Construction of the Cellulose Nanofibers (CNFs) Aerogel Loading TiO2 NPs and Its Application in Disposal of Organic Pollutants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
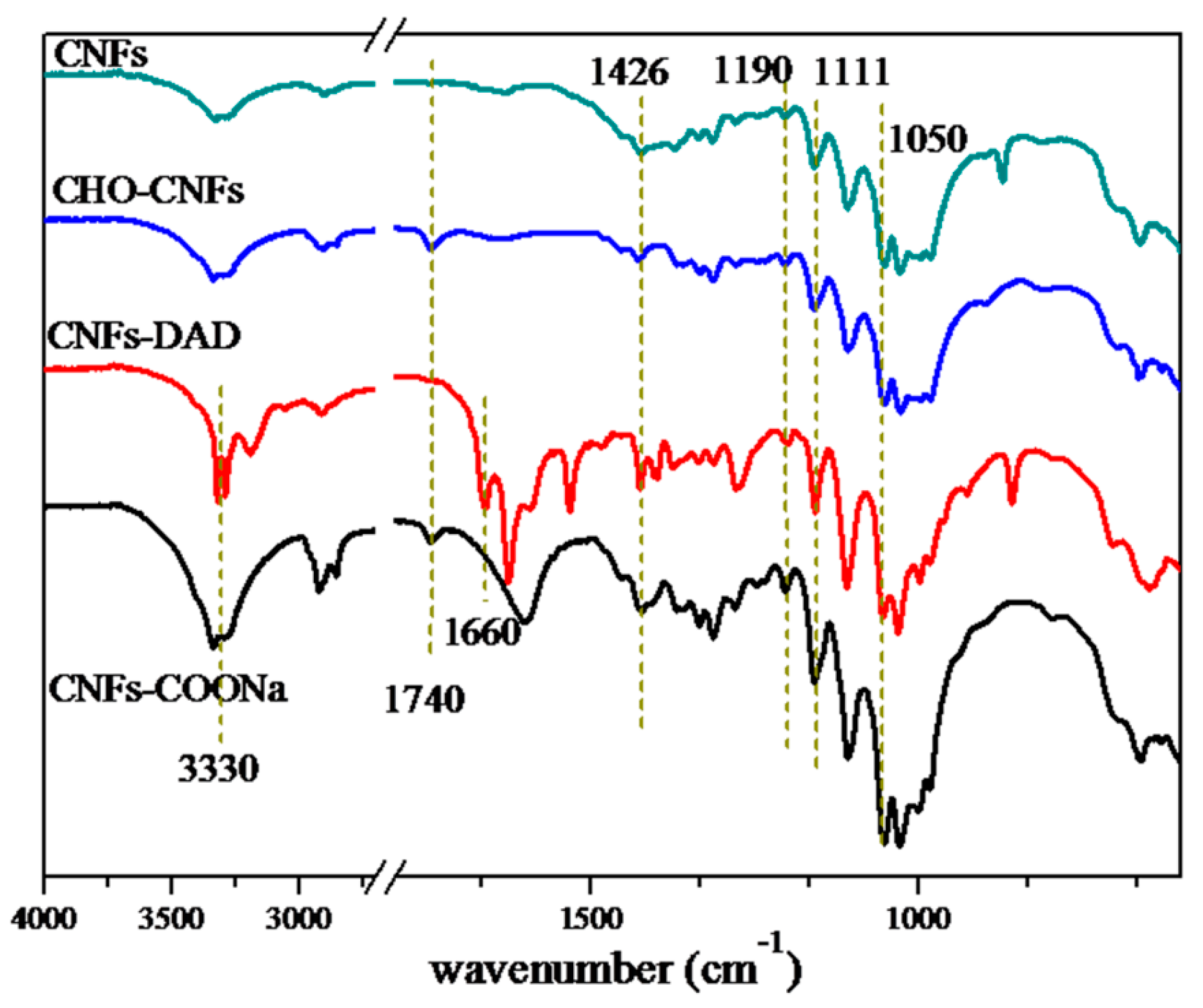
2.2. Preparation of CNF Suspension and Oxidized Cellulose Nanofibers (CNFs-COONa)
2.3. Preparation of Oxidized Cellulose Nanofibers (CNFs-CHO)
2.4. Hydrazide-Modified CNF Synthesis (CNFs-DAD)
2.5. The Preparation of Chemically Crosslinked CNF Aerogel
2.6. Synthesis of TiO2@CNF Aerogel
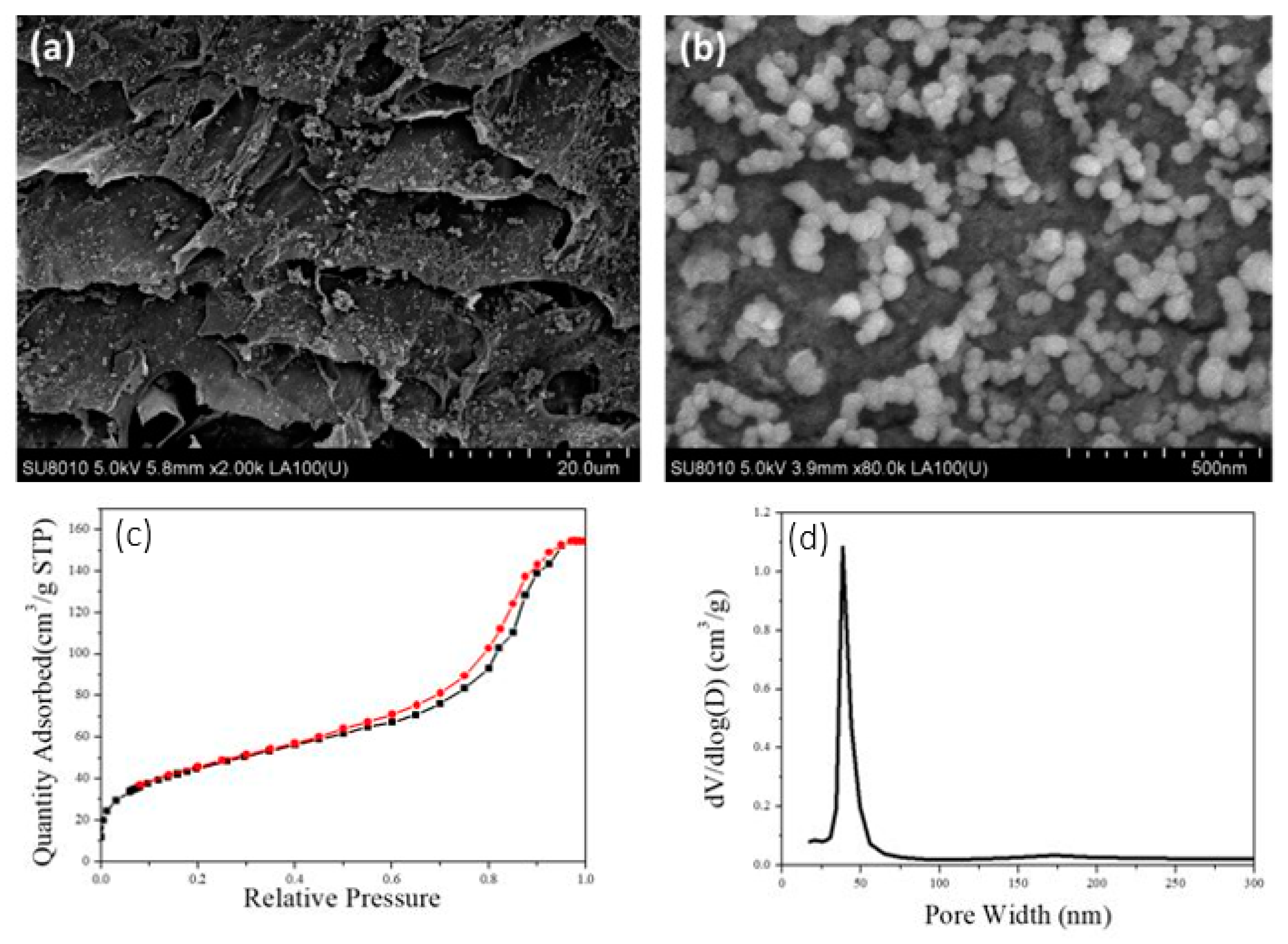
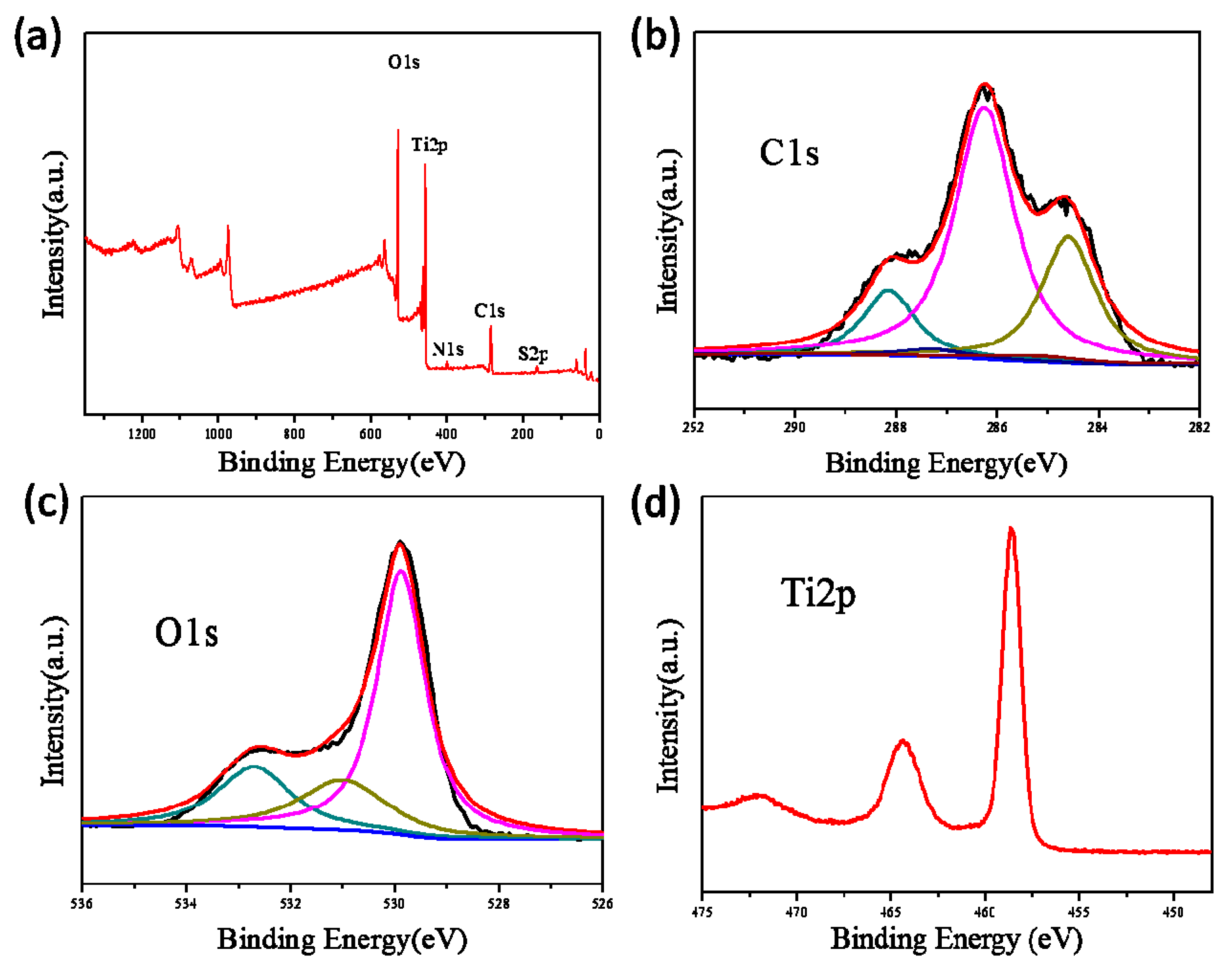
2.7. Characterization Methods
2.8. Adsorption and Degradation of Rhodamine B (RhB)
3. Results and Discussion
3.1. Characterization of the Chemically Crosslinked CNF Aerogel
3.2. Characterization of the TiO2@CNF Aerogels
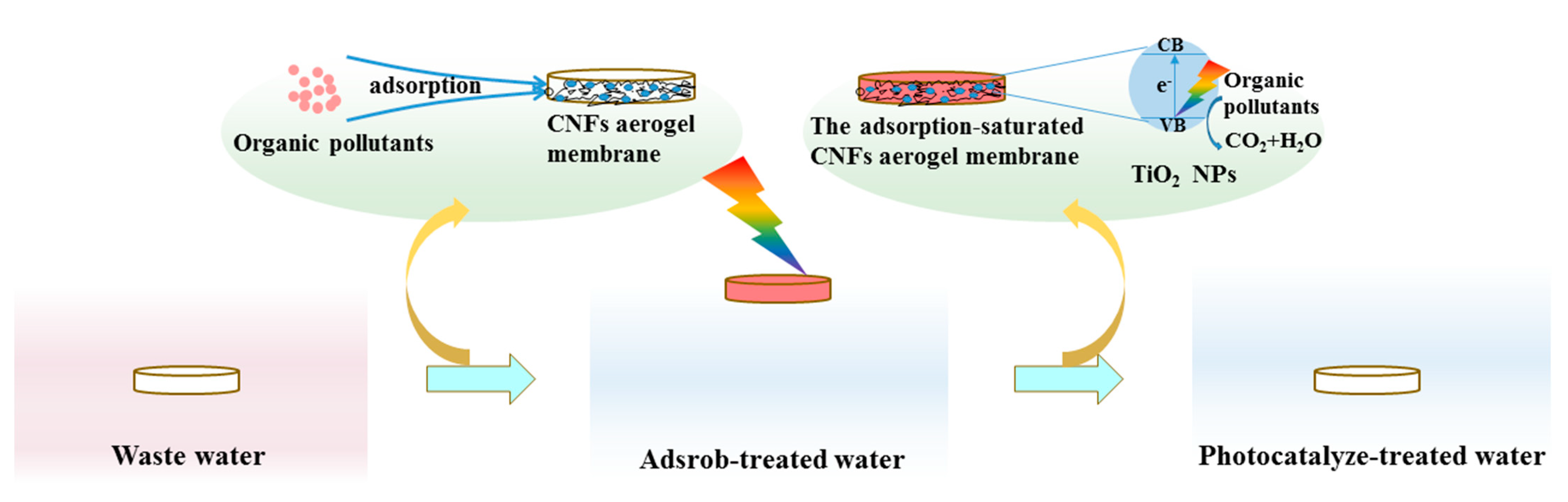
3.3. Application of CNF Aerogel and TiO2@CNF Aerogel—Adsorption and Degradation of RhB
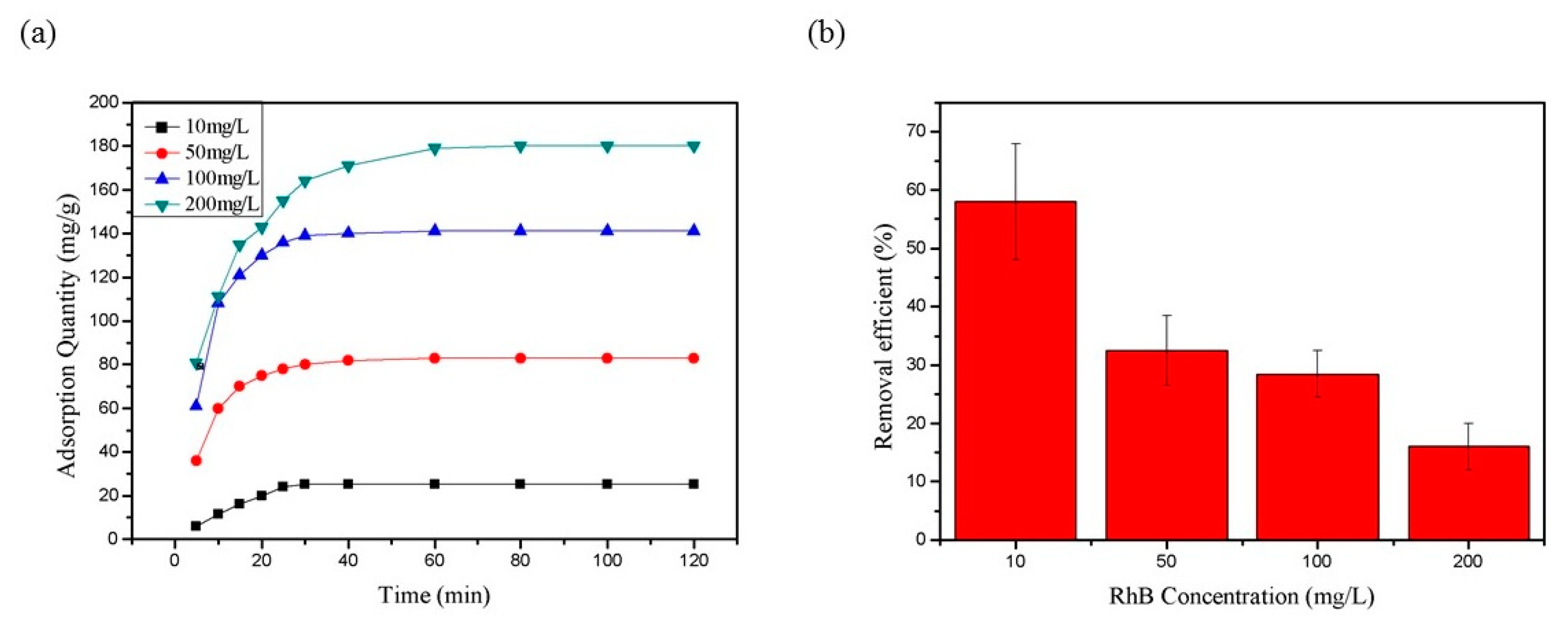
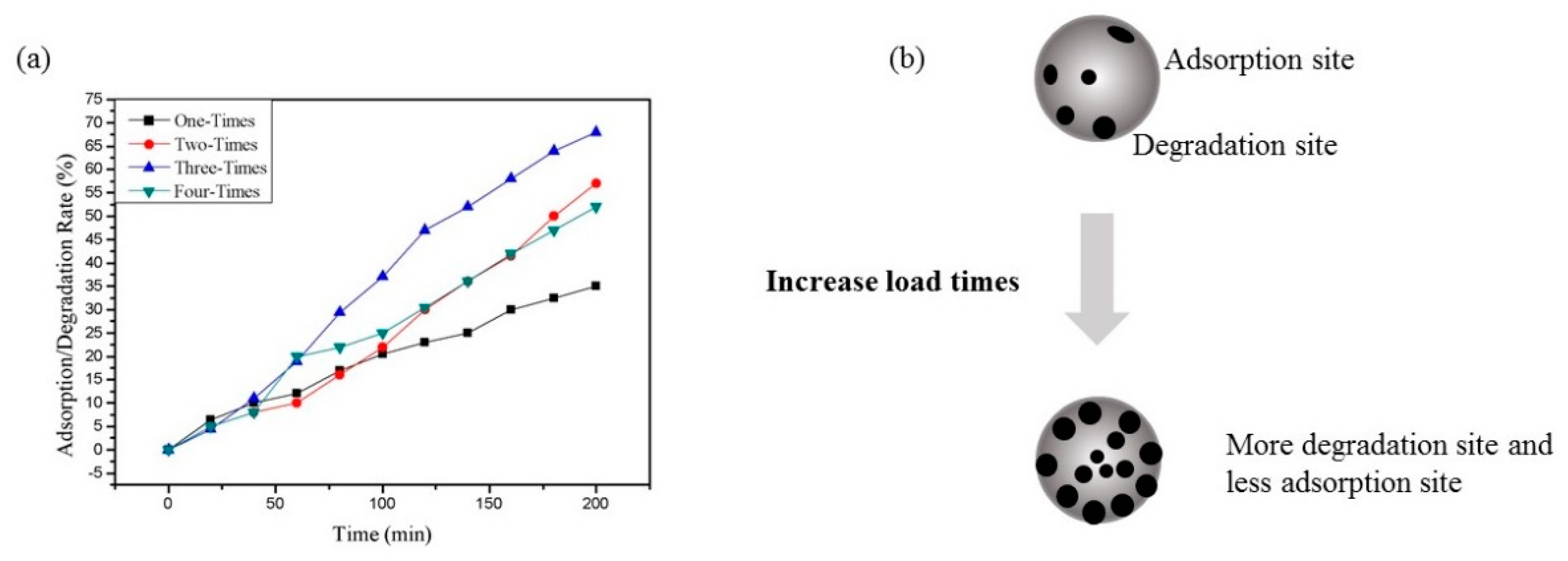
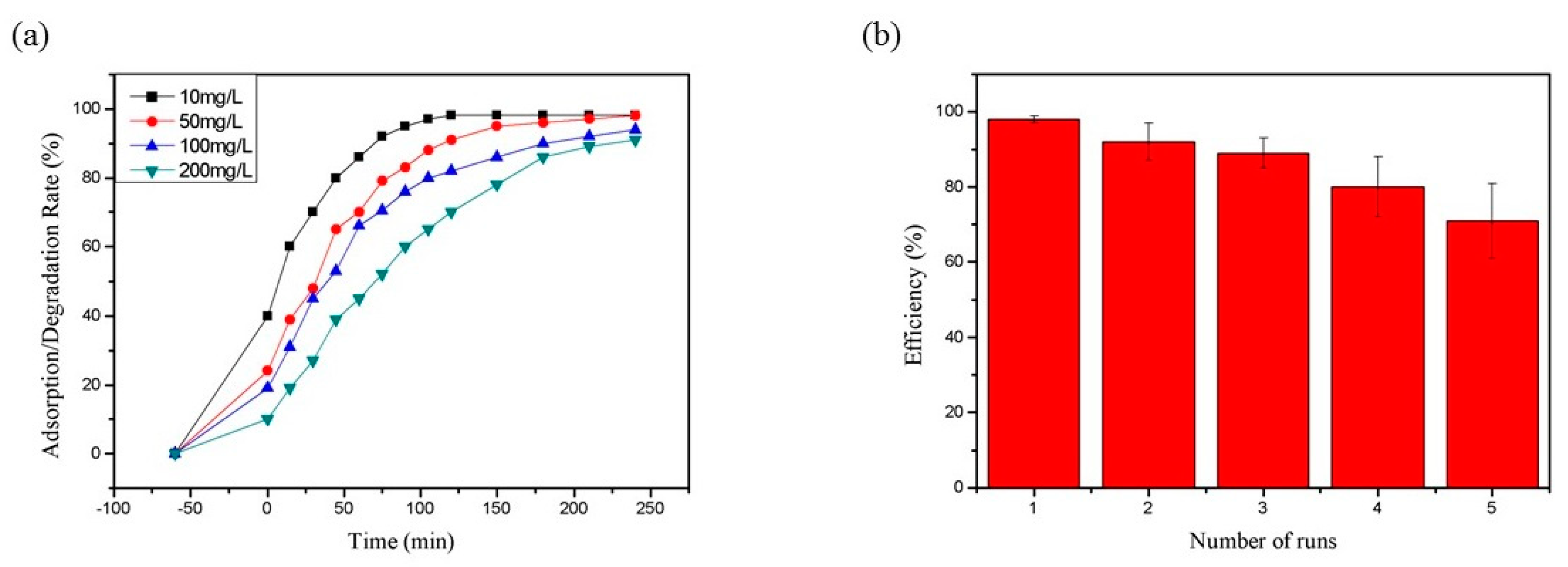
3.3.1. Adsorption of RhB by CNF Aerogel
