Collagen–Alginate Composite Hydrogel: Application in Tissue Engineering and Biomedical Sciences
Abstract
:1. Introduction
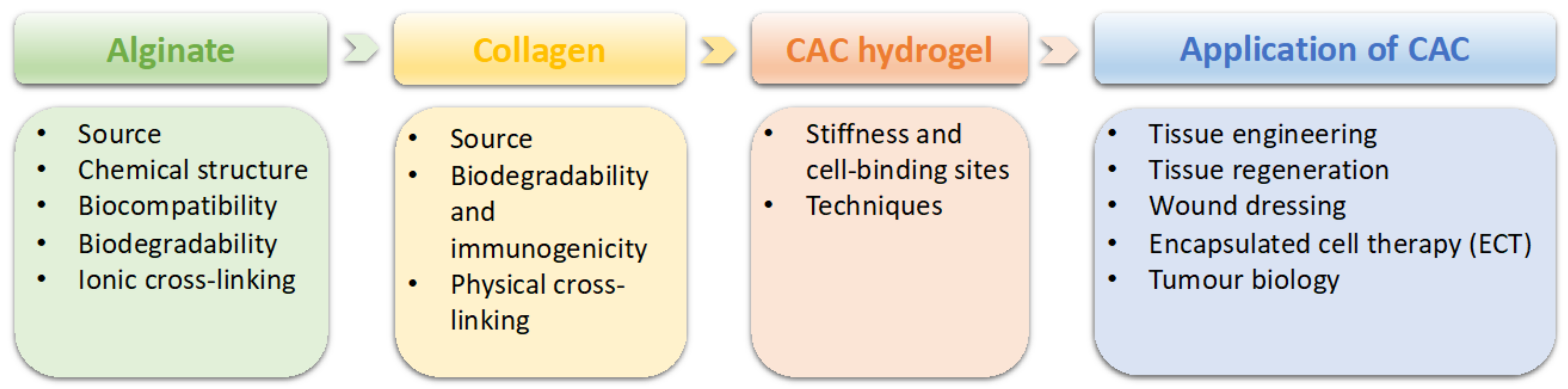
2. General Properties of Alginate and Collagen
2.1. Alginate
2.1.1. Alginate Sources
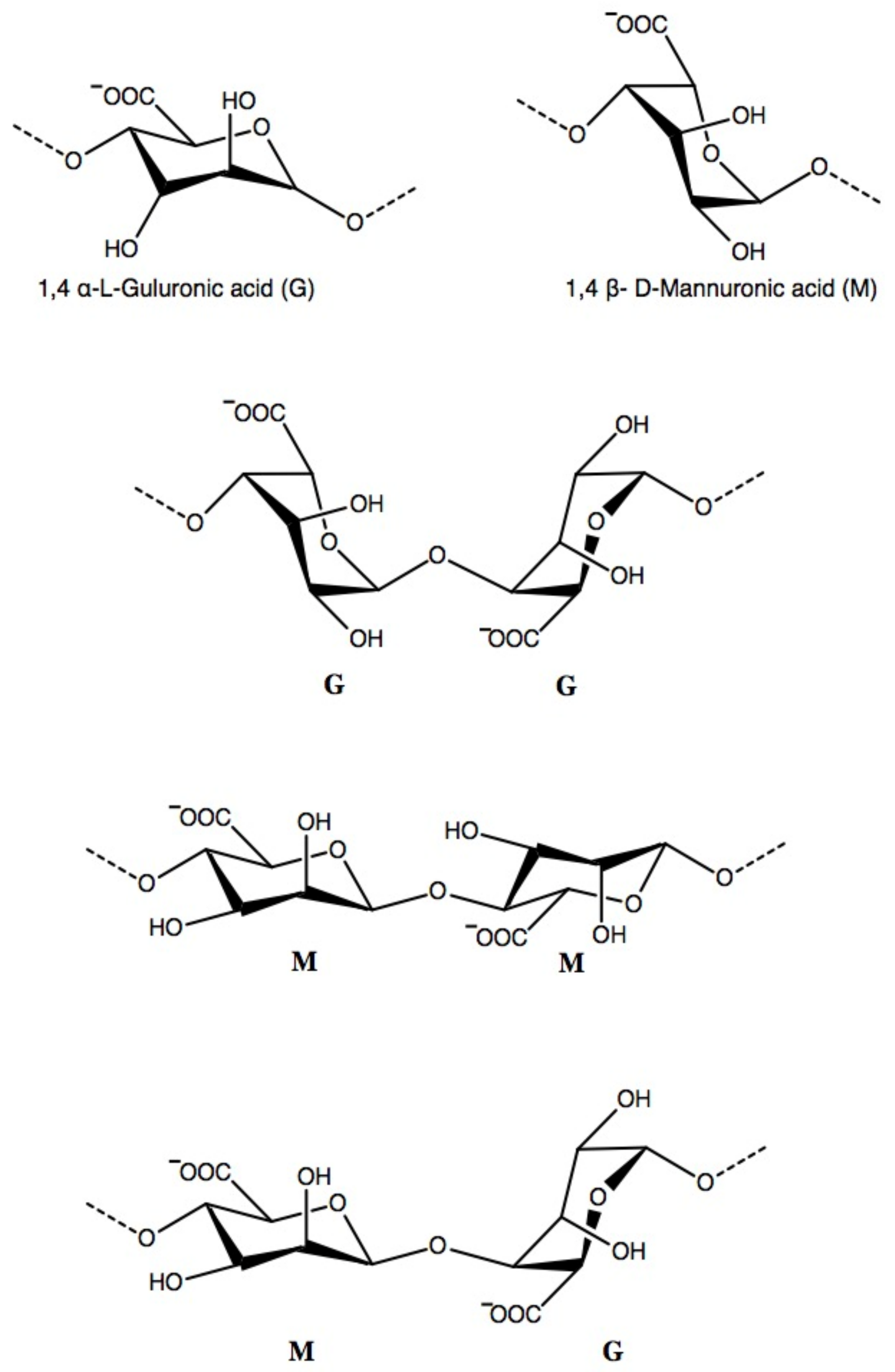
2.1.2. Chemical Structure and Molecular Weight
2.1.3. Biocompatibility and Biodegradability
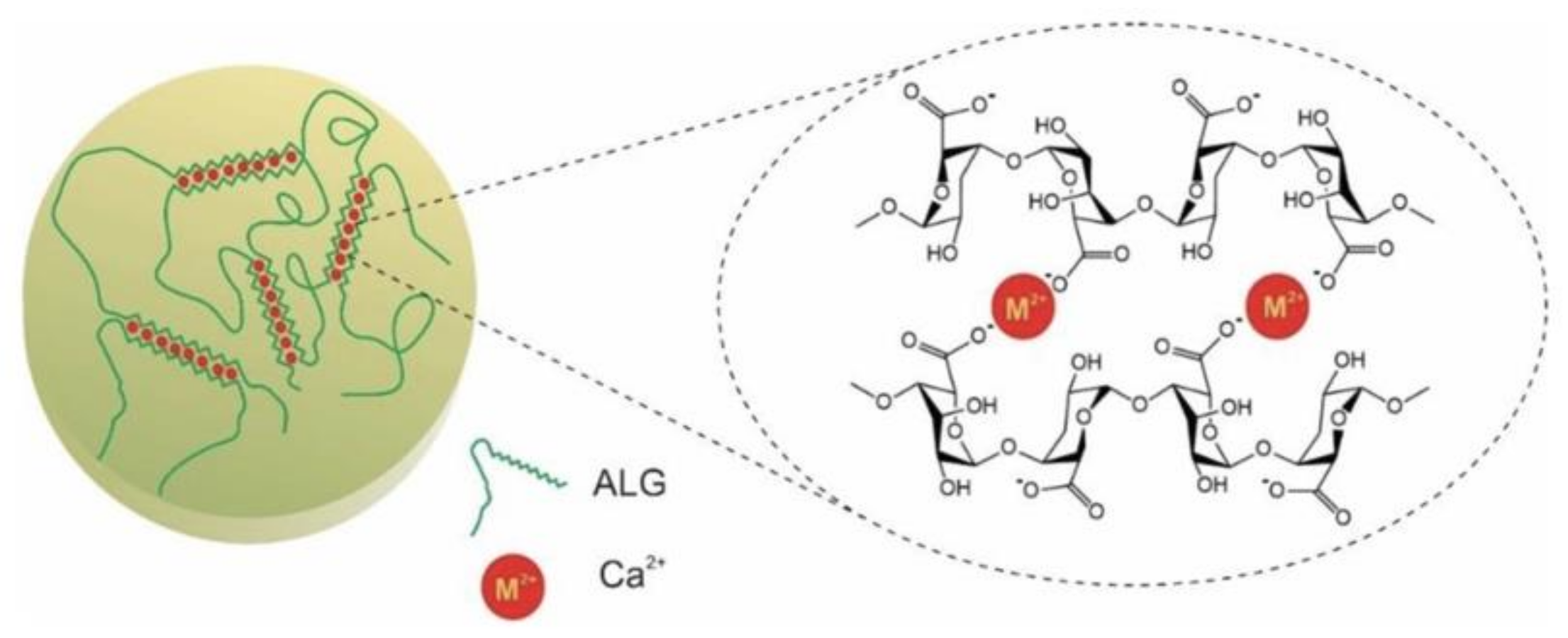
2.1.4. Ionic Cross-Linking
2.2. Collagen
2.2.1. Collagen Source
2.2.2. Biodegradability and Immunogenicity
2.2.3. Physical Cross-Linking
2.3. Collagen–Alginate Composite Hydrogel
2.3.1. Stiffness and Cell-Binding Sites
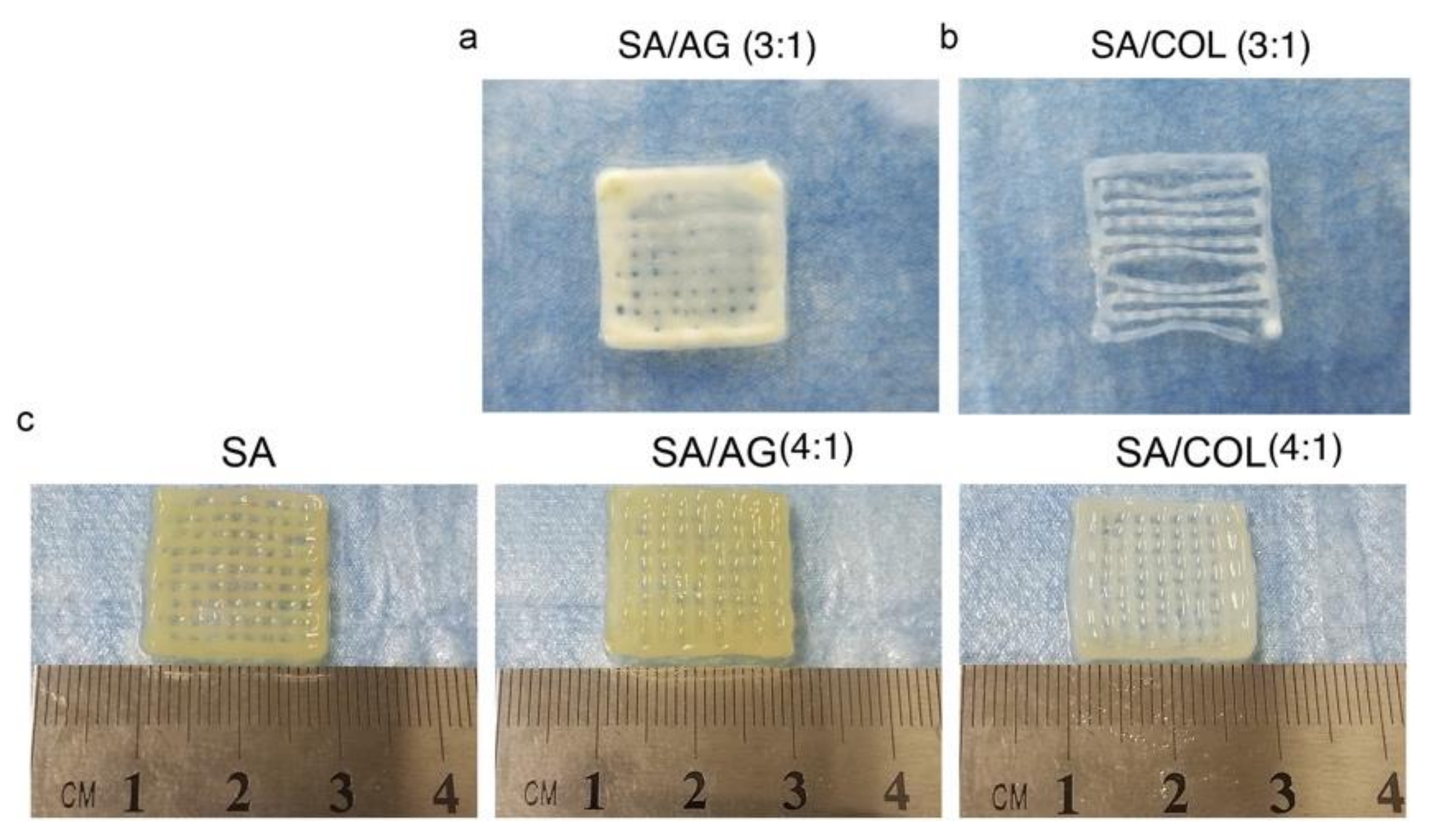
2.3.2. Technique
Three-Dimensional (3D) Bioprinting
3. Application of CAC Hydrogel
3.1. Tissue Engineering
3.1.1. Bone Tissue Engineering
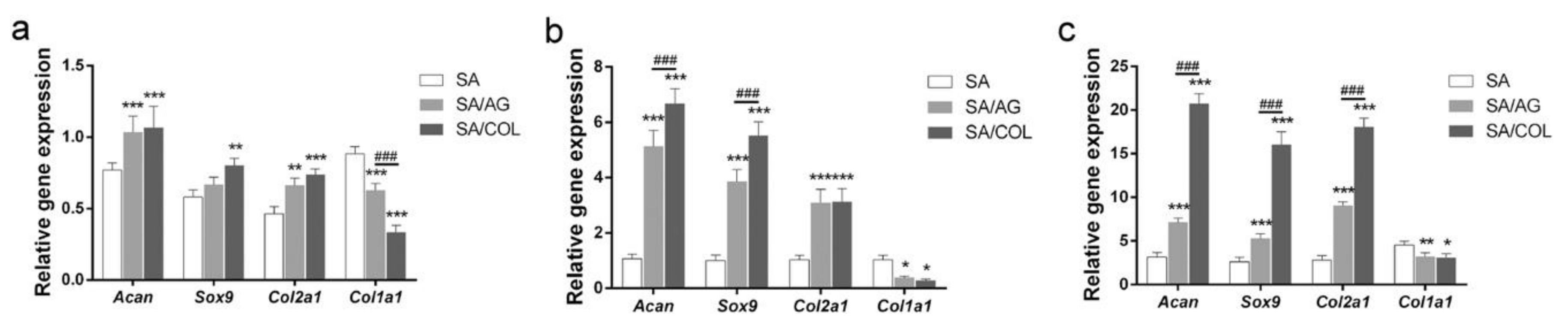
3.1.2. Cartilage Tissue Engineering
3.1.3. Intervertebral Disc (IVD)
3.2. Tissue Regeneration
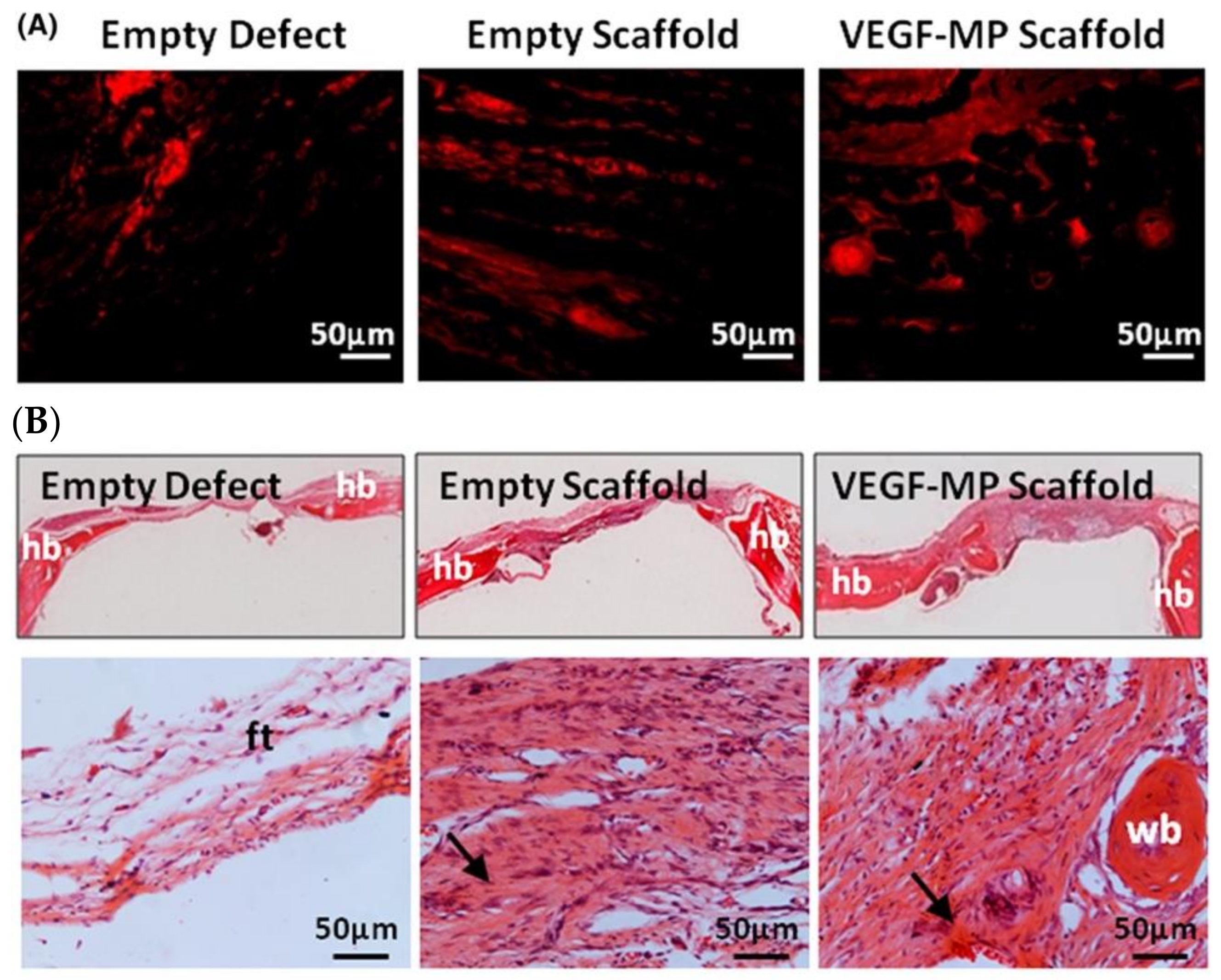
3.2.1. Blood Vessel
3.2.2. Neuron
3.2.3. Liver
3.2.4. Vocal Folds Restoration
3.3. Biomedical Sciences
3.3.1. Wound Dressing
3.3.2. Encapsulated Cell Therapy
Islet Cells
GDNF-Secreting HEK Cells
3.3.3. Tumor Biology
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hubbell, J.A. Biomaterials in tissue engineering. Bio-technology 1995, 13, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Huebsch, N.; Mooney, D.J. Inspiration and application in the evolution of biomaterials. Nature 2009, 462, 426–432. [Google Scholar] [CrossRef] [Green Version]
- Ratner, B.D.; Bryant, S.J. Biomaterials: Where we have been and where we are going. Annu. Rev. Biomed. Eng. 2004, 6, 41–75. [Google Scholar] [CrossRef]
- Ullah, S.; Chen, X. Fabrication, applications and challenges of natural biomaterials in tissue engineering. Appl. Mater. Today 2020, 20, 100656. [Google Scholar] [CrossRef]
- Porter, R.M.; Akers, R.M.; Howard, R.D.; Forsten-Williams, K. Alginate encapsulation impacts the insulin-like growth factor-I system of monolayer-expanded equine articular chondrocytes and cell response to interleukin-1 beta. Tissue Eng. 2007, 13, 1333–1345. [Google Scholar] [CrossRef]
- Wee, S.; Gombotz, W.R. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 1998, 31, 267–285. [Google Scholar] [PubMed]
- Kim, W.S.; Mooney, D.J.; Arany, P.R.; Lee, K.; Huebsch, N.; Kim, J. Adipose tissue engineering using injectable, oxidized alginate hydrogels. Tissue Eng. Part A 2012, 18, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Nishimura, Y.; Tanihara, M.; Suzuki, K.; Nakamura, T.; Shimizu, Y.; Yamawaki, Y.; Kakimaru, Y. Evaluation of a novel alginate gel dressing: Cytotoxicity to fibroblasts in vitro and foreign-body reaction in pig skin in vivo. J. Biomed. Mater. Res. 1998, 39, 317–322. [Google Scholar] [CrossRef]
- Peters, M.C.; Polverini, P.J.; Mooney, D.J. Engineering vascular networks in porous polymer matrices. J. Biomed. Mater. Res. 2002, 60, 668–678. [Google Scholar] [CrossRef] [PubMed]
- You, J.O.; Liu, Y.C.; Peng, C.A. Efficient gene transfection using chitosan-alginate core-shell nanoparticles. Int. J. Nanomed. 2006, 1, 173–180. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.W.; Juliano, R. Mitogenic signal transduction by integrin—And growth factor receptor—Mediated pathways. Mol. Cells 2004, 17, 188–202. [Google Scholar]
- Burgeson, R.E.; Nimni, M.E. Collagen types—Molecular-structure and tissue Distribution. Clin. Orthop. Relat. R. 1992, 282, 250–272. [Google Scholar]
- Ramachandran, G.N. Structure of collagen at the molecular level. In Treatise on Collagen; Ramachandran, G.N., Ed.; Academic Press: New York, NJ, USA, 1967; pp. 103–183. [Google Scholar]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hesse, E.; Hefferan, T.E.; Tarara, J.E.; Haasper, C.; Meller, R.; Krettek, C.; Lu, L.C.; Yaszemski, M.J. Collagen type I hydrogel allows migration, proliferation, and osteogenic differentiation of rat bone marrow stromal cells. J. Biomed. Mater. Res. A 2010, 94, 442–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynn, A.K.; Yannas, I.V.; Bonfield, W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. Part B 2004, 71B, 343–354. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Nagarajan, S.; Bechelany, M.; Kalkura, S.N. Collagen based biomaterials for tissue engineering applications: A review. In Processes and Phenomena on the Boundary between Biogenic and Abiogenic Nature—Lecture Notes in Earth System Sciences; Frank-Kamenetskaya, O., Vlasov, D., Panova, E., Lessovaia, S., Eds.; Springer: Cham, Switzerland, 2020; pp. 3–22. [Google Scholar]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Review of collagen i hydrogels for bioengineered tissue microenvironments: Characterization of mechanics, structure, and transport. Tissue Eng. Part B Rev. 2014, 20, 683–696. [Google Scholar] [CrossRef] [Green Version]
- Heino, J. Cellular signaling by collagen-binding integrins. I Domain Integrins 2014, 819, 143–155. [Google Scholar]
- Holder, A.J.; Badiei, N.; Hawkins, K.; Wright, C.; Williams, P.R.; Curtis, D.J. Control of collagen gel mechanical properties through manipulation of gelation conditions near the sol-gel transition. Soft Matter 2018, 14, 574–580. [Google Scholar] [CrossRef] [Green Version]
