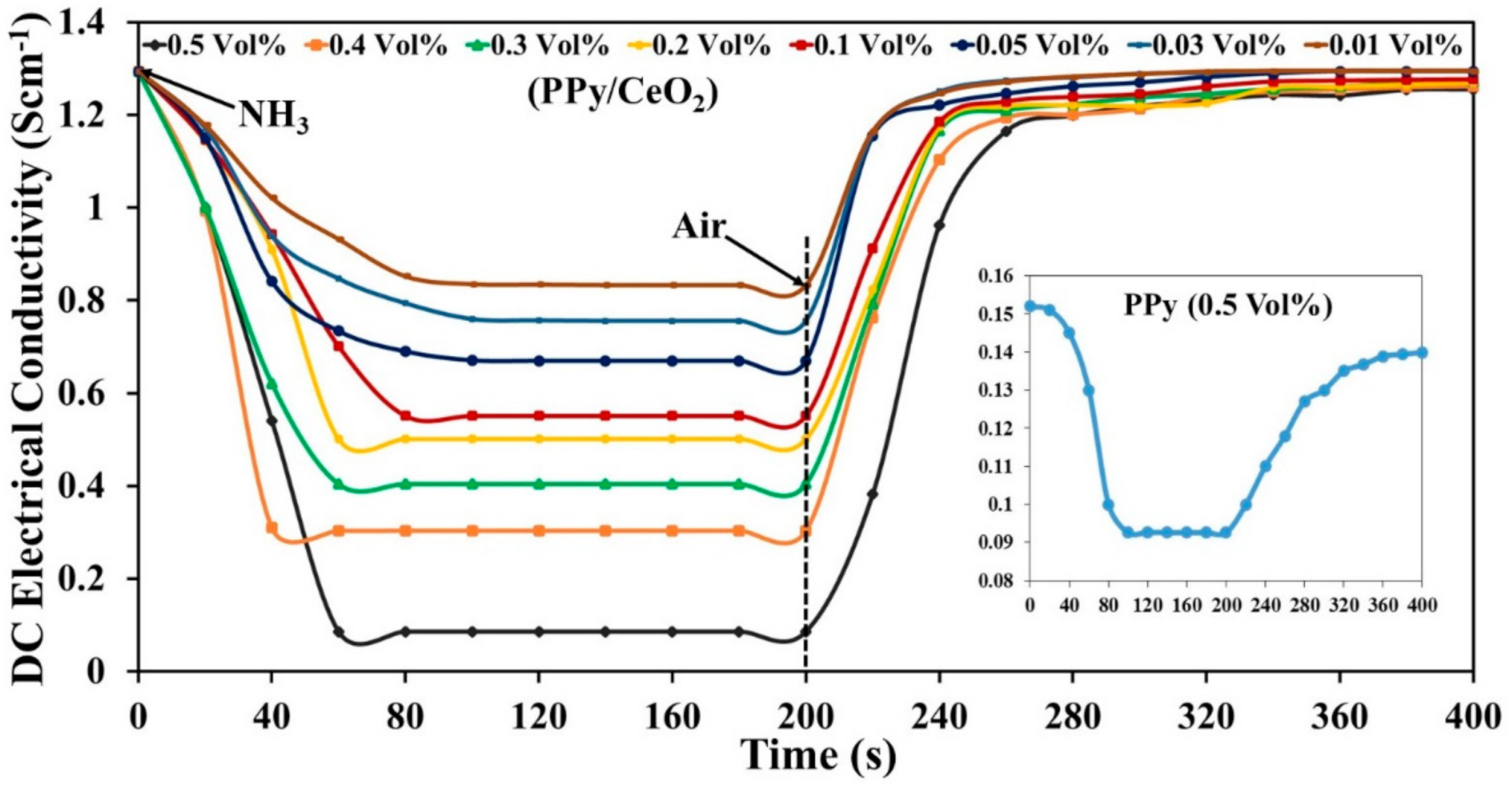
3.6.1. Sensing Response
3.6.2. Reversibility
3.6.3. Selectivity
3.6.4. Stability
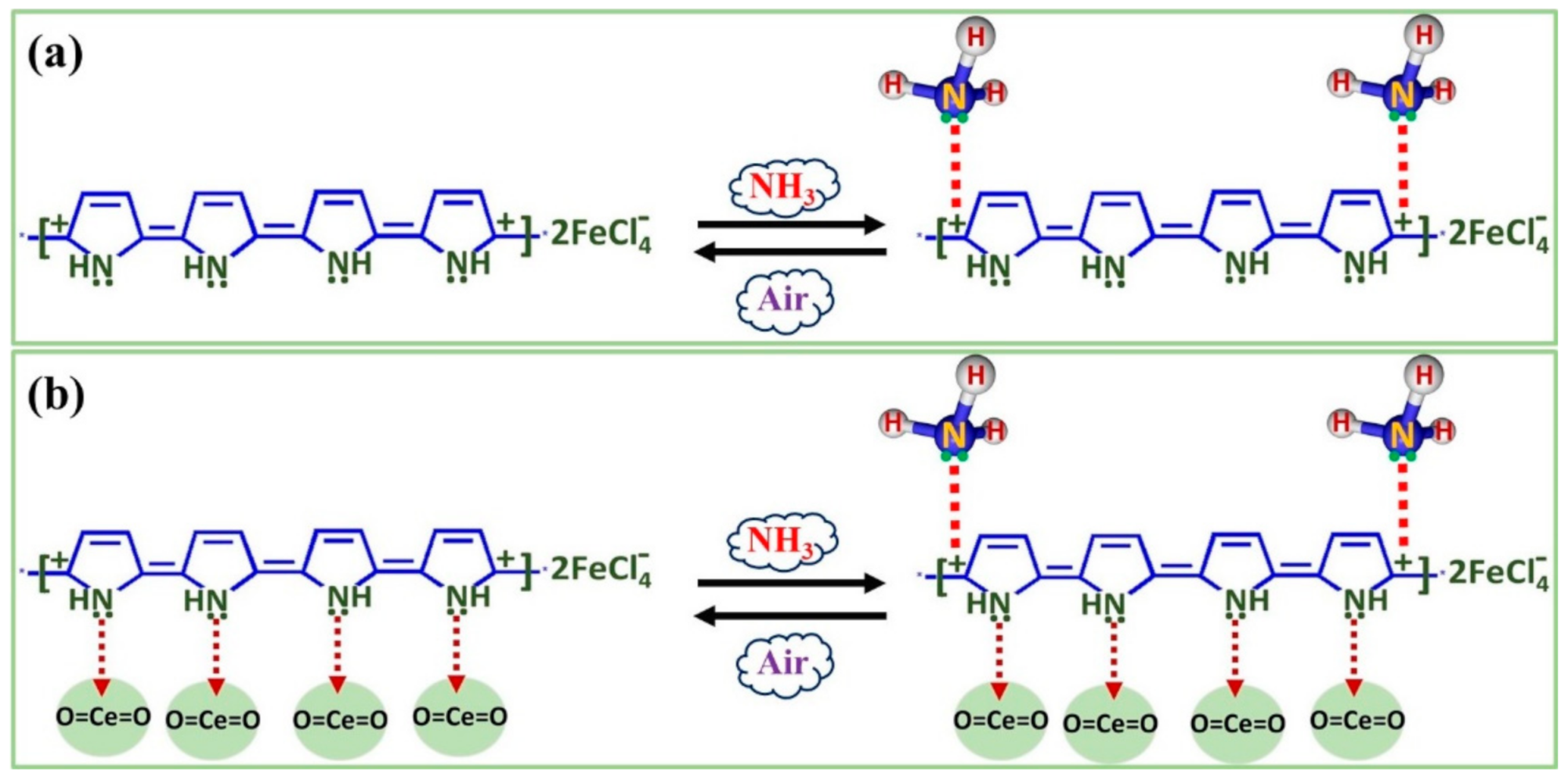
3.6.5. Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, J.; Shu, X.; Tian, Y.; Tong, Z.; Bai, S.; Luo, R.; Li, D.; Liu, C.C. Facile preparation of polypyrrole-reduced graphene oxide hybrid for enhancing NH3 sensing at room temperature. Sens. Actuators B Chem. 2017, 241, 658–664. [Google Scholar] [CrossRef]
- Lamdhade, G.T.; Raulkar, K.B.; Yawale, S.S.; Yawale, S.P. Fabrication of multilayer SnO2–ZnO–PPy sensor for ammonia gas detection. Indian J. Phys. 2015, 89, 1025–1030. [Google Scholar] [CrossRef]
- Joshi, A.; Gangal, S.A.; Gupta, S.K. Ammonia sensing properties of polypyrrole thin films at room temperature. Sens. Actuators B Chem. 2011, 156, 938–942. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Wu, S.; Xu, H.; Cao, B. One-pot fabrication of uniform polypyrrole/Au nanocomposites and investigation for gas sensing. Sens. Actuators B Chem. 2013, 186, 695–700. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Zong, X.; Zhang, Y. Fabrication of Polypyrrole/Zn2SnO4 Nanofilm for Ultra-Highly Sensitive Ammonia Sensing Application. Sens. Actuators B Chem. 2018, 274, 575–586. [Google Scholar] [CrossRef]
- Liu, B.; Liu, X.; Yuan, Z.; Jiang, Y.; Su, Y.; Ma, J.; Tai, H. A flexible NO2 gas sensor based on polypyrrole/nitrogen-doped multiwall carbon nanotube operating at room temperature. Sens. Actuators B Chem. 2019, 295, 86–92. [Google Scholar] [CrossRef]
- Tai, H.; Duan, Z.; He, Z.; Li, X.; Xu, J.; Liu, B.; Jiang, Y. Enhanced ammonia response of Ti3C2Tx nanosheets supported by TiO2 nanoparticles at room temperature. Sens. Actuators B Chem. 2019, 298, 126874. [Google Scholar] [CrossRef]
- Lefferts, M.J.; Humphreys, L.H.; Mai, N.; Murugappan, K.; Armitage, B.I.; Pons, J.F.; Castell, M.R. ANFO vapor detection with conducting polymer percolation network sensors and GC/MS. Analyst 2021, 146, 2186–2193. [Google Scholar] [CrossRef]
- Armitage, B.I.; Murugappan, K.; Lefferts, M.J.; Cowsik, A.; Castell, M.R. Conducting polymer percolation gas sensor on a flexible substrate. J. Mater. Chem. C 2020, 8, 12669–12676. [Google Scholar] [CrossRef]
