Silica Containing Composite Anion Exchange Membranes by Sol–Gel Synthesis: A Short Review
Abstract
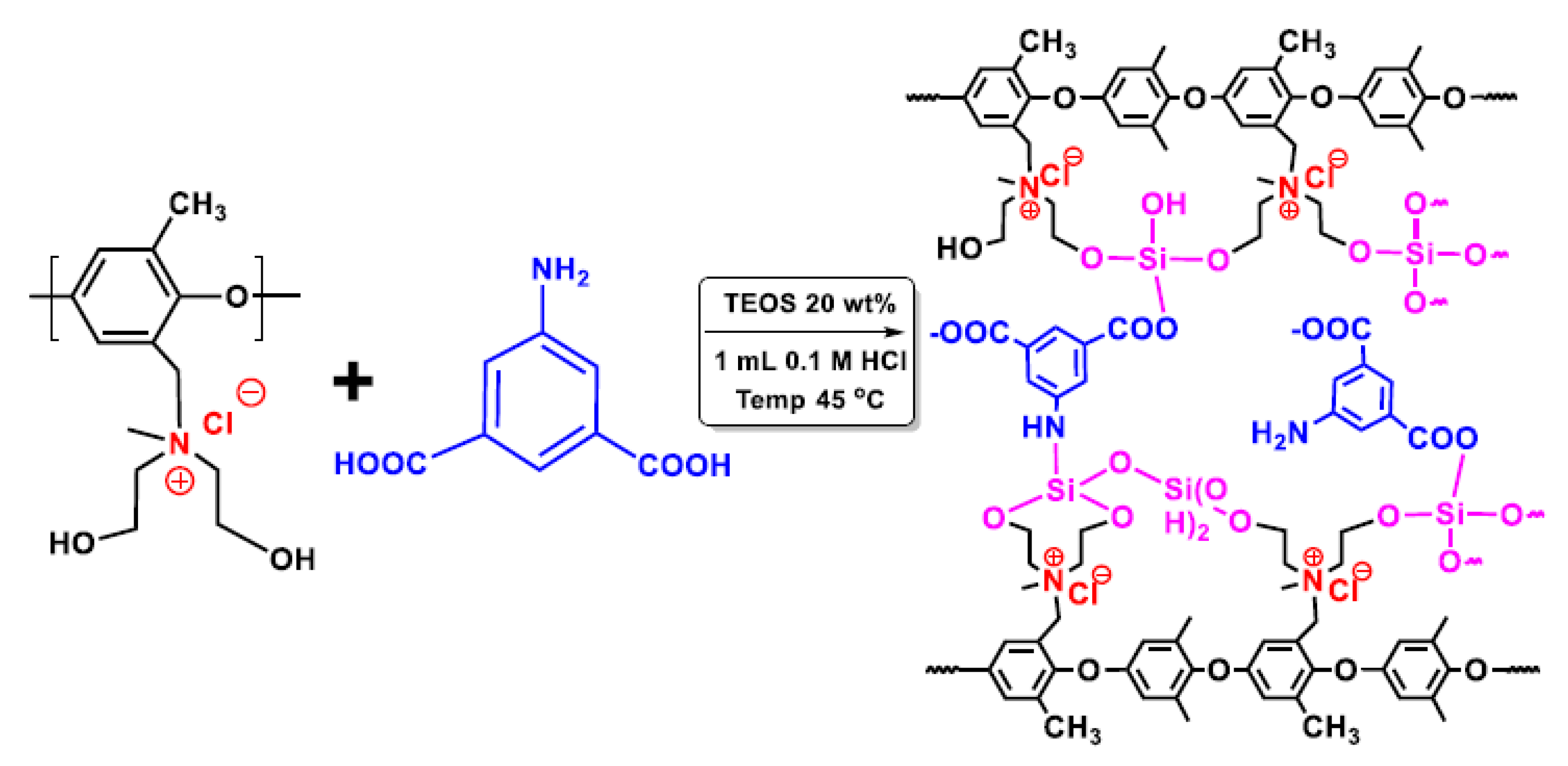
:1. Introduction
2. Membranes for Diffusion Dialysis (DD) and Related Fields
3. Membranes for Electrochemical Energy
4. Electrodialysis (ED)
5. Salient Features
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAPTEOS | 3-(2-aminoethylamino)propyltriethoxysilane |
| AAPTMOS | 3-(2-aminoethylamino)propyltrimethoxysilane |
| AEAPS | 3-(2-aminoethylamino)propyldimethoxymethylsilane |
| AEM: | anion exchange membranes |
| AEMFC | anion exchange membrane fuel cells |
| AIPA | amino-isophthalic acid |
| APTEOS | 3-aminopropyltriethoxysilane |
| APTMOS | 3-aminopropyltrimethoxysilane |
| APTEOS-I | N-triethoxysilylpropyl-N,N,N-trimethylammonium iodide |
| BrPPO | brominated poly(2,6-dimethyl-1,4-phenylene oxide) |
| CS | chitosan |
| DABCO | 1,4-diazabicyclo[2.2.2]octane |
| DD | diffusion dialysis |
| DEA | diethanolamine |
| DMAE | dimethylaminoethanol |
| DMAEM | 2-(dimethylaminoethyl) methacrylate |
| ED | electrodialysis |
| EPh | monophenyltriethoxysilane |
| GA | glutaraldehyde |
| GMA | glycidylmethacrylate |
| GPTMOS | 3-glycidoxypropyltrimethoxysilane |
| GTMA-Cl | glycidyltrimethylammonium chloride |
| MPS | 3-methacryloxypropyltrimethoxysilane |
| ODG | 4,4′-oxydiphenylguanidine |
| PA | polyamine |
| PEI | poly(ethylene imine) |
| PEO | poly(ethylene oxide)b |
| PNB | polynorbornene |
| PPO | poly(2,6-dimethyl-1,4-phenylene oxide) |
| PSU | polysulfone |
| PVA | poly(vinyl alcohol) |
| QPVA | quaternized poly(vinyl alcohol) |
| SMPEI | silica-modified poly(ethylene imine) |
| TEOS | tetraethoxysilane |
| TMA | trimethylamine/trimethylammonium |
| TMSP | N-trimethoxysilylpropyl-N,N,N-trimethylammonium chloride |
| VBC | vinylbenzyl chloride |
| VTMS | vinyltrimethoxysilane |
References
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion exchange membranes for alkaline fuel cells: A review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Hickner, M.A.; Herring, A.M.; Coughlin, E.B. Anion exchange membranes: Current status and moving forward. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1727–1735. [Google Scholar] [CrossRef]
- Knauth, P.; Pasquini, L.; Narducci, R.; Sgreccia, E.; Becerra-Arciniegas, R.-A.; Di Vona, M.L. Effective ion mobility in anion exchange ionomers: Relations with hydration, porosity, tortuosity, and percolation. J. Membr. Sci. 2021, 617, 118622. [Google Scholar] [CrossRef]
- Kreuer, K.-D. Ion conducting membranes for fuel cells and other electrochemical devices. Chem. Mater. 2014, 26, 361–380. [Google Scholar] [CrossRef]
- Maurya, S.; Shin, S.-H.; Kim, Y.; Moon, S.-H. A review on recent developments of anion exchange membranes for fuel cells and redox flow batteries. RSC Adv. 2015, 5, 37206–37230. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Atanassov, P.; Dekel, D.R.; Herring, A.M.; Hickner, M.A.; Kohl, P.A.; Kucernak, A.R.; Mustain, W.; Nijmeijer, K.; Scott, K.; et al. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 2014, 7, 3135–3191. [Google Scholar] [CrossRef] [Green Version]