- Wong, F.S.Y.; Wong, C.C.H.; Chan, B.P.; Lo, A.C.Y. Sustained delivery of bioactive GDNF from collagen and alginate-based cell-encapsulating gel promoted photoreceptor survival in an inherited retinal degeneration model. PLoS ONE 2016, 11, e0159342. [Google Scholar] [CrossRef] [Green Version]
- Wong, F.S.Y.; Tsang, K.K.; Chu, A.M.W.; Chan, B.P.; Yao, K.M.; Lo, A.C.Y. Injectable cell-encapsulating composite alginate-collagen platform with inducible termination switch for safer ocular drug delivery. Biomaterials 2019, 201, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.G.; Griffith, M.; Li, F.F. Alginate microsphere-collagen composite hydrogel for ocular drug delivery and implantation. J. Mater. Sci. Mater. Med. 2008, 19, 3365–3371. [Google Scholar] [CrossRef]
- Moxon, S.R.; Corbett, N.J.; Fisher, K.; Potjewyd, G.; Domingos, M.; Hooper, N.M. Blended alginate/collagen hydrogels promote neurogenesis and neuronal maturation. Mat. Sci. Eng. C 2019, 104, 109904. [Google Scholar] [CrossRef]
- Ledo, A.M.; Vining, K.H.; Alonso, M.J.; Garcia-Fuentes, M.; Mooney, D.J. Extracellular matrix mechanics regulate transfection and SOX9-directed differentiation of mesenchymal stem cells. Acta Biomater. 2020, 110, 153–163. [Google Scholar] [CrossRef]
- Strand, B.L.; Morch, Y.A.; Skjak-Braek, G. Alginate as immobilization matrix for cells. Minerva Biotecnol. 2000, 12, 223–233. [Google Scholar]
- Smidsrod, O.; Skjakbraek, G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990, 8, 71–78. [Google Scholar] [CrossRef]
- Rinaudo, M. Main properties and current applications of some polysaccharides as biomaterials. Polym. Int. 2008, 57, 397–430. [Google Scholar] [CrossRef]
- Remminghorst, U.; Rehm, B.H.A. Bacterial alginates: From biosynthesis to applications. Biotechnol. Lett. 2006, 28, 1701–1712. [Google Scholar] [CrossRef]
- Martinsen, A.; Skjakbraek, G.; Smidsrod, O. Alginate as immobilization material.1. Correlation between chemical and physical-properties of alginate gel beads. Biotechnol. Bioeng. 1989, 33, 79–89. [Google Scholar] [CrossRef]
- Tonnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- LeRoux, M.A.; Guilak, F.; Setton, L.A. Compressive and shear properties of alginate gel: Effects of sodium ions and alginate concentration. J. Biomed. Mater. Res. 1999, 47, 46–53. [Google Scholar] [CrossRef]
- Kong, H.J.; Smith, M.K.; Mooney, D.J. Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials 2003, 24, 4023–4029. [Google Scholar] [CrossRef]
- Orive, G.; Carcaboso, A.M.; Hernandez, R.M.; Gascon, A.R.; Pedraz, J.L. Biocompatibility evaluation of different alginates and alginate-based microcapsules. Biomacromolecules 2005, 6, 927–931. [Google Scholar] [CrossRef]
- Lee, J.; Lee, K.Y. Local and sustained vascular endothelial growth factor delivery for angiogenesis using an injectable system. Pharm Res. 2009, 26, 1739–1744. [Google Scholar] [CrossRef]
- Mushollaeni, W.; Supartini, N.; Rusdiana, E. Toxicity test of alginate from Sargassum and Padina on the liver of mice. Food Public Health 2014, 4, 204–208. [Google Scholar]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological interactions between polysaccharides and divalent cations—Egg-box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.K.; Ma, P.X. Ionically cross-linked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Mourino, V.; Newby, P.; Pishbin, F.; Cattalini, J.P.; Lucangioli, S.; Boccaccini, A.R. Physicochemical, biological and drug-release properties of gallium cross-linked alginate/nanoparticulate bioactive glass composite films. Soft Matter 2011, 7, 6705–6712. [Google Scholar] [CrossRef]
- Hijazi, S.; Visaggio, D.; Pirolo, M.; Frangipani, E.; Bernstein, L.; Visca, P. Antimicrobial activity of gallium compounds on ESKAPE pathogens. Front. Cell Infect. Microbiol. 2018, 8, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chitambar, C.R. Gallium and its competing roles with iron in biological systems. BBA Mol. Cell Res. 2016, 1863, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Rastin, H.; Ramezanpour, M.; Hassan, K.; Mazinani, A.; Tung, T.T.; Vreugde, S.; Losic, D. 3D bioprinting of a cell-laden antibacterial polysaccharide hydrogel composite. Carbohydr. Polym. 2021, 264, 117989. [Google Scholar] [CrossRef] [PubMed]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-based biomaterials for tissue engineering applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef] [Green Version]
- Vanderrest, M.; Garrone, R. Collagen family of proteins. FASEB J. 1991, 5, 2814–2823. [Google Scholar] [CrossRef] [Green Version]
- Prockop, D.J.; Kivirikko, K.I. Collagens—Molecular-Biology, diseases, and potentials for therapy. Annu. Rev. Biochem. 1995, 64, 403–434. [Google Scholar] [CrossRef] [PubMed]
- Chevallay, B.; Herbage, D. Collagen-based biomaterials as 3D scaffold for cell cultures: Applications for tissue engineering and gene therapy. Med. Biol. Eng. Comput. 2000, 38, 211–218. [Google Scholar] [CrossRef]
- Wolf, K.; Alexander, S.; Schacht, V.; Coussens, L.M.; von Andrian, U.H.; van Rheenen, J.; Deryugina, E.; Friedl, P. Collagen-based cell migration models in vitro and in vivo. Semin. Cell Dev. Biol. 2009, 20, 931–941. [Google Scholar] [CrossRef] [Green Version]