- Oh, H.J.; Yeang, B.J.; Park, Y.K.; Choi, H.J.; Kim, J.H.; Kang, Y.S.; Bae, Y.; Kim, J.Y.; Lim, S.J.; Lee, W.; et al. Washable colorimetric nanofiber nonwoven for ammonia gas detection. Polymers 2020, 12, 1585. [Google Scholar] [CrossRef]
- Park, Y.K.; Oh, H.J.; Bae, J.H.; Lim, J.Y.; Lee, H.D.; Hong, S.I.; Son, H.S.; Kim, J.H.; Lim, S.J.; Lee, W. Colorimetric textile sensor for the simultaneous detection of nh3 and hcl gases. Polymers 2020, 12, 2595. [Google Scholar] [CrossRef]
- Ly, T.N.; Park, S. Highly sensitive ammonia sensor for diagnostic purpose using reduced graphene oxide and conductive polymer. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, H.; Huang, P.; Xiang, C.; Zou, Y.; Xu, F.; Sun, L. Inducement of nanoscale Cu–BTC on nanocomposite of PPy–rGO and its performance in ammonia sensing. Mater. Res. Bull. 2018, 99, 152–160. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Li, P.; Zong, X.; Dong, G.; Zhang, Y. Facile fabrication of polyaniline/multi-walled carbon nanotubes/molybdenum disulfide ternary nanocomposite and its high-performance ammonia-sensing at room temperature. Sens. Actuators B Chem. 2017, 258, 895–905. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, B.; Zhang, Z. Combination of PPy with three-dimensional rGO to construct bioinspired nanocomposite for NH3-sensing enhancement. Org. Electron. 2019, 70, 240–245. [Google Scholar] [CrossRef]
- van Hieu, N.; Quoc, N.; Dinh, P.; Trung, T.; Duc, N. Thin film polypyrrole/SWCNTs nanocomposites-based NH 3 sensor operated at room temperature. Sens. Actuators B Chem. 2009, 140, 500–507. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, I.; Husain, A.; Khan, A.; Asiri, A.M. Electrical conductivity based ammonia sensing properties of polypyrrole/MoS2 nanocomposite. Polymers 2020, 12, 3047. [Google Scholar] [CrossRef]
- Sharma, S.; Hussain, S.; Singh, S.; Islam, S.S. MWCNT-conducting polymer composite based ammonia gas sensors: A new approach for complete recovery process. Sens. Actuators B Chem. 2014, 194, 213–219. [Google Scholar] [CrossRef]