- Liao, J.; Lu, M.; Chu, Y.; Wang, J. Ultra-low vanadium ion diffusion amphoteric ion-exchange membranes for all-vanadium redox flow batteries. J. Power Sources 2015, 282, 241–247. [Google Scholar] [CrossRef]
- Pasquini, L.; Knauth, P.; Pelzer, K.; Di Vona, M.L. Anion-conducting sulfaminated aromatic polymers by acid functionalization. RSC Adv. 2015, 5, 56636–56644. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Mai, Z.; Zhang, H.; Vankelecom, I.F.J. Ion exchange membranes for vanadium redox flow battery (VRB) applications. Energy Environ. Sci. 2011, 4, 1147–1160. [Google Scholar] [CrossRef]
- Prifti, H.; Parasuraman, A.; Winardi, S.; Lim, T.M.; Skyllas-Kazacos, M. Membranes for redox flow battery applications. Membranes 2012, 2, 275–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Jasti, A.; Kumar, M.; Shahi, V.K. A green method for the preparation of highly stable organic-inorganic hybrid anion-exchange membranes in aqueous media for electrochemical processes. Polym. Chem. 2010, 1, 1302–1312. [Google Scholar] [CrossRef]
- Vincent, I.; Bessarabov, D. Low cost hydrogen production by anion exchange membrane electrolysis: A review. Renew. Sustain. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Schalenbach, M.; Zeradjanin, A.R.; Kasian, O.; Cherevko, S.; Mayrhofer, K.J.J. A perspective on low-temperature water electrolysis—Challenges in alkaline and acidic technology. Int. J. Electrochem. Sci. 2018, 13, 1173–1226. [Google Scholar] [CrossRef]
- Xu, F.; Su, Y.; Lin, B. Progress of Alkaline Anion Exchange Membranes for Fuel Cells: The Effects of Micro-Phase Separation. Front. Mater. 2020, 7. [Google Scholar] [CrossRef] [Green Version]
- Ang, W.L.; Mohammad, A.W.; Hilal, N.; Leo, C.P. A review on the applicability of integrated/hybrid membrane processes in water treatment and desalination plants. Desalination 2015, 363, 2–18. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.T.; Zhu, J.Y.; Hou, J.W.; Liu, J.D.; Van der Bruggen, B. Zwitterionic functionalized layered double hydroxides nanosheets for a novel charged mosaic membrane with high salt permeability. J. Membr. Sci. 2016, 510, 27–37. [Google Scholar] [CrossRef]
- Luo, J.Y.; Wu, C.M.; Xu, T.W.; Wu, Y.H. Diffusion dialysis-concept, principle and applications. J. Membr. Sci. 2011, 366, 1–16. [Google Scholar] [CrossRef]
- Xu, J.; Lu, S.; Fu, D. Recovery of hydrochloric acid from the waste acid solution by diffusion dialysis. J. Hazard. Mater. 2009, 165, 832–837. [Google Scholar] [CrossRef]
- Knauth, P.; Pasquini, L.; Di Vona, M.L. Comparative study of the cation permeability of protonic, anionic and ampholytic membranes. Solid State Ion. 2017, 300, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef]
- Le, N.L.; Nunes, S.P. Materials and membrane technologies for water and energy sustainability. Sustain. Mater. Technol. 2016, 7, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; He, G.; Zhang, F. A mini-review on anion exchange membranes for fuel cell applications: Stability issue and addressing strategies. Int. J. Hydrogen Energy 2015, 40, 7348–7360. [Google Scholar] [CrossRef]
- Di Vona, M.L.; Licoccia, S.; Knauth, P. Organic–inorganic hybrid membranes based on sulfonated polyaryl–ether–ketones: Correlation between water uptake and electrical conductivity. Solid State Ion. 2008, 179, 1161–1165. [Google Scholar] [CrossRef]