- Gelse, K.; Poschl, E.; Aigner, T. Collagens—structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [Green Version]
- Osidak, E.O.; Kozhukhov, V.I.; Osidak, M.S.; Domogatsky, S.P. Collagen as bioink for bioprinting: A comprehensive review. Int. J. Bioprint. 2020, 6, 270. [Google Scholar]
- Badylak, S.F. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl. Immunol. 2004, 12, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.A.; Taylor, N.L.; Jalan, A.A.; Hwang, L.K.; Wang, B.K.; Hartgerink, J.D. A Nanostructured synthetic collagen mimic for hemostasis. Biomacromolecules 2014, 15, 1484–1490. [Google Scholar] [CrossRef]
- Xu, X.; Gan, Q.L.; Clough, R.C.; Pappu, K.M.; Howard, J.A.; Baez, J.A.; Wang, K. Hydroxylation of recombinant human collagen type I alpha 1 in transgenic maize co-expressed with a recombinant human prolyl 4-hydroxylase. BMC Biotechnol. 2011, 11, 69. [Google Scholar] [CrossRef] [Green Version]
- Cooperman, L.; Michaeli, D. The immunogenicity of injectable collagen. II. A retrospective review of seventy-two tested and treated patients. J. Am. Acad. Derm. 1984, 10, 647–651. [Google Scholar] [CrossRef]
- Addad, S.; Exposito, J.Y.; Faye, C.; Ricard-Blum, S.; Lethias, C. Isolation, characterization and biological evaluation of jellyfish collagen for use in biomedical applications. Mar. Drugs 2011, 9, 967–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.S.; Choi, J.S.; Kim, J.D.; Yoon, H.I.; Choi, Y.C.; Cho, Y.W. Human collagen isolated from adipose tissue. Biotechnol. Prog. 2012, 28, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Lauer-Fields, J.L.; Fields, G.B. Triple-helical peptide analysis of collagenolytic protease activity. Biol. Chem. 2002, 383, 1095–1105. [Google Scholar] [CrossRef]
- Fields, G.B. A Model for interstitial collagen catabolism by mammalian collagenases. J. Theor. Biol. 1991, 153, 585–602. [Google Scholar] [CrossRef]
- Aimes, R.T.; Quigley, J.P. Matrix metalloproteinase-2 Is an interstitial collagenase—Inhibitor-Free Enzyme catalyzes the cleavage of collagen fibrils and soluble native type-I collagen generating the specific 3/4-length and 1/4-length fragments. J. Biol. Chem. 1995, 270, 5872–5876. [Google Scholar] [CrossRef] [Green Version]
- Ohuchi, E.; Imai, K.; Fujii, Y.; Sato, H.; Seiki, M.; Okada, Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J. Biol. Chem. 1997, 272, 2446–2451. [Google Scholar] [CrossRef] [Green Version]
- Song, E.; Kim, S.Y.; Chun, T.; Byun, H.J.; Lee, Y.M. Collagen scaffolds derived from a marine source and their biocompatibility. Biomaterials 2006, 27, 2951–2961. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.M.; Black, L.; Santacana-Laffitte, G.; Patrick, C.W. Preparation and assessment of glutaraldehyde-cross-linked collagen-chitosan hydrogels for adipose tissue engineering. J. Biomed. Mater. Res. Part A 2007, 81A, 59–65. [Google Scholar] [CrossRef]
- Powell, H.M.; Boyce, S.T. EDC cross-linking improves skin substitute strength and stability. Biomaterials 2006, 27, 5821–5827. [Google Scholar] [CrossRef] [PubMed]
- Tu, R.; Quijano, R.C.; Lu, C.L.; Shen, S.; Wang, E.; Hata, C.; Lin, D. A preliminary-study of the fixation mechanism of collagen reaction with a polyepoxy fixative. Int. J. Artif. Organs 1993, 16, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Speer, D.P.; Chvapil, M.; Eskelson, C.D.; Ulreich, J. Biological effects of residual glutaraldehyde in glutaraldehyde-tanned collagen biomaterials. J. Biomed. Mater. Res. 1980, 14, 753–764. [Google Scholar] [CrossRef]
- Weadock, K.S.; Miller, E.J.; Bellincampi, L.D.; Zawadsky, J.P.; Dunn, M.G. Physical cross-linking of collagen fibers: Comparison of ultraviolet irradiation and dehydrothermal treatment. J. Biomed. Mater. Res. 1995, 29, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Zork, N.M.; Myers, K.M.; Yoshida, K.; Cremers, S.; Jiang, H.F.; Ananth, C.V.; Wapner, R.J.; Kitajewski, J.; Vink, J. A systematic evaluation of collagen cross-links in the human cervix. Am. J. Obs. Gynecol. 2015, 212. [Google Scholar] [CrossRef] [Green Version]
- Haugh, M.G.; Jaasma, M.J.; O’Brien, F.J. The effect of dehydrothermal treatment on the mechanical and structural properties of collagen-GAG scaffolds. J. Biomed. Mater. Res. Part A 2009, 89A, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Miles, C.A.; Bailey, A.J. Thermally labile domains in the collagen molecule. Micron 2001, 32, 325–332. [Google Scholar] [CrossRef]
- Haugh, M.G.; Murphy, C.M.; McKiernan, R.C.; Altenbuchner, C.; O’Brien, F.J. Cross-linking and mechanical properties significantly influence cell attachment, proliferation, and migration within collagen glycosaminoglycan scaffolds. Tissue Eng. Part A 2011, 17A, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Weadock, K.S.; Miller, E.J.; Keuffel, E.L.; Dunn, M.G. Effect of physical cross-linking methods on collagen-fiber durability in proteolytic solutions. J. Biomed. Mater. Res. 1996, 32, 221–226. [Google Scholar] [CrossRef]