3.3.2. Degradation of RhB by TiO2@CNF Aerogel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Isari, A.A.; Payan, A.; Fattahi, M.; Jorfi, S.; Kakavandi, B. Photocatalytic degradation of rhodamine B and real textile wastewater using Fe-doped TiO2 anchored on reduced graphene oxide (Fe-TiO2/rGO): Characterization and feasibility, mechanism and pathway studies. Appl. Surf. Sci. 2018, 462, 549–564. [Google Scholar] [CrossRef]
- Li, D.; Tian, X.; Wang, Z.; Guan, Z.; Li, X.; Qiao, H.; Ke, H.; Luo, L.; Wei, Q. Multifunctional adsorbent based on metal-organic framework modified bacterial cellulose/chitosan composite aerogel for high efficient removal of heavy metal ion and organic pollutant. Chem. Eng. J. 2020, 383, 123127. [Google Scholar] [CrossRef]
- Suazo-Hernández, J.; Sepúlveda, P.; Manquián-Cerda, K.; Ramírez-Tagle, R.; Rubio, M.A.; Bolan, N.; Sarkar, B.; Arancibia-Miranda, N. Synthesis and characterization of zeolite-based composites functionalized with nanoscale zero-valent iron for removing arsenic in the presence of selenium from water. J. Hazard. Mater. 2019, 373, 810–819. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Geng, L.-H.; Amiralian, N.; Martin, D.J.; Hsiao, B.S. Nanocellulose from Spinifex as an Effective Adsorbent to Remove Cadmium(II) from Water. ACS Sustain. Chem. Eng. 2018, 6, 3279–3290. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Hsiao, B.S. Efficient Removal of UO22+ from Water Using Carboxycellulose Nanofibers Prepared by the Nitro-Oxidation Method. Ind. Eng. Chem. Res. 2017, 56, 13885–13893. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Zhan, C.; Sharma, S.K.; Geng, L.; Hsiao, B.S. Lead removal from water using carboxycellulose nanofibers prepared by nitro-oxidation method. Cellulose 2018, 25, 1961–1973. [Google Scholar] [CrossRef]
- Sharma, P.R.; Joshi, R.; Sharma, S.K.; Hsiao, B.S. A Simple Approach to Prepare Carboxycellulose Nanofibers from Untreated Biomass. Biomacromolecules 2017, 18, 2333–2342. [Google Scholar] [CrossRef]
- Sharma, S.K.; Sharma, P.R.; Chen, H.; Johnson, K.; Zhan, C.; Wang, R.; Hsiao, B. Cellulose-Supported Nanosized Zinc Oxide: Highly Efficient Bionanomaterial for Removal of Arsenic from Water; ACS Symp. Ser. American Chemical Society: Washington, DC, USA, 2020; pp. 253–267. [Google Scholar]
- Zhu, Z.; Zhong, L.; Zhang, Z.; Li, H.; Shi, W.; Cui, F.; Wang, W. Gravity driven ultrafast removal of organic contaminants across catalytic superwetting membranes. J. Mater. Chem. A 2017, 5, 25266–25275. [Google Scholar] [CrossRef]