- Sgreccia, E.; Di Vona, M.L.; Licoccia, S.; Sganappa, M.; Casciola, M.; Chailan, J.F.; Knauth, P. Self-assembled nanocomposite organic-inorganic proton conducting sulfonated poly-ether-ether-ketone (SPEEK)-based membranes: Optimized mechanical, thermal and electrical properties. J. Power Sources 2009, 192, 353–359. [Google Scholar] [CrossRef]
- Sgreccia, E.; Di Vona, M.L.; Knauth, P. Hybrid composite membranes based on SPEEK and functionalized PPSU for PEM fuel cells. Int. J. Hydrogen Energy 2011, 36, 8063–8069. [Google Scholar] [CrossRef]
- Licoccia, S.; Di Vona, M.L.; D’Epifanio, A.; Ahmed, Z.; Bellitto, S.; Marani, D.; Mecheri, B.; De Bonis, C.; Trombetta, M.; Traversa, E. SPPSU-based hybrid proton conducting polymeric electrolytes for intermediate temperature PEMFCs. J. Power Sources 2007, 167, 79–83. [Google Scholar] [CrossRef]
- Su, Y.-H.; Liu, Y.-L.; Sun, Y.-M.; Lai, J.-Y.; Wang, D.-M.; Gao, Y.; Liu, B.; Guiver, M.D. Proton exchange membranes modified with sulfonated silica nanoparticles for direct methanol fuel cells. J. Membr. Sci. 2007, 296, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.R.; Jiang, F.J. Ion-conducting membrane for vanadium redox flow batteries. Prog. Chem. 2021, 33, 462–470. [Google Scholar]
- Derbali, Z.; Fahs, A.; Chailan, J.-F.; Ferrari, I.; Di Vona, M.; Knauth, P. Composite anion exchange membranes with functionalized hydrophilic or hydrophobic titanium dioxide. Int. J. Hydrogen Energy 2017, 42, 19178–19189. [Google Scholar] [CrossRef]
- Sacca, A.; Carbone, A.; Passalacqua, E.; D’Epifanio, A.; Licoccia, S.; Traversa, E.; Sala, E.; Traini, F.; Ornelas, R. Nafion-TiO2 hybrid membranes for medium temperature polymer electrolyte fuel cells (PEFCs). J. Power Sources 2005, 152, 16–21. [Google Scholar] [CrossRef]
- Mazzapioda, L.; Panero, S.; Navarra, M.A. Polymer electrolyte membranes based on nafion and a superacidic inorganic additive for fuel cell applications. Polymers 2019, 11, 914. [Google Scholar] [CrossRef] [Green Version]
- Ramanavicius, S.; Ramanavicius, A. Progress and Insights in the Application of MXenes as New 2D Nano-Materials Suitable for Biosensors and Biofuel Cell Design. Int. J. Mol. Sci. 2020, 21, 9224. [Google Scholar] [CrossRef]
- Alberti, G.; Casciola, M.; Capitani, D.; Donnadio, A.; Narducci, R.; Pica, M.; Sganappa, M. Novel Nafion-zirconium phosphate nanocomposite membranes with enhanced stability of proton conductivity at medium temperature and high relative humidity. Electrochim. Acta 2007, 52, 8125–8132. [Google Scholar] [CrossRef]
- Vivani, R.; Alberti, G.; Costantino, F.; Nocchetti, M. New advances in zirconium phosphate and phosphonate chemistry: Structural archetypes. Microporous Mesoporous Mater. 2008, 107, 58–70. [Google Scholar] [CrossRef]
- Di Vona, M.L.; Casciola, M.; Donnadio, A.; Nocchetti, M.; Pasquini, L.; Narducci, R.; Knauth, P. Anionic conducting composite membranes based on aromatic polymer and layered double hydroxides. Int. J. Hydrogen Energy 2017, 42, 3197–3205. [Google Scholar] [CrossRef]