- Gorham, S.D.; Light, N.D.; Diamond, A.M.; Willins, M.J.; Bailey, A.J.; Wess, T.J.; Leslie, N.J. Effect of chemical modifications on the susceptibility of collagen to proteolysis. II. Dehydrothermal cross-linking. Int. J. Biol. Macromol. 1992, 14, 129–138. [Google Scholar] [CrossRef]
- Lv, H.W.; Wang, H.P.; Zhang, Z.J.; Yang, W.; Liu, W.B.; Li, Y.L.; Li, L.S. Biomaterial stiffness determines stem cell fate. Life Sci. 2017, 178, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Mitrousis, N.; Fokina, A.; Shoichet, M.S. Biomaterials for cell transplantation. Nat. Rev. Mater. 2018, 3, 441–456. [Google Scholar] [CrossRef]
- Velasco-Hogan, A.; Xu, J.; Meyers, M.A. Additive manufacturing as a method to design and optimize bioinspired structures. Adv. Mater. 2018, 30, 1800940. [Google Scholar] [CrossRef] [PubMed]
- De Mori, A.; Fernandez, M.P.; Blunn, G.; Tozzi, G.; Roldo, M. 3D printing and electrospinning of composite hydrogels for cartilage and bone tissue engineering. Polymers 2018, 10, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagarajan, N.; Dupret-Bories, A.; Karabulut, E.; Zorlutuna, P.; Vrana, N.E. Enabling personalized implant and controllable biosystem development through 3D printing. Biotechnol. Adv. 2018, 36, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, A.; Ling, Q.-D.; Kumar, S.S.; Chang, Y.; Kao, T.-C.; Munusamy, M.A.; Alarfaj, A.A.; Hsu, S.-T.; Umezawa, A. External stimulus-responsive biomaterials designed for the culture and differentiation of ES, iPS, and adult stem cells. Prog. Polym. Sci. 2014, 39, 1585–1613. [Google Scholar] [CrossRef]
- Draget, K.I.; Skjåk-Bræk, G.; Smidsrød, O. Alginate based new materials. Int. J. Biol. Macromol. 1997, 21, 47–55. [Google Scholar] [CrossRef]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.H.; Kim, S.K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef] [PubMed]
- You, F.; Wu, X.; Zhu, N.; Lei, M.; Eames, B.F.; Chen, X. 3D printing of porous cell-laden hydrogel constructs for potential applications in cartilage tissue engineering. ACS Biomater. Sci. Eng. 2016, 2, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wu, C.; Lode, A.; Gelinsky, M. Hierarchical mesoporous bioactive glass/alginate composite scaffolds fabricated by three-dimensional plotting for bone tissue engineering. Biofabrication 2013, 5, 015005. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Kim, G.H. PCL/alginate composite scaffolds for hard tissue engineering: Fabrication, characterization, and cellular activities. ACS Comb. Sci. 2015, 17, 87–99. [Google Scholar] [CrossRef]
- D’Amora, U.; D’Este, M.; Eglin, D.; Safari, F.; Sprecher, C.M.; Gloria, A.; De Santis, R.; Alini, M.; Ambrosio, L. Collagen density gradient on three-dimensional printed poly(ε-caprolactone) scaffolds for interface tissue engineering. J. Tissue Eng. Regen. Med. 2018, 12, 321–329. [Google Scholar] [CrossRef]
- Gloria, A.; Russo, T.; Rodrigues, D.F.L.; D’Amora, U.; Colella, F.; Improta, G.; Triassi, M.; De Santis, R.; Ambrosio, L. From 3D hierarchical scaffolds for tissue engineering to advanced hydrogel-based and complex devices for in situ cell or drug release. Procedia CIRP 2016, 49, 72–75. [Google Scholar] [CrossRef] [Green Version]
- Perez, R.A.; Kim, M.; Kim, T.H.; Kim, J.H.; Lee, J.H.; Park, J.H.; Knowles, J.C.; Kim, H.W. Utilizing core-shell fibrous collagen–alginate hydrogel cell delivery system for bone tissue engineering. Tissue Eng. Part A 2014, 20A, 103–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, R.A.; Kim, J.H.; Buitrago, J.O.; Wall, I.B.; Kim, H.W. Novel therapeutic core-shell hydrogel scaffolds with sequential delivery of cobalt and bone morphogenetic protein-2 for synergistic bone regeneration. Acta Biomater. 2015, 23, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Buitrago, J.O.; Perez, R.A.; El-Fiqi, A.; Singh, R.K.; Kim, J.H.; Kim, H.W. Core-shell fibrous stem cell carriers incorporating osteogenic nanoparticulate cues for bone tissue engineering. Acta Biomater. 2015, 28, 183–192. [Google Scholar] [CrossRef]
- Yao, R.; Alkhawtani, A.Y.F.; Chen, R.Y.; Luang, J.; Xu, M.E. Rapid and efficient in vivo angiogenesis directed by electro-assisted bioprinting of alginate/collagen microspheres with human umbilical vein endothelial cell coating layer. Int. J. Bioprint. 2019, 5, 3–14. [Google Scholar] [CrossRef]
- Lee, B.R.; Hwang, J.W.; Choi, Y.Y.; Wong, S.F.; Hwang, Y.H.; Lee, D.Y.; Lee, S.H. In situ formation and collagen–alginate composite encapsulation of pancreatic islet spheroids. Biomaterials 2012, 33, 837–845. [Google Scholar] [CrossRef]
- Chan, H.F.; Zhang, Y.; Leong, K.W. Efficient One-step production of microencapsulated hepatocyte spheroids with enhanced functions. Small 2016, 12, 2720–2730. [Google Scholar] [CrossRef] [PubMed]
- San Jose, L.H.; Stephens, P.; Song, B.; Barrow, D. Microfluidic encapsulation supports stem cell viability, proliferation, and neuronal differentiation. Tissue Eng. Part C Methods 2018, 24, 158–170. [Google Scholar] [CrossRef] [Green Version]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.M.; Schuetz, M.A.; Hutmacher, D.W. Bone regeneration based on tissue engineering conceptions—A 21st century perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [Green Version]
- Sakiyama-Elbert, S.E.; Hubbell, J.A. Functional biomaterials: Design of novel biomaterials. Annu. Rev. Mater. Res. 2001, 31, 183–201. [Google Scholar] [CrossRef]