- Yan, J.; Peng, J.; Lai, L.; Ji, F.; Zhang, Y.; Lai, B.; Chen, Q.; Yao, G.; Chen, X.; Song, L. Activation CuFe2O4 by Hydroxylamine for Oxidation of Antibiotic Sulfamethoxazole. Environ. Sci. Technol. 2018, 52, 14302–14310. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, S.; Sadeghpour, A.; Malfait, W.J.; Konishi, A.; Otake, K.; Yoda, S. Formation of Nanofibrous Structure in Biopolymer Aerogel during Supercritical CO2 Processing: The Case of Chitosan Aerogel. Biomacromolecules 2019, 20, 2051–2057. [Google Scholar] [CrossRef]
- Alburquerque, N.G.; Zhao, S.; Adilien, N.; Koebel, M.M.; Lattuada, M.; Malfait, W.J. Strong, Machinable, and Insulating Chitosan–Urea Aerogels: Toward Ambient Pressure Drying of Biopolymer Aerogel Monoliths. ACS Appl. Mater. Interfaces 2020, 12, 22037–22049. [Google Scholar] [CrossRef]
- Du, H.; Liu, W.; Zhang, M.; Si, C.; Zhang, X.; Li, B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydr. Polym. 2019, 209, 130–144. [Google Scholar] [CrossRef]
- Ye, D.; Yang, P.; Lei, X.; Zhang, D.; Li, L.; Chang, C.; Sun, P.; Zhang, L. Robust Anisotropic Cellulose Hydrogels Fabricated via Strong Self-aggregation Forces for Cardiomyocytes Unidirectional Growth. Chem. Mater. 2018, 30, 5175–5183. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Liu, Y.; Lai, W.; Huang, F.; Ou, A.; Qin, R.; Liu, X.; Wang, X. Ester Crosslinking Enhanced Hydrophilic Cellulose Nanofibrils Aerogel. ACS Sustain. Chem. Eng. 2018, 6, 11979–11988. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Lu, C.; Deng, Y. Aerogels from crosslinked cellulose nano/micro-fibrils and their fast shape recovery property in water. J. Mater. Chem. 2012, 22, 11642–11650. [Google Scholar] [CrossRef]
- Yang, X.; Cranston, E.D. Chemically Cross-Linked Cellulose Nanocrystal Aerogels with Shape Recovery and Superabsorbent Properties. Chem. Mater. 2014, 26, 6016–6025. [Google Scholar] [CrossRef]
- Du, M.; Du, Y.; Feng, Y.; Yang, K.; Lv, X.; Jiang, N.; Liu, Y. Facile preparation of BiOBr/cellulose composites by in situ synthesis and its enhanced photocatalytic activity under visible-light. Carbohydr. Polym. 2018, 195, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Kampouri, S.; Nguyen, T.N.; Spodaryk, M.; Palgrave, R.G.; Züttel, A.; Smit, B.; Stylianou, K.C. Concurrent Photocatalytic Hydrogen Generation and Dye Degradation Using MIL-125-NH2 under Visible Light Irradiation. Adv. Funct. Mater. 2018, 28, 1806368. [Google Scholar] [CrossRef]
- Zhan, C.; Li, Y.; Sharma, P.R.; He, H.; Sharma, S.K.; Wang, R.; Hsiao, B.S. A study of TiO2 nanocrystal growth and environmental remediation capability of TiO2/CNC nanocomposites. RSC Adv. 2019, 9, 40565–40576. [Google Scholar] [CrossRef]