- Pizzoferrato, R.; Ciotta, E.; Ferrari, I.V.; Narducci, R.; Pasquini, L.; Varone, A.; Richetta, M.; Antonaroli, S.; Braglia, M.; Knauth, P.; et al. Layered double hydroxides containing an ionic liquid: Ionic conductivity and use in composite anion exchange membranes. ChemElectroChem 2018, 5, 2781–2788. [Google Scholar] [CrossRef]
- Kang, D.W.; Kang, M.; Yun, H.; Park, H.; Hong, C.S. Emerging porous solid electrolytes for hydroxide ion transport. Adv. Funct. Mater. 2021, 31, 2100083. [Google Scholar] [CrossRef]
- Charradi, K.; Ahmed, Z.; Aranda, P.; Chtourou, R. Silica/montmorillonite nanoarchitectures and layered double hydroxide-SPEEK based composite membranes for fuel cells applications. Appl. Clay Sci. 2019, 174, 77–85. [Google Scholar] [CrossRef]
- Sanchez, C.; Soler-Illia, G.J.A.; Ribot, F.; Grosso, D. Design of functional nano-structured materials through the use of controlled hybrid organic–inorganic interfaces. Comptes Rendus Chim. 2003, 6, 1131–1151. [Google Scholar] [CrossRef]
- Sanchez, C.; Livage, J.; Henry, M.; Babonneau, F. Chemical modification of alkoxide precursors. J. Non Cryst. Solids 1988, 100, 65–76. [Google Scholar] [CrossRef]
- Ye, F.; Collinson, M.M.; Higgins, D.A. What can be learned from single molecule spectroscopy? Applications to sol–gel-derived silica materials. Phys. Chem. Chem. Phys. 2009, 11, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Fidalgo, A.; Pandarus, V.; Béland, F.; Ilharco, L.M.; Pagliaro, M. The sol–gel route to advanced silica-based materials and recent applications. Chem. Rev. 2013, 113, 6592–6620. [Google Scholar] [CrossRef] [PubMed]
- Cuiming, W.; Tongwen, X.; Weihua, Y. Fundamental studies of a new hybrid (inorganic–organic) positively charged membrane: Membrane preparation and characterizations. J. Membr. Sci. 2003, 216, 269–278. [Google Scholar] [CrossRef]
- Wu, Y.H.; Wu, C.M.; Gong, M.; Xu, T.W. New anion exchanger organic-inorganic hybrid materials and membranes from a copolymer of glycidylmethacrylate and gamma-methacryloxypropyl trimethoxy silane. J. Appl. Polym. Sci. 2006, 102, 3580–3589. [Google Scholar] [CrossRef]
- Takeichi, T.; Furukawa, N. 5.25—Epoxy resins and phenol-formaldehyde resins. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 723–751. [Google Scholar]
- Wu, Y.; Wu, C.; Xu, T.; Fu, Y. Novel anion-exchange organic–inorganic hybrid membranes prepared through sol–gel reaction of multi-alkoxy precursors. J. Membr. Sci. 2009, 329, 236–245. [Google Scholar] [CrossRef]
- Wu, C.; Wu, Y.; Xu, T.; Fu, Y. Novel anion-exchange organic-inorganic hybrid membranes prepared through sol-gel reaction and UV/thermal curing. J. Appl. Polym. Sci. 2008, 107, 1865–1871. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, C.; Xu, T.; Gong, M.; Xu, X. Synthesis and characterizations of anion exchange organic–inorganic hybrid materials based on poly(2,6-dimethyl-1,4-phenylene oxide) (PPO). J. Solid State Chem. 2005, 178, 2292–2300. [Google Scholar] [CrossRef]
- Xu, T.; Lu, F.; Wu, Y. Novel hollow-fiber anion-exchange hybrid membranes: Preparation and characterization. J. Appl. Polym. Sci. 2009, 111, 3128–3136. [Google Scholar] [CrossRef]
- Luo, J.; Wu, C.; Wu, Y.; Xu, T. Diffusion dialysis of hydrochloride acid at different temperatures using PPO–SiO2 hybrid anion exchange membranes. J. Membr. Sci. 2010, 347, 240–249. [Google Scholar] [CrossRef]