- Boontheekul, T.; Kong, H.J.; Mooney, D.J. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials 2005, 26, 2455–2465. [Google Scholar] [CrossRef]
- Rico-Llanos, G.A.; Borrego-Gonzalez, S.; Moncayo-Donoso, M.; Becerra, J.; Visser, R. Collagen type I Biomaterials as scaffolds for bone tissue engineering. Polymers 2021, 13, 599. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, W.T.; Nagapudi, K.; Thomas, B.S.; Chaikof, E.L. Photo-cross-linking of type I collagen gels in the presence of smooth muscle cells: Mechanical properties, cell viability, and function. Biomacromolecules 2003, 4, 890–895. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kozlowska, J. Properties and modification of porous 3-D collagen/hydroxyapatite composites. Int. J. Biol. Macromol. 2013, 52, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Ma, P.X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Benayahu, D.; Pomeraniec, L.; Shemesh, S.; Heller, S.; Rosenthal, Y.; Rath-Wolfson, L.; Benayahu, Y. Biocompatibility of a marine collagen-based scaffold in vitro and in vivo. Mar. Drugs 2020, 18, 420. [Google Scholar] [CrossRef]
- Bendtsen, S.T.; Wei, M. Synthesis and characterization of a novel injectable alginate-collagen-hydroxyapatite hydrogel for bone tissue regeneration. J. Mater. Chem. B 2015, 3, 3081–3090. [Google Scholar] [CrossRef]
- Yu, C.C.; Chang, J.J.; Lee, Y.H.; Lin, Y.C.; Wu, M.H.; Yang, M.C.; Chien, C.T. Electrospun scaffolds composing of alginate, chitosan, collagen and hydroxyapatite for applying in bone tissue engineering. Mater. Lett. 2013, 93, 133–136. [Google Scholar] [CrossRef]
- Parmentier, L.; Riffault, M.; Hoey, D.A. Utilizing osteocyte derived factors to enhance cell viability and osteogenic matrix deposition within IPN hydrogels. Materials 2020, 13, 1690. [Google Scholar] [CrossRef] [Green Version]
- Perez, R.A.; Won, J.E.; Knowles, J.C.; Kim, H.W. Naturally and synthetic smart composite biomaterials for tissue regeneration. Adv. Drug Deliv. Rev. 2013, 65, 471–496. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, E.; Lopez-Noriega, A.; Thompson, E.; Kelly, H.M.; Cryan, S.A.; O’Brien, F.J. Development of collagen-hydroxyapatite scaffolds incorporating PLGA and alginate microparticles for the controlled delivery of rhBMP-2 for bone tissue engineering. J. Control. Release 2015, 198, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Sotome, S.; Uemura, T.; Kikuchi, M.; Chen, J.; Itoh, S.; Tanaka, J.; Tateishi, T.; Shinomiya, K. Synthesis and in vivo evaluation of a novel hydroxyapatite/collagen–alginate as a bone filler and a drug delivery carrier of bone morphogenetic protein. Mat. Sci. Eng. C 2004, 24, 341–347. [Google Scholar] [CrossRef]
- Pak, J.; Lee, J.H.; Kartolo, W.A.; Lee, S.H. Cartilage regeneration in human with adipose tissue-derived stem cells: Current status in clinical implications. Biomed. Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zheng, L.; Fan, H.S.; Zhang, X.D. A scaffold-filter model for studying the chondrogenic differentiation of stem cells in vitro. Mat. Sci. Eng. C 2017, 70, 962–968. [Google Scholar] [CrossRef]
- Roach, H.I.; Erenpreisa, J.; Aigner, T. Osteogenic differentiation of hypertrophic chondrocytes involves asymmetric cell divisions and apoptosis. J. Cell Biol. 1995, 131, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Domm, C.; Schunke, M.; Christesen, K.; Kurz, B. Redifferentiation of dedifferentiated bovine articular chondrocytes in alginate culture under low oxygen tension. Osteoarthr. Cartil. 2002, 10, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonaventure, J.; Kadhom, N.; Cohensolal, L.; Ng, K.H.; Bourguignon, J.; Lasselin, C.; Freisinger, P. Reexpression of cartilage-specific genes by dedifferentiated human articular chondrocytes cultured in alginate beads. Exp. Cell Res. 1994, 212, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.Z.; Kim, H.W. Efficacy of collagen and alginate hydrogels for the prevention of rat chondrocyte dedifferentiation. J. Tissue Eng. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen–alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. C 2018, 83, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.C.W.; Ferguson, S.J.; Gantenbein-Ritter, B. The effects of dynamic loading on the intervertebral disc. Eur. Spine J. 2011, 20, 1796–1812. [Google Scholar] [CrossRef] [Green Version]
- Eyre, D.R. Biochemistry of the intervertebral disc. Int. Rev. Connect. Tissue Res. 1979, 8, 227–291. [Google Scholar] [PubMed]
- Kishen, T.J.; Diwan, A.D. Fusion versus disk replacement for degenerative conditions of the lumbar and cervical spine: Quid est testimonium? Orthop. Clin. N. Am. 2010, 41, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J.; Diwan, A.D.; Tipper, J.L. Advanced strategies for the regeneration of lumbar disc annulus fibrosus. Int. J. Mol. Sci. 2020, 21, 4889. [Google Scholar] [CrossRef]