- Abdulla, S.; Mathew, T.L.; Pullithadathil, B. Highly sensitive, room temperature gas sensor based on polyaniline-multiwalled carbon nanotubes (PANI/MWCNTs) nanocomposite for trace-level ammonia detection. Sens. Actuators B Chem. 2015, 221, 1523–1534. [Google Scholar] [CrossRef]
- Eising, M.; Cava, C.E.; Salvatierra, R.V.; Zarbin, A.J.; Roman, L.S. Doping effect on self-assembled films of polyaniline and carbon nanotube applied as ammonia gas sensor. Sens. Actuators B Chem. 2017, 245, 25–33. [Google Scholar] [CrossRef]
- Husain, S.; Ahmad, S.; Mohammad, F. Electrical conductivity and ammonia sensing studies on polythiophene/MWCNTs nanocomposites. Materialia 2020, 14, 100868. [Google Scholar] [CrossRef]
- Husain, A.; Ahmad, S.; Shariq, M.U.; Khan, M.M. Ultra-sensitive, highly selective and completely reversible ammonia sensor based on polythiophene/SWCNT nanocomposite. Materialia 2020, 10, 100704. [Google Scholar] [CrossRef]
- Sultan, T.; Anwer, S.; Ahmad, F. Mohammad, preparation, characterization, and dynamic adsorption—Desorption studies on polypyrrole encapsulated TiO2 nanoparticles. J. Appl. Polym. Sci. 2016, 43411, 1–11. [Google Scholar] [CrossRef]
- Sultan, S.; Ahmad, S.; Mohammad, F. Highly sensitive chlorine gas sensor and enhanced thermal DC electrical conductivity from polypyrrole/silicon carbide nanocomposites. RSC Adv. 2016, 6, 84200–84208. [Google Scholar] [CrossRef]
- Barkade, S.S.; Pinjari, D.V.; Singh, A.K.; Gogate, P.R.; Naik, J.B.; Sonawane, S.H.; Ashokkumar, M.; Pandit, A.B. Ultrasound assisted miniemulsion polymerization for preparation of polypyrrole—Zinc Oxide (PPy/ZnO) functional latex for lique fi ed petroleum gas sensing. Ind. Eng. Chem. Res. 2013, 52, 7704–7712. [Google Scholar] [CrossRef]
- Singh, K.; Nowotny, J.; Thangadurai, V. Amphoteric oxide semiconductors for energy conversion devices: A tutorial review. Chem. Soc. Rev. 2013, 42, 1961–1972. [Google Scholar] [CrossRef]
- Nuraje, N.; Asmatulu, R.; Kudaibergenov, S. Metal oxide-based functional materials for solar energy conversion: A review. Curr. Inorg. Chem. 2012, 2, 124–146. [Google Scholar] [CrossRef]