- Pan, J.; He, Y.; Wu, L.; Jiang, C.; Wu, B.; Mondal, A.N.; Cheng, C.; Xu, T. Anion exchange membranes from hot-pressed electrospun QPPO–SiO2 hybrid nanofibers for acid recovery. J. Membr. Sci. 2015, 480, 115–121. [Google Scholar] [CrossRef]
- Bakangura, E.; Cheng, C.; Wu, L.; He, Y.; Ge, X.; Ran, J.; Emmanuel, K.; Xu, T. Highly charged hierarchically structured porous anion exchange membranes with excellent performance. J. Membr. Sci. 2016, 515, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Bakangura, E.; Cheng, C.; Wu, L.; Ge, X.; Ran, J.; Khan, M.I.; Kamana, E.; Afsar, N.; Irfan, M.; Shehzad, A.; et al. Hierarchically structured porous anion exchange membranes containing zwetterionic pores for ion separation. J. Membr. Sci. 2017, 537, 32–41. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, C.; Li, Y.; Xu, T.; Fu, Y. PVA–silica anion-exchange hybrid membranes prepared through a copolymer crosslinking agent. J. Membr. Sci. 2010, 350, 322–332. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, J.; Zhao, L.; Zhang, G.; Wu, C.; Xu, T. QPPO/PVA anion exchange hybrid membranes from double crosslinking agents for acid recovery. J. Membr. Sci. 2013, 428, 95–103. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, M.; Cao, J.; Xu, T.; Mao, F. Combination of OH – ions and –OH groups within QPPO/PVA hybrid membranes for acid recovery. Desalination Water Treat. 2015, 57, 1–11. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Liu, Q.L.; Meng, X.J.; Broadwell, I. Structure and pervaporation performance of novel quaternized poly(vinyl alcohol)/γ-aminopropyltriethoxysilane hybrid membranes. J. Appl. Polym. Sci. 2010, 118, 1121–1126. [Google Scholar] [CrossRef]
- Wu, C.; Wu, Y.; Luo, J.; Xu, T.; Fu, Y. Anion exchange hybrid membranes from PVA and multi-alkoxy silicon copolymer tailored for diffusion dialysis process. J. Membr. Sci. 2010, 356, 96–104. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, J.; Yao, L.; Wu, C.; Mao, F.; Xu, T. PVA/SiO2 anion exchange hybrid membranes from multisilicon copolymers with two types of molecular weights. J. Membr. Sci. 2012, 399–400, 16–27. [Google Scholar] [CrossRef]
- Emmanuel, K.; Cheng, C.; Erigene, B.; Mondal, A.N.; Hossain, M.M.; Khan, M.I.; Afsar, N.U.; Liang, G.; Wu, L.; Xu, T. Imidazolium functionalized anion exchange membrane blended with PVA for acid recovery via diffusion dialysis process. J. Membr. Sci. 2016, 497, 209–215. [Google Scholar] [CrossRef]
- Sharma, P.P.; Gahlot, S.; Gupta, H.; Kulshrestha, V. Hybrid anion conducting membranes (ACM) for industrial applications: Excellent salt removal efficiency and electro-chemical properties. Sep. Purif. Technol. 2017, 189, 449–458. [Google Scholar] [CrossRef]
- Irfan, M.; Afsar, N.U.; Bakangura, E.; Mondal, A.N.; Khan, M.I.; Emmanuel, K.; Yang, Z.; Wu, L.; Xu, T. Development of novel PVA-QUDAP based anion exchange membranes for diffusion dialysis and theoretical analysis therein. Sep. Purif. Technol. 2017, 178, 269–278. [Google Scholar] [CrossRef]