- Choi, Y.; Park, M.H.; Lee, K. Tissue engineering strategies for intervertebral disc treatment using functional polymers. Polymers 2019, 11, 872. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.Z.; Liu, W.Q.; Xia, T.T.; Yang, L. Disordered mechanical stress and tissue engineering therapies in intervertebral disc degeneration. Polymers 2019, 11, 1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borzacchiello, A.; Gloria, A.; De Santis, R.; Ambrosio, L. Spinal disc implants using hydrogels. In Biomedical Hydrogels; Rimmer, S., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 103–117. [Google Scholar]
- Moriguchi, Y.; Borde, B.; Berlin, C.; Wipplinger, C.; Sloan, S.R.; Kirnaz, S.; Pennicooke, B.; Navarro-Ramirez, R.; Khair, T.; Grunert, P.; et al. In vivo annular repair using high-density collagen gel seeded with annulus fibrosus cells. Acta Biomater. 2018, 79, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Sloan, S.R.; Wipplinger, C.; Navarro-Ramirez, R.; Zubkov, M.; Kim, E.; Kirnaz, S.; Bonassar, L.J.; Härtl, R. Mesenchymal stem cell-seeded high-density collagen gel for annular repair: 6-week results from in vivo sheep models. Neurosurgery 2019, 85, E350–E359. [Google Scholar] [CrossRef] [PubMed]
- Tsaryk, R.; Gloria, A.; Russo, T.; Anspach, L.; De Santis, R.; Ghanaati, S.; Unger, R.E.; Ambrosio, L.; Kirkpatrick, C.J. Collagen-low molecular weight hyaluronic acid semi-interpenetrating network loaded with gelatin microspheres for cell and growth factor delivery for nucleus pulposus regeneration. Acta Biomater. 2015, 20, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Bowles, R.D.; Williams, R.M.; Zipfel, W.R.; Bonassar, L.J. Self-assembly of aligned tissue-engineered annulus fibrosus and intervertebral disc composite via collagen gel contraction. Tissue Eng. Part A 2010, 16A, 1339–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gloria, A.; Russo, T.; D’Amora, U.; Santin, M.; De Santis, R.; Ambrosio, L. Customised multiphasic nucleus/annulus scaffold for intervertebral disc repair/regeneration. Connect. Tissue Res. 2020, 61, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, Y.; Mojica-Santiago, J.; Grunert, P.; Pennicooke, B.; Berlin, C.; Khair, T.; Navarro-Ramirez, R.; Arbona, R.J.R.; Nguyen, J.; Hartl, R.; et al. Total disc replacement using tissue-engineered intervertebral discs in the canine cervical spine. PLoS ONE 2017, 12, e0185716. [Google Scholar] [CrossRef] [Green Version]
- Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 2005, 438, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674. [Google Scholar] [CrossRef]
- Folkman, J.; Hochberg, M. Self-regulation of growth in three dimensions. J. Exp. Med. 1973, 138, 745–753. [Google Scholar] [CrossRef]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef]
- Gelmi, A.; Cieslar-Pobuda, A.; de Muinck, E.; Los, M.; Rafat, M.; Jager, E.W.H. Direct mechanical stimulation of stem cells: A beating electromechanically active scaffold for cardiac tissue engineering. Adv. Healthc. Mater. 2016, 5, 1471–1480. [Google Scholar] [CrossRef] [Green Version]
- Quinlan, E.; Lopez-Noriega, A.; Thompson, E.M.; Hibbitts, A.; Cryan, S.A.; O’Brien, F.J. Controlled release of vascular endothelial growth factor from spray-dried alginate microparticles in collagen-hydroxyapatite scaffolds for promoting vascularization and bone repair. J. Tissue Eng. Regen. Med. 2017, 11, 1097–1109. [Google Scholar] [CrossRef]
- Ali, Z.; Islam, A.; Sherrell, P.; Le-Moine, M.; Lolas, G.; Syrigos, K.; Rafat, M.; Jensen, L.D. Adjustable delivery of pro-angiogenic FGF-2 by alginate: Collagen microspheres. Biol. Open 2018, 7, bio027060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruoslahti, E. Brain extracellular matrix. Glycobiology 1996, 6, 489–492. [Google Scholar] [CrossRef] [Green Version]
- Ripellino, J.A.; Klinger, M.M.; Margolis, R.U.; Margolis, R.K. The hyaluronic acid binding region as a specific probe for the localization of hyaluronic acid in tissue sections. Application to chick embryo and rat brain. J. Histochem. Cytochem. 1985, 33, 1060–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Kirker, K.R.; Prestwich, G.D. Cross-linked hyaluronic acid hydrogel films: New biomaterials for drug delivery. J. Control. Release 2000, 69, 169–184. [Google Scholar] [CrossRef]
- Park, H.; Kang, S.W.; Kim, B.S.; Mooney, D.J.; Lee, K.Y. Shear-reversibly cross-linked alginate hydrogels for tissue engineering. Macromol. Biosci. 2009, 9, 895–901. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Hsueh, C.H. Neuronal production from induced pluripotent stem cells in self-assembled collagen-hyaluronic acid-alginate microgel scaffolds with grafted GRGDSP/Ln5-P4. Mater. Sci. Eng. C 2017, 76, 760–774. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.S.; Teply, B.A.; Stevens, M.M.; Zeitels, S.M.; Langer, R. Collagen composite hydrogels for vocal fold lamina propria restoration. Biomaterials 2006, 27, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.C.; Steed, D.L.; Franz, M.G. Wound healing: Biologic features and approaches to maximize healing trajectories—In brief. Curr. Prob. Surg. 2001, 38, 65–140. [Google Scholar] [CrossRef]
- Szycher, M.; Lee, S.J. Modern wound dressings: A systematic approach to wound healing. J. Biomater. Appl. 1992, 7, 142–213. [Google Scholar] [CrossRef]