- Montemor, M.F.; Pinto, R.; Ferreira, M.G.S. Chemical composition and corrosion protection of silane films modified with CeO2 nanoparticles. Electrochim. Acta 2009, 54, 5179–5189. [Google Scholar] [CrossRef]
- Venkadesan, G.; Muthusamy, J. Experimental investigation of Al2O3/8YSZ and CeO2/8YSZ plasma sprayed thermal barrier coating on diesel engine. Ceram. Int. 2019, 45, 3166–3176. [Google Scholar] [CrossRef]
- He, G.; Fan, H.; Ma, L.; Wang, K.; Liu, C.; Ding, D.; Chen, L. Dumbbell-like ZnO nanoparticles-CeO 2 nanorods composite by one-pot hydrothermal route and their electrochemical charge storage. Appl. Surf. Sci. 2016, 366, 129–138. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Murashkina, A.A.; Maragou, V.I.; Demin, A.K.; Strekalovsky, V.N.; Tsiakaras, P.E. CeO2 based materials doped with lanthanides for applications in intermediate temperature electrochemical devices. Int. J. Hydrogen Energy 2011, 36, 6175–6183. [Google Scholar] [CrossRef]
- Scibioh, M.A.; Kim, S.K.; Cho, E.A.; Lim, T.H.; Hong, S.A.; Ha, H.Y. Pt-CeO2/C anode catalyst for direct methanol fuel cells. Appl. Catal. B Environ. 2008, 84, 773–782. [Google Scholar] [CrossRef]
- Fornasiero, P.; Balducci, G.; Kašpar, J.; Meriani, S.; di Monte, R.; Graziani, M. Metal-loaded CeO2-ZrO2 solid solutions as innovative catalysts for automotive catalytic converters. Catal. Today 1996, 29, 47–52. [Google Scholar] [CrossRef]
- Trovarelli, A. Structural and oxygen storage/release properties of CeO2-based solid solutions. Comments Inorg. Chem. 2006, 20, 263–284. [Google Scholar] [CrossRef]
- Khan, A.L.; Dhanjai; Jain, R. Fabrication and optimization of polypyrrole/cerium oxide/glassy carbon sensing platform for the electrochemical detection of flupirtine. J. Appl. Electrochem. 2020, 50, 655–672. [Google Scholar] [CrossRef]
- Karimi, S.W.; Husain, M.; Hosseini, P.A.; Azar, M.R. Ganjali, Rapid and sensitive detection of hydrogen peroxide in milk by Enzyme-free electrochemiluminescence sensor based on a polypyrrole-cerium oxide nanocomposite. Sens. Actuators B Chem. 2018, 271, 90–96. [Google Scholar] [CrossRef]