- Liu, W.; Cai, J.; Ding, Z.; Li, Z. TiO2/RGO composite aerogels with controllable and continuously tunable surface wettability for varied aqueous photocatalysis. Appl. Catal. B Environ. 2015, 174–175, 421–426. [Google Scholar] [CrossRef]
- Haarstrick, A.; Kut, O.M.; Heinzle, E. TiO2-Assisted Degradation of Environmentally Relevant Organic Compounds in Wastewater Using a Novel Fluidized Bed Photoreactor. Environ. Sci. Technol. 1996, 30, 817–824. [Google Scholar] [CrossRef]
- Zhang, L.; Kanki, T.; Sano, N.; Toyoda, A. Development of TiO2 photocatalyst reaction for water purification. Sep. Purif. Technol. 2003, 31, 105–110. [Google Scholar] [CrossRef]
- Beck-Candanedo, S.; Roman, A.M.; Gray, D.G. Effect of Reaction Conditions on the Properties and Behavior of Wood Cellulose Nanocrystal Suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. TEMPO-Mediated Oxidation of Native Cellulose. The Effect of Oxidation Conditions on Chemical and Crystal Structures of the Water-Insoluble Fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Larsson, P.A.; Gimåker, M.; Wågberg, L. The influence of periodate oxidation on the moisture sorptivity and dimensional stability of paper. Cellulose 2008, 15, 837–847. [Google Scholar] [CrossRef]