- Singer, A.J.; Clark, R.A.F. Mechanisms of disease—Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.T.S.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Pinho, E.; Soares, G. Functionalization of cotton cellulose for improved wound healing. J. Mater. Chem. B 2018, 6, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. Biomedicine 2015, 5, 24–28. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ding, F.X.; Hu, D.; Zhou, Y.; Long, C.L.; Shen, L.J.; Zhang, Y.Y.; Zhang, D.Y.; Wei, G.H. Human umbilical cord mesenchymal stem cell conditioned medium attenuates renal fibrosis by reducing inflammation and epithelial-to-mesenchymal transition via the TLR4/NF-kappa B signaling pathway in vivo and in vitro. Stem Cell Res. Ther. 2018, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.C.; Yuan, Q.J.; Yin, T.; You, J.; Gu, Z.P.; Xiong, S.B.; Hu, Y. Self-assembly of collagen-based biomaterials: Preparation, characterizations and biomedical applications. J. Mater. Chem. B 2018, 6, 2650–2676. [Google Scholar] [CrossRef] [PubMed]
- Lozeau, L.D.; Grosha, J.; Smith, I.M.; Stewart, E.J.; Camesano, T.A.; Rolle, M.W. Alginate affects bioactivity of chimeric collagen-binding LL37 antimicrobial peptides adsorbed to collagen-alginate wound dressings. ACS Biomater. Sci. Eng. 2020, 6, 3398–3410. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.F.; Wu, T.T.; Wang, W.S.; Li, B.L.; Wang, M.; Chen, L.L.; Xia, H.; Zhang, T. Biofunctions of antimicrobial peptide-conjugated alginate/hyaluronic acid/collagen wound dressings promote wound healing of a mixed-bacteria-infected wound. Int. J. Biol. Macromol. 2019, 140, 330–342. [Google Scholar] [CrossRef]
- Van Gils, C.C.; Roeder, B.; Chesler, S.M.; Mason, S. Improved healing with a collagen–alginate dressing in the chemical matricectomy. J. Am. Podiat. Med. Assoc. 1998, 88, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Donaghue, V.M.; Chrzan, J.S.; Rosenblum, B.I.; Giurini, J.M.; Habershaw, G.M.; Veves, A. A clinical evaluation of a collagen–alginate topical wound dressing in the management of diabetic foot ulcers. Diabetologia 1996, 39, 1019. [Google Scholar]
- Feng, X.L.; Zhang, X.F.; Li, S.Q.; Zheng, Y.Q.; Shi, X.N.; Li, F.; Guo, S.B.; Yang, J.M. Preparation of aminated fish scale collagen and oxidized sodium alginate hybrid hydrogel for enhanced full-thickness wound healing. Int. J. Biol. Macromol. 2020, 164, 626–637. [Google Scholar] [CrossRef]
- Xie, H.X.; Chen, X.L.; Shen, X.R.; He, Y.; Chen, W.; Luo, Q.; Ge, W.H.; Yuan, W.H.; Tang, X.; Hou, D.Y.; et al. Preparation of chitosan-collagen–alginate composite dressing and its promoting effects on wound healing. Int. J. Biol. Macromol. 2018, 107, 93–104. [Google Scholar] [CrossRef]
- Zhang, H.J.; Peng, M.X.; Cheng, T.; Zhao, P.; Qiu, L.P.; Zhou, J.; Lu, G.Z.; Chen, J.H. Silver nanoparticles-doped collagen–alginate antimicrobial biocomposite as potential wound dressing. J. Mater. Sci. 2018, 53, 14944–14952. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Li, Z.; Li, Y.; Wang, Y.Y.; Yao, M.H.; Zhang, K.; Chen, Z.Y.; Yue, H.; Shi, J.J.; Guan, F.X.; et al. Sodium alginate/collagen hydrogel loaded with human umbilical cord mesenchymal stem cells promotes wound healing and skin remodeling. Cell Tissue Res. 2021, 383, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Michalska-Sionkowska, M.; Walczak, M.; Sionkowska, A. Antimicrobial activity of collagen material with thymol addition for potential application as wound dressing. Polym. Test. 2017, 63, 360–366. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.P.; Xu, Y.M.; Ma, P.X.; Guo, B.L. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.O.; Jafari, S.H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Advan. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Otvos, L., Jr.; Ostorhazi, E. Therapeutic utility of antibacterial peptides in wound healing. Expert Rev. Anti-Infect. Ther. 2015, 13, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Branco da Cunha, C.; Klumpers, D.D.; Li, W.A.; Koshy, S.T.; Weaver, J.C.; Chaudhuri, O.; Granja, P.L.; Mooney, D.J. Influence of the stiffness of three-dimensional alginate/collagen-I interpenetrating networks on fibroblast biology. Biomaterials 2014, 35, 8927–8936. [Google Scholar] [CrossRef]
- Orive, G.; Hernandez, R.M.; Gascon, A.R.; Calafiore, R.; Chang, T.M.S.; De Vos, P.; Hortelano, G.; Hunkeler, D.; Lacik, I.; Shapiro, A.M.J.; et al. Cell encapsulation: Promise and progress. Nat. Med. 2003, 9, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Uludag, H.; De Vos, P.; Tresco, P.A. Technology of mammalian cell encapsulation. Adv. Drug Deliv. Rev. 2000, 42, 29–64. [Google Scholar] [CrossRef]
- Chang, T.M.S.; Malave, N. Development and First clinical use of semipermeable microcapsules (artificial cells) as a compact artificial kidney. Trans. Am. Soc. Artif. Intern. Organs 1970, 16, 141–148. [Google Scholar] [CrossRef]
- Zurn, A.D.; Henry, H.; Schluep, M.; Aubert, V.; Winkel, L.; Eilers, B.; Bachmann, C.; Aebischer, P. Evaluation of an intrathecal immune response in amyotrophic lateral sclerosis patients implanted with encapsulated genetically engineered xenogeneic cells. Cell Transplant. 2000, 9, 471–484. [Google Scholar] [CrossRef]