- Mohanapriya, M.K.; Deshmukh, K.; Ahamed, M.B.; Chidambaram, K.; Pasha, S.K.K. Influence of cerium oxide (CeO2) nanoparticles on the structural, morphological, mechanical and dielectric properties of PVA/PPy blend nanocomposites. Mater. Today Proc. 2016, 3, 1864–1873. [Google Scholar] [CrossRef]
- Seema, S.; Prasad, M.V.N.A. Studies on DC conductivity and LPG sensing behaviour of nanostructured polypyrrole-CeO2 composites. AIP Conf. Proc. 2018, 1989, 030019. [Google Scholar] [CrossRef]
- Benmouhoub, C.; Agrisuelas, J.; Benbrahim, N.; Pillier, F.; Gabrielli, C.; Kadri, A.; Pailleret, A.; Perrot, H.; Sel, O. Influence of the incorporation of CeO2 nanoparticles on the ion exchange behavior of dodecylsulfate doped polypyrrole films: Ac-electrogravimetry investigations. Electrochim. Acta 2014, 145, 270–280. [Google Scholar] [CrossRef][Green Version]
- Husain, M.U.; Shariq, F.; Mohammad, D.C. Electrical conductivity and liquefied petroleum gas sensing application of polythiophene/zinc oxide nanocomposite. Materialia 2020, 9, 100599. [Google Scholar] [CrossRef]
- Husain, S.; Ahmad, S.; Mohammad, F. Electrical conductivity and alcohol sensing studies on polythiophene/tin oxide nanocomposites. J. Sci. Adv. Mater. Devices 2020, 5, 84–94. [Google Scholar] [CrossRef]
- Husain, S.; Ahmad, F.; Mohammad, F. Polythiophene/graphene/zinc tungstate nanocomposite: Synthesis, characterization, DC electrical conductivity and cigarette smoke sensing application. Polym. Polym. Compos. 2020, 1–12, in press. [Google Scholar] [CrossRef]
- Husain, S.; Ahmad, S.; Mohammad, F. Thermally stable and highly sensitive ethene gas sensor based on polythiophene/zirconium oxide nanocomposites. Mater. Today Commun. 2019, 20, 100574. [Google Scholar] [CrossRef]
- Husain, S.; Ahmad, S.; Mohammad, F. Synthesis, characterisation and ethanol sensing application of polythiophene/graphene nanocomposite. Mater. Chem. Phys. 2020, 239, 122324. [Google Scholar] [CrossRef]
- Calvache-Muñoz, J.; Prado, F.A.; Rodríguez-Páez, J.E. Cerium oxide nanoparticles: Synthesis, characterization and tentative mechanism of particle formation. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 146–159. [Google Scholar] [CrossRef]