- Rodriguez-Docampo, Z.; Otto, S. Orthogonal or simultaneous use of disulfide and hydrazone exchange in dynamic covalent chemistry in aqueous solution. Chem. Commun. 2008, 42, 5301–5303. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Kim, H.C.; Kim, H.Y.; Chung, Y.S.; Park, W.H.; Youk, J.H. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Reunchan, P.; Ouyang, S.; Tong, H.; Umezawa, N.; Kako, T.; Ye, J. Anatase TiO2 Single Crystals Exposed with High-Reactive {111} Facets Toward Efficient H2 Evolution. Chem. Mater. 2013, 25, 405–411. [Google Scholar] [CrossRef]
- Qiu, B.; Zhou, Y.; Ma, Y.; Yang, X.; Sheng, W.; Xing, M.; Zhang, J. Facile synthesis of the Ti3+ self-doped TiO2-graphene nanosheet composites with enhanced photocatalysis. Sci. Rep. 2015, 5, 8591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pan, C.; Fang, P.; Wei, J.; Xiong, R. Mo + C Codoped TiO2 Using Thermal Oxidation for Enhancing Photocatalytic Activity. ACS Appl. Mater. Interfaces 2010, 2, 1173–1176. [Google Scholar] [CrossRef]





| Composite Material | Adsorption Performance | Degradation Performance | Reference |
|---|---|---|---|
| Carboxycellulose nanofibers | ★★★★ | [4] | |