- Wong, H.L.; Wang, M.X.; Cheung, P.T.; Yao, K.M.; Chan, B.P. A 3D collagen microsphere culture system for GDNF-secreting HEK293 cells with enhanced protein productivity. Biomaterials 2007, 28, 5369–5380. [Google Scholar] [CrossRef]
- Lee, M.; Lo, A.C.; Cheung, P.T.; Wong, D.; Chan, B.P. Drug carrier systems based on collagen–alginate composite structures for improving the performance of GDNF-secreting HEK293 cells. Biomaterials 2009, 30, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Khavari, A.; Nyden, M.; Weitz, D.A.; Ehrlicher, A.J. Composite alginate gels for tunable cellular microenvironment mechanics. Sci. Rep. 2016, 6, 30854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Yang, Y.W.; Han, L.C.; Han, S.; Liu, N.; Xu, F.; Li, F. Effect of three-dimensional ECM stiffness on cancer cell migration through regulating cell volume homeostasis. Biochem. Biophys. Res. Commun. 2020, 528, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Mejia, D.L.; Chiang, B.; Luker, K.E.; Luker, G.D. Hybrid collagen alginate hydrogel as a platform for 3D tumor spheroid invasion. Acta Biomater. 2018, 75, 213–225. [Google Scholar] [CrossRef] [PubMed]


| Type | Molecular Formula | Polymerized Form | Tissue Distribution | |
|---|---|---|---|---|
| Fibril-Forming (fibrillar) | I | [α1(I)]2α2(I) | fibril | bone, skin, tendons, ligaments, cornea (represent 90% of total collagen of the human body) |
| II | [α1(II)] | fibril | cartilage, intervertebral disc, notochord, vitreous humor in the eye | |
| III | [α1(III)]3 | fibril | skin, blood vessels | |
| V | [α1(V)]2α2(V) and α1(V)α2(V)α3(V) | fibril (assemble with type I) | idem as type I | |
| XI | α1(XI)α2(XI)α3(XI) | fibril (assemble with type II) | idem as type II | |
| Fibril-associated | IX | α1(IX)α2(IX)α3(IX) | lateral association with type II fibril | cartilage |
| XII | [α1(XII)]3 | lateral association with type I fibril | tendons, ligaments | |
| Network-forming | IV | [α1(IV)]2α2(IV) | sheet-like network | basal lamina |
| VII | [α1(VII)]3 | anchoring | beneath stratified squamous epithelia |
| Technique | Description | Incorporation of Bioactive Agent | Cell Type | Hydrogel Characteristics | Reference |
|---|---|---|---|---|---|
| Dual concentric nozzle system | Alginate and collagen solution were loaded into outer and inner syringe respectively. Simultaneous injection of ALG and COL solution into CaCl2 bath to form a fiber | Bioactive glass nanoparticles loaded in the shell [90] | Mesenchymal stem cells (MSCs) | CAC fiber (core diameter: 700–1000 μm and shell diameter: 200–500 μm) [88] | [88,89,90] |
| BMP loaded in the core and cobalt loaded in the shell [89] | CAC fiber (core diameter: 1000 μm and shell diameter: 150 μm [89] | ||||
| Electro-assisted inkjet printing | Alginate microsphere was fabricated by inkjet printing followed by collagen coating | Nil | Endothelial cell | CAC microsphere | [91] |
| Concave well mold | CAC containing islet cells were introduced into concave well mold followed by Ca2+ bath | Nil | Pancreatic islets cell | Islets spheroid | [92] |
| Microfluidics | CAC microspheres/spheroids were generated when pass through the inlet followed by shear force | Nil | Stem cell | CAC microsphere | [93] |
| Nil | Liver cell | CAC spheroid | [94] |
| Tissue Engineering or Tissue Regeneration | Tissue Application | Reference |
|---|---|---|
| Tissue engineering | Bone | [88,89,90,104,105,109] |
| Cartilage | [26,115,116] | |
| IVD | [129] | |
| Tissue regeneration | Blood vessel | [135,136] |
| Neuron | [25,94,141] | |
| Liver | [93] | |
| Vocal folds | [142] |
| Composition | Finding | Reference |
|---|---|---|
| Collagen–alginate-AMPs | Antimicrobial activity and no cytotoxicity to fibroblasts | [152] |
| Collagen–alginate-hyaluronic acid-AMPs | Antimicrobial activity, good biocompatibility, reduced inflammation, and angiogenesis | [153] |
| Collagen–alginate wound dressing | Accelerating wound healing and great patients’ satisfaction | [154,155] |
| Aminated collagen-oxidized alginate-polymyxin B sulfate-bacitracin | Antimicrobial activity, angiogenesis, and reepithelization | [156] |
| Chitosan-collagen–alginate dressing-PU membrane | Hemocompatibility, reepithelization, and water-resistance | [157] |
| Collagen–alginate-AgNPs | Antibacterial activity but dose-dependent cytotoxicity to mammalian cells | [158] |
| Collagen–alginate-hUCMSCs | Promote wound healing, reepithelization, and angiogenesis | [159] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, T.; Lo, A.C.Y. Collagen–Alginate Composite Hydrogel: Application in Tissue Engineering and Biomedical Sciences. Polymers 2021, 13, 1852. https://doi.org/10.3390/polym13111852
Hu T, Lo ACY. Collagen–Alginate Composite Hydrogel: Application in Tissue Engineering and Biomedical Sciences. Polymers. 2021; 13(11):1852. https://doi.org/10.3390/polym13111852
Chicago/Turabian StyleHu, Tingyu, and Amy C. Y. Lo. 2021. "Collagen–Alginate Composite Hydrogel: Application in Tissue Engineering and Biomedical Sciences" Polymers 13, no. 11: 1852. https://doi.org/10.3390/polym13111852
APA StyleHu, T., & Lo, A. C. Y. (2021). Collagen–Alginate Composite Hydrogel: Application in Tissue Engineering and Biomedical Sciences. Polymers, 13(11), 1852. https://doi.org/10.3390/polym13111852