- Jayakumar, G.; Irudayaraj, A.A.; Raj, A.D. A comprehensive investigation on the properties of nanostructured cerium oxide. Opt. Quantum Electron. 2019, 51, 312. [Google Scholar] [CrossRef]
- Jayakumar, G.; Irudayaraj, A.A.; Raj, A.D. Investigation on the synthesis and photocatalytic activity of activated carbon–cerium oxide (AC–CeO2) nanocomposite. Appl. Phys. A Mater. Sci. Process. 2019, 125, 742. [Google Scholar] [CrossRef]
- Ansari, M.O.; Mohammad, F. Thermal stability and electrical properties of dodecyl-benzene-sulfonic-acid doped nanocomposites of polyaniline and multi-walled carbon nanotubes. Compos. Part B 2012, 43, 3541–3548. [Google Scholar] [CrossRef]
- Ansari, M.O.; Mohammad, F. Chemical thermal stability, electrical conductivity and ammonia sensing studies on p-toluenesulfonic acid doped polyaniline: Titanium dioxide (p TSA/Pani: TiO2) nanocomposites. Sens. Actuators B Chem. 2011, 157, 122–129. [Google Scholar] [CrossRef]
- Wang, S.; Kang, Y.; Wang, L.; Zhang, H.; Wang, Y.; Wang, Y. Organic/inorganic hybrid sensors: A review. Sens. Actuators B Chem. 2013, 182, 467–481. [Google Scholar] [CrossRef]
- Bai, H.; Shi, G. Gas sensors based on conducting polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef]






| S. No. | Materials | N-H (cm−1) | C=C (cm−1) | C-C (cm1) | C=N (cm1) | C-N (cm1) | =C-H (cm1) | C=N+-C (cm−1) | C-H (cm1) |
|---|---|---|---|---|---|---|---|---|---|
| 1. | PPy | 3430.8 | 1550.8 | 1459.9 | 1307.1 | 1187.3 | 1044.8 | 922.5 | 791.9 |
| 2. | PPy/CeO2 | 3427.7 | 1542.6 | 1448.1 | 1305.6 | 1171.3 | 1037.2 | 906.5 | 784.4 |
| S. No. | Ammonia-Concentration (vol %) | Sensing Response (%) | Response Time (s) | Recovery Time (s) | Reversibility (%) |
|---|---|---|---|---|---|
| 1. | 0.5 | 93.4 | 40 | 60 | 90.6 |
| 2. | 0.4 | 76.2 | 45 | 47 | 91.4 |
| 3. | 0.3 | 68.8 | 52 | 40 | 94.6 |
| 4. | 0.2 | 61.4 | 56 | 38 | 95.3 |
| 5. | 0.1 | 57.5 | 59 | 30 | 96.1 |
| 6. | 0.05 | 48.2 | 65 | 26 | 98.2 |
| 7. | 0.03 | 41.6 | 69 | 23 | 98.9 |
| 8. | 0.01 | 35.6 | 75 | 20 | 99.3 |
| S. No. | Material Used | Method of Preparation | Response (%) | Response Time | Recovery Time | Reference |
|---|---|---|---|---|---|---|
| 1. | PPy/RGO | Chemical oxidative | 50 at 10 ppm | 200 s | 90 s | [1] |