| Carboxycellulose nanofibers | ★★★★ | [5] | |
| ZnO/Microfibrillated Cellulose | ★★★★ | [8] | |
| BiOBr/cellulose composite | ★★ | ★★★★★ | [21] |
| TiO2/CNC nanocomposites | ★ | ★★★★★ | [20] |
| TiO2@CNF Aerogel | ★★★★ | ★★★★★ | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Zhang, X.; Qin, Y.; Li, Y. Construction of the Cellulose Nanofibers (CNFs) Aerogel Loading TiO2 NPs and Its Application in Disposal of Organic Pollutants. Polymers 2021, 13, 1841. https://doi.org/10.3390/polym13111841
Li K, Zhang X, Qin Y, Li Y. Construction of the Cellulose Nanofibers (CNFs) Aerogel Loading TiO2 NPs and Its Application in Disposal of Organic Pollutants. Polymers. 2021; 13(11):1841. https://doi.org/10.3390/polym13111841
Chicago/Turabian StyleLi, Kang, Xuejie Zhang, Yan Qin, and Ying Li. 2021. "Construction of the Cellulose Nanofibers (CNFs) Aerogel Loading TiO2 NPs and Its Application in Disposal of Organic Pollutants" Polymers 13, no. 11: 1841. https://doi.org/10.3390/polym13111841
APA StyleLi, K., Zhang, X., Qin, Y., & Li, Y. (2021). Construction of the Cellulose Nanofibers (CNFs) Aerogel Loading TiO2 NPs and Its Application in Disposal of Organic Pollutants. Polymers, 13(11), 1841. https://doi.org/10.3390/polym13111841