- Emmanuel, K.; Cheng, C.; Erigene, B.; Mondal, A.N.; Afsar, N.U.; Khan, M.I.; Hossain, M.; Jiang, C.; Ge, L.; Wu, L.; et al. Novel synthetic route to prepare doubly quaternized anion exchange membranes for diffusion dialysis application. Sep. Purif. Technol. 2017, 189, 204–212. [Google Scholar] [CrossRef]
- Irfan, M.; Bakangura, E.; Afsar, N.U.; Xu, T. Augmenting acid recovery from different systems by novel Q-DAN anion exchange membranes via diffusion dialysis. Sep. Purif. Technol. 2018, 201, 336–345. [Google Scholar] [CrossRef]
- Mondal, A.N.; Cheng, C.; Khan, M.I.; Hossain, M.M.; Emmanuel, K.; Ge, L.; Wu, B.; He, Y.; Ran, J.; Ge, X.; et al. Improved acid recovery performance by novel Poly(DMAEM-co-γ-MPS) anion exchange membrane via diffusion dialysis. J. Membr. Sci. 2017, 525, 163–174. [Google Scholar] [CrossRef]
- Nagarale, R.; Shahi, V.K.; Rangarajan, R. Preparation of polyvinyl alcohol–silica hybrid heterogeneous anion-exchange membranes by sol–gel method and their characterization. J. Membr. Sci. 2005, 248, 37–44. [Google Scholar] [CrossRef]
- Nagarale, R.; Gohil, G.; Shahi, V.K.; Rangarajan, R. Preparation of organic–inorganic composite anion-exchange membranes via aqueous dispersion polymerization and their characterization. J. Colloid Interface Sci. 2005, 287, 198–206. [Google Scholar] [CrossRef]
- Tripathi, B.P.; Kumar, M.; Shahi, V.K. Organic-inorganic hybrid alkaline membranes by epoxide ring opening for direct methanol fuel cell applications. J. Membr. Sci. 2010, 360, 90–101. [Google Scholar] [CrossRef]
- Chakrabarty, T.; Prakash, S.; Shahi, V.K. End group cross-linked 2-(dimethylamino) ethylmethacrylate based anion exchange membrane for electrodialysis. J. Membr. Sci. 2013, 428, 86–94. [Google Scholar] [CrossRef]
- Premakshi, H.G.; Mitchell, G.R.; Kariduraganavar, M.Y. Development of composite anion-exchange membranes using poly(vinyl alcohol) and silica precursor for pervaporation separation of water–isopropanol mixtures. RSC Adv. 2016, 6, 11802–11814. [Google Scholar] [CrossRef]
- Cheng, C.; Li, P.; He, Y.; Hu, X.; Emmanuel, K. Branched polyvinyl alcohol hybrid membrane for acid recovery via diffusion dialysis. Chem. Eng. Technol. 2019, 42, 1180–1187. [Google Scholar] [CrossRef]
- Pandey, R.P.; Thakur, A.K.; Shahi, V.K. Stable and efficient composite anion-exchange membranes based on silica modified poly(ethyleneimine)–poly(vinyl alcohol) for electrodialysis. J. Membr. Sci. 2014, 469, 478–487. [Google Scholar] [CrossRef]
- Bocian, S.; Studzińska, S.; Buszewski, B. Functionalized anion exchange stationary phase for separation of anionic compounds. Talanta 2014, 127, 133–139. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, C.; Yu, F.; Xu, T.; Fu, Y. Free-standing anion-exchange PEO–SiO2 hybrid membranes. J. Membr. Sci. 2008, 307, 28–36. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, C.; Xu, T.; Lin, X.; Fu, Y. Novel silica/poly(2,6-dimethyl-1,4-phenylene oxide) hybrid anion-exchange membranes for alkaline fuel cells: Effect of heat treatment. J. Membr. Sci. 2009, 338, 51–60. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, C.; Xu, T.; Yu, F.; Fu, Y. Novel anion-exchange organic–inorganic hybrid membranes: Preparation and characterizations for potential use in fuel cells. J. Membr. Sci. 2008, 321, 299–308. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, C.; Varcoe, J.R.; Poynton, S.D.; Xu, T.; Fu, Y. Novel silica/poly(2,6-dimethyl-1,4-phenylene oxide) hybrid anion-exchange membranes for alkaline fuel cells: Effect of silica content and the single cell performance. J. Power Sources 2010, 195, 3069–3076. [Google Scholar] [CrossRef]