| 2. | SnO2/ZnO/PPy | Electro-spinning method | 0.68 at 30 ppm | 67 s | 106 s | [2] |
| 3. | PPy thin films | Solution casting | 12 at 25 ppm | 90 s | 10 min | [3] |
| 4. | PPy/Au | Chemical oxidative | 50 at 300 ppm | 40 s | 80 s | [4] |
| 5. | PPy/Zn2SnO4 | Layer-by-layer self-assembly | 82.1 at 100 ppm | 35 s | 26 s | [5] |
| 6. | PPy/MoS2 | Chemical oxidative | - | 60 s | 50 s | [17] |
| 7. | PPy/CeO2 | Chemical oxidative | 93.4 at 0.5 vol % and 35.6 at 0.01 vol % | 40 s at 0.5 vol % and 75 s at 0.01 vol % | 60 s at 0.5 vol % and 19 s at 0.01 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husain, A.; Al-Zahrani, S.A.; Al Otaibi, A.; Khan, I.; Mujahid Ali Khan, M.; Alosaimi, A.M.; Khan, A.; Hussein, M.A.; Asiri, A.M.; Jawaid, M. Fabrication of Reproducible and Selective Ammonia Vapor Sensor-Pellet of Polypyrrole/Cerium Oxide Nanocomposite for Prompt Detection at Room Temperature. Polymers 2021, 13, 1829. https://doi.org/10.3390/polym13111829
Husain A, Al-Zahrani SA, Al Otaibi A, Khan I, Mujahid Ali Khan M, Alosaimi AM, Khan A, Hussein MA, Asiri AM, Jawaid M. Fabrication of Reproducible and Selective Ammonia Vapor Sensor-Pellet of Polypyrrole/Cerium Oxide Nanocomposite for Prompt Detection at Room Temperature. Polymers. 2021; 13(11):1829. https://doi.org/10.3390/polym13111829
Chicago/Turabian StyleHusain, Ahmad, Salma Ahmed Al-Zahrani, Ahmed Al Otaibi, Imran Khan, Mohammad Mujahid Ali Khan, Abeer Mohamed Alosaimi, Anish Khan, Mahmoud Ali Hussein, Abdullah M. Asiri, and Mohammad Jawaid. 2021. "Fabrication of Reproducible and Selective Ammonia Vapor Sensor-Pellet of Polypyrrole/Cerium Oxide Nanocomposite for Prompt Detection at Room Temperature" Polymers 13, no. 11: 1829. https://doi.org/10.3390/polym13111829
APA StyleHusain, A., Al-Zahrani, S. A., Al Otaibi, A., Khan, I., Mujahid Ali Khan, M., Alosaimi, A. M., Khan, A., Hussein, M. A., Asiri, A. M., & Jawaid, M. (2021). Fabrication of Reproducible and Selective Ammonia Vapor Sensor-Pellet of Polypyrrole/Cerium Oxide Nanocomposite for Prompt Detection at Room Temperature. Polymers, 13(11), 1829. https://doi.org/10.3390/polym13111829