- Lin, X.; Wu, C.; Wu, Y.; Xu, T. Free-standing hybrid anion-exchange membranes for application in fuel cells. J. Appl. Polym. Sci. 2011, 123, 3644–3651. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, Q.; Qian, H.; Xue, B.; Li, S.; Zhang, S. Self-assembly prepared anion exchange membranes with high alkaline stability and organic solvent resistance. J. Membr. Sci. 2017, 522, 159–167. [Google Scholar] [CrossRef]
- Li, X.; Cheng, J.; Liu, Y.; Gong, S.; He, G.; Li, L.; Li, S.; Zhang, F. Improved conductivity and stability of anion exchange membrane modified with bi-phenylguanidinium bridged silsesquioxane. Int. J. Hydrogen Energy 2017, 42, 21016–21026. [Google Scholar] [CrossRef]
- He, X.; Han, Z.; Yang, Y.; Wang, S.; Tu, G.; Huang, S.; Zhang, F.; Chen, D. The preparation and application of a ROMP-type epoxy-functionalized norbornene copolymer and its hybrid alkaline anion exchange membranes. RSC Adv. 2017, 7, 55977–55985. [Google Scholar] [CrossRef] [Green Version]
- He, X.H.; Jiang, X.; Wang, Z.J.; Deng, Y.J.; Han, Z.L.; Yang, Y.P.; Chen, D.F. Crosslinked hydroxyl-conductive copolymer/silica composite membranes based on addition-type polynorbornene for alkaline anion exchange membrane fuel cell applications. Polym. Eng. Sci. 2018, 58, 13–21. [Google Scholar] [CrossRef]
- Chen, J.; Shen, C.; Gao, S.; Yuan, Y.; Ren, X. Novel imidazole-grafted hybrid anion exchange membranes based on poly(2,6-dimethyl-1,4-phenylene oxide) for fuel cell applications. J. Appl. Polym. Sci. 2018, 135, 12. [Google Scholar] [CrossRef]
- Ataollahi, N.; Cappelletto, E.; Vezzù, K.; Di Noto, V.; Cavinato, G.; Callone, E.; Dirè, S.; Scardi, P.; Di Maggio, R. Properties of anion exchange membrane based on polyamine: Effect of functionalized silica particles prepared by sol–gel method. Solid State Ion. 2018, 322, 85–92. [Google Scholar] [CrossRef]
- Ding, A.; Zhou, J.; Cheng, X.; Shen, C.; Gao, S. Quaternized poly (2,6-dimethyl-1,4-phenylene oxide) crosslinked by tertiary amine and siloxane for anion exchange membranes. J. Appl. Polym. Sci. 2021, 138, 12. [Google Scholar] [CrossRef]
- Ye, N.; Zhang, D.; Yang, Y.; Wan, R.; Chen, S.; Zhan, Q.; He, R. 3-Glycidoxy-propylthrimethoxysilane improved anion exchange membranes based on quaternized poly(2,6-dimethyl-1,4-phenyleneoxide). Polymer 2019, 174, 38–44. [Google Scholar] [CrossRef]
- Liu, Y.X.; Dai, J.; Zhang, K.B.; Ma, L.L.; Qaisrani, N.A.; Zhang, F.X.; He, G.H. Hybrid anion exchange membrane of hydroxyl-modified polysulfone incorporating guanidinium-functionalized graphene oxide. Ionics 2017, 23, 3085–3096. [Google Scholar] [CrossRef]
- Shi, B.; Zhang, J.; Wu, W.; Wang, J.; Huang, J. Controlling conduction environments of anion exchange membrane by functionalized SiO2 for enhanced hydroxide conductivity. J. Membr. Sci. 2019, 569, 166–176. [Google Scholar] [CrossRef]
- Sgreccia, E.; Di Vona, M.; Antonaroli, S.; Ercolani, G.; Sette, M.; Pasquini, L.; Knauth, P. Nanocomposite anion exchange membranes with a conductive semi-interpenetrating silica network. Membranes 2021, 11, 260. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Q.L.; Zhu, A.M.; Huang, S.M.; Zeng, Q.H. Performance of organic–inorganic hybrid anion-exchange membranes for alkaline direct methanol fuel cells. J. Power Sources 2009, 186, 328–333. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Wang, J.; Wang, L. Preparation and characterization of a sol-gel derived silica/PVA-Py hybrid anion exchange membranes for alkaline fuel cell application. J. Electroanal. Chem. 2020, 873, 114342. [Google Scholar] [CrossRef]
- Leung, P.; Xu, Q.; Zhao, T.; Zeng, L.; Zhang, C. Preparation of silica nanocomposite anion-exchange membranes with low vanadium-ion crossover for vanadium redox flow batteries. Electrochimica Acta 2013, 105, 584–592. [Google Scholar] [CrossRef]
- Huang, S.-L.; Chen, M.-L.; Lin, Y.-S. Chitosan–silica anion exchange membrane for the vanadium redox flow energy storage battery applications. React. Funct. Polym. 2017, 119, 1–8. [Google Scholar] [CrossRef]
- Khan, M.A.; Kumar, M.; Alothman, Z.A. Preparation and characterization of organic–inorganic hybrid anion-exchange membranes for electrodialysis. J. Ind. Eng. Chem. 2015, 21, 723–730. [Google Scholar] [CrossRef]
- Yang, S.; Kim, W.-S.; Choi, J.; Choi, Y.-W.; Jeong, N.; Kim, H.; Nam, J.-Y.; Jeong, H.; Kim, Y.H. Fabrication of photocured anion-exchange membranes using water-soluble siloxane resins as cross-linking agents and their application in reverse electrodialysis. J. Membr. Sci. 2019, 573, 544–553. [Google Scholar] [CrossRef]
- Sharma, P.P.; Yadav, V.; Rajput, A.; Kulshrestha, V. Poly (triethoxyvinylsilane-co-quaternaryvinylbenzylchloride)/fGNR based anion exchange membrane and its application towards salt and acid recovery. J. Membr. Sci. 2018, 556, 303–311. [Google Scholar] [CrossRef]
- Afsar, N.U.; Ge, X.; Zhao, Z.; Hussain, A.; He, Y.; Ge, L.; Xu, T. Zwitterion membranes for selective cation separation via electrodialysis. Sep. Purif. Technol. 2021, 254, 117619. [Google Scholar] [CrossRef]
- Sgreccia, E.; Pasquini, L.; Ercolani, G.; Knauth, P.; Di Vona, M.L. Stimuli-responsive amphoteric ion exchange polymers bearing carboxylic and amine groups grafted to a cross-linkable silica network. Eur. Polym. J. 2019, 112, 255–262. [Google Scholar] [CrossRef]
- Pang, R.Z.; Li, X.; Li, J.S.; Lu, Z.Y.; Huang, C.; Sun, X.Y.; Wang, L.J. In situ Preparation and Antifouling Performance of ZrO2/PVDF Hybrid Membrane. Acta Phys. Chim. Sin. 2013, 29, 2592–2598. [Google Scholar]
- Couture, G.; Alaaeddine, A.; Boschet, F.; Ameduri, B. Polymeric materials as anion-exchange membranes for alkaline fuel cells. Prog. Polym. Sci. 2011, 36, 1521–1557. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, W.; Wang, Y. Diffusion dialysis for acid recovery from acidic waste solutions: Anion exchange membranes and technology integration. Membranes 2020, 10, 169. [Google Scholar] [CrossRef] [PubMed]
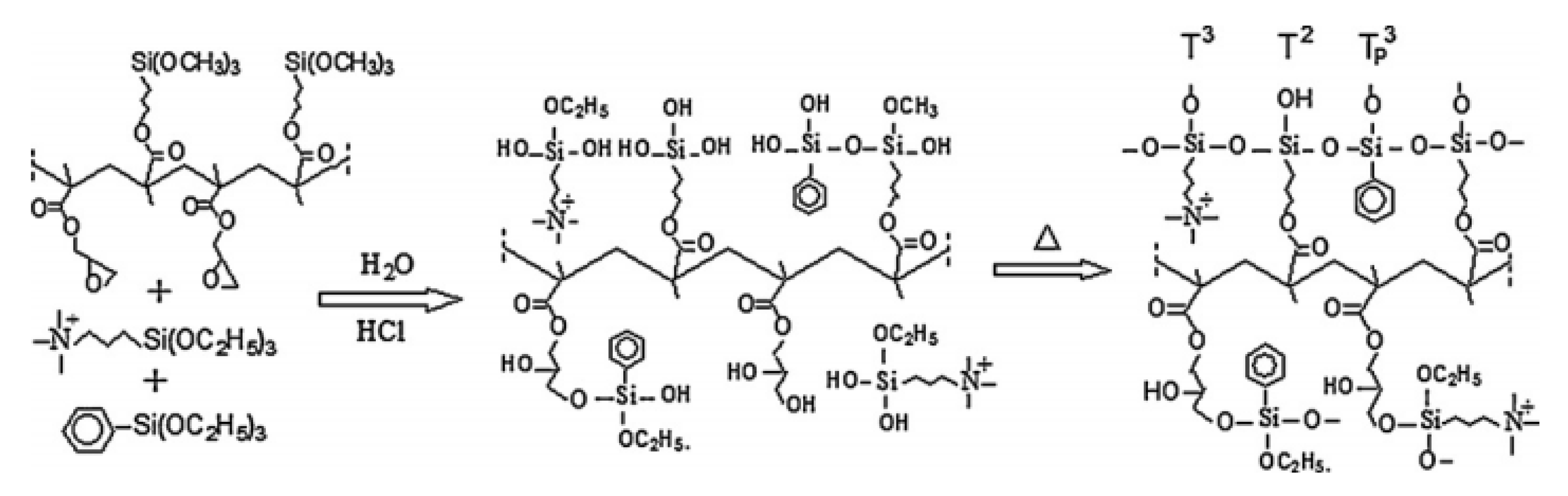
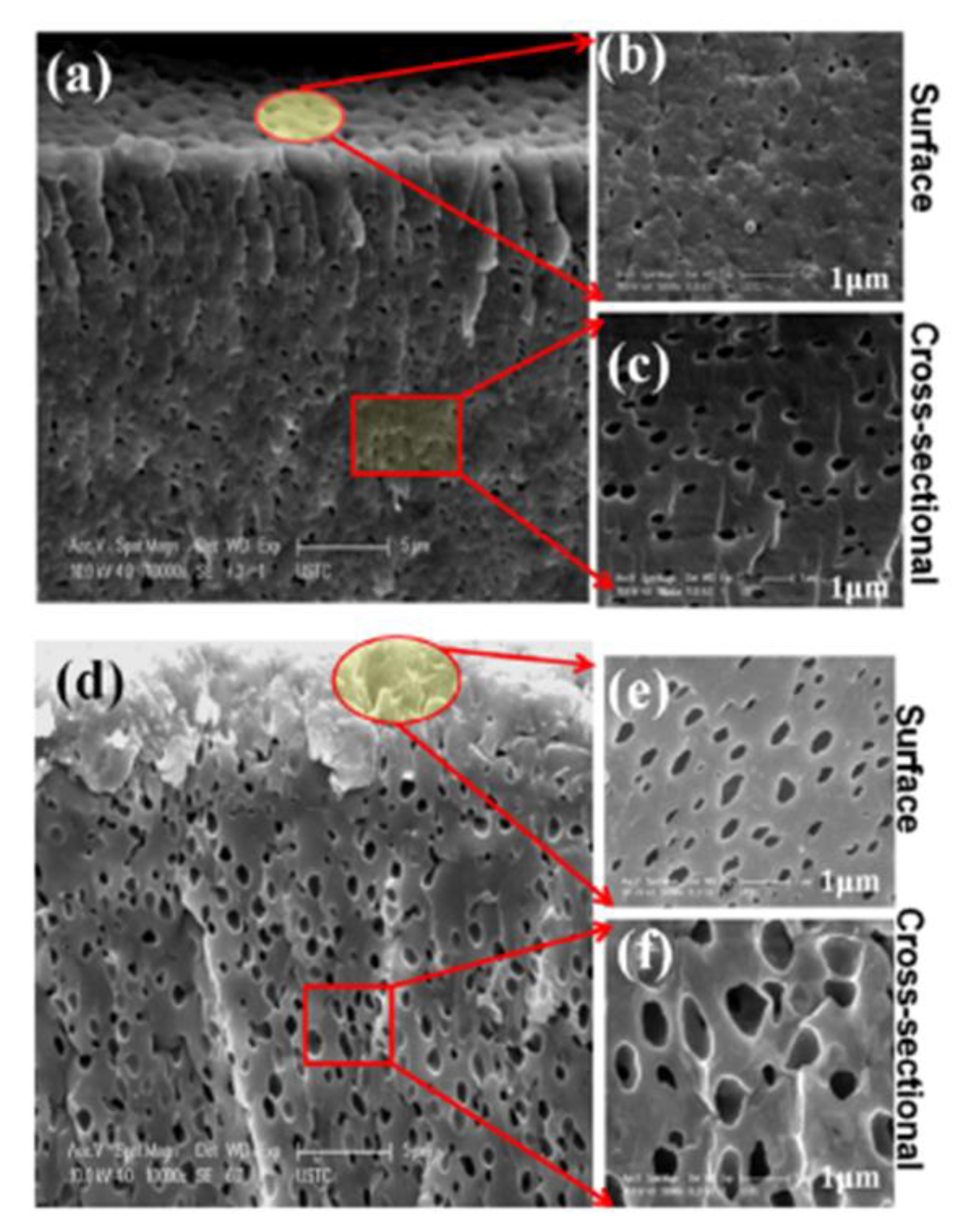
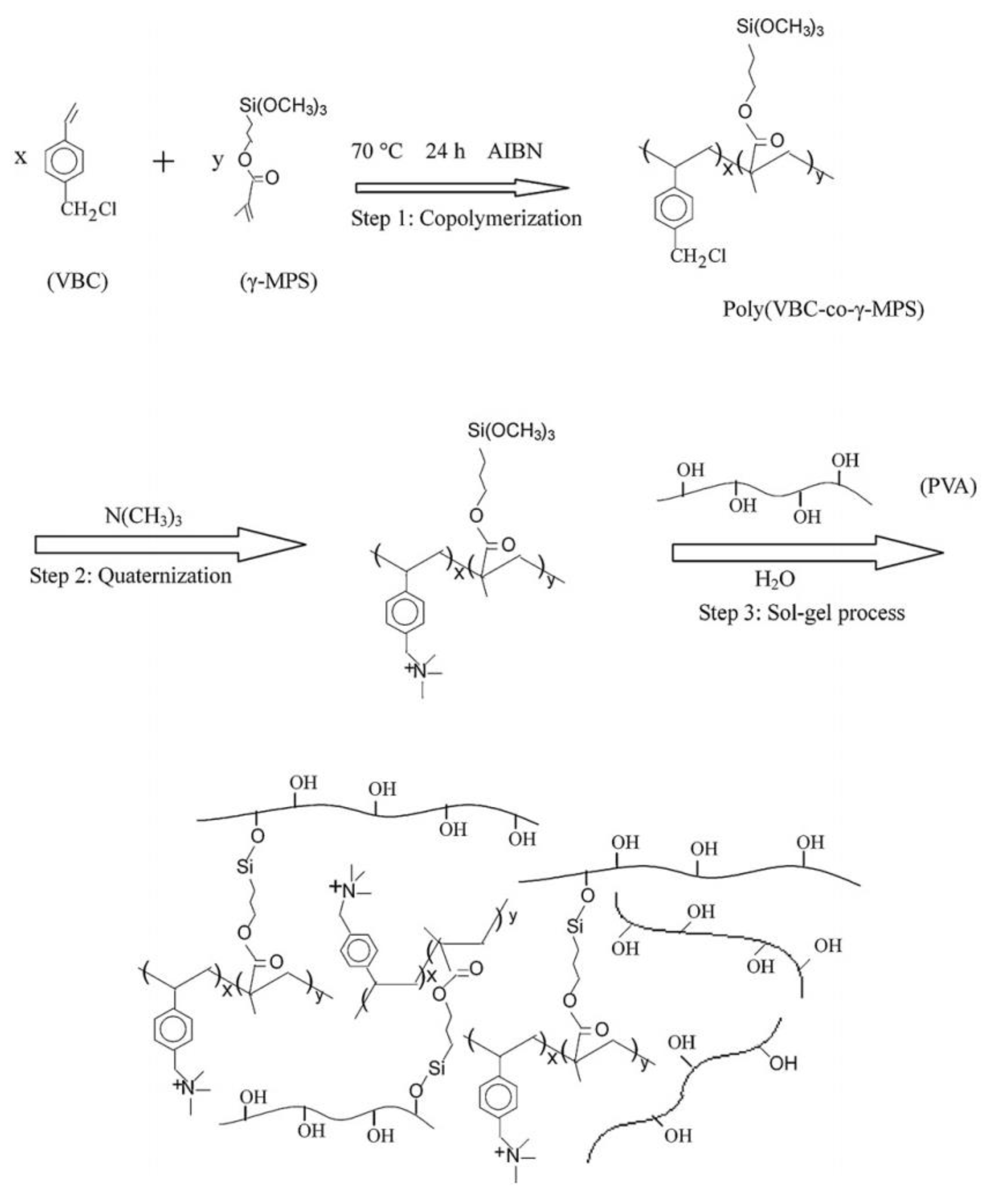
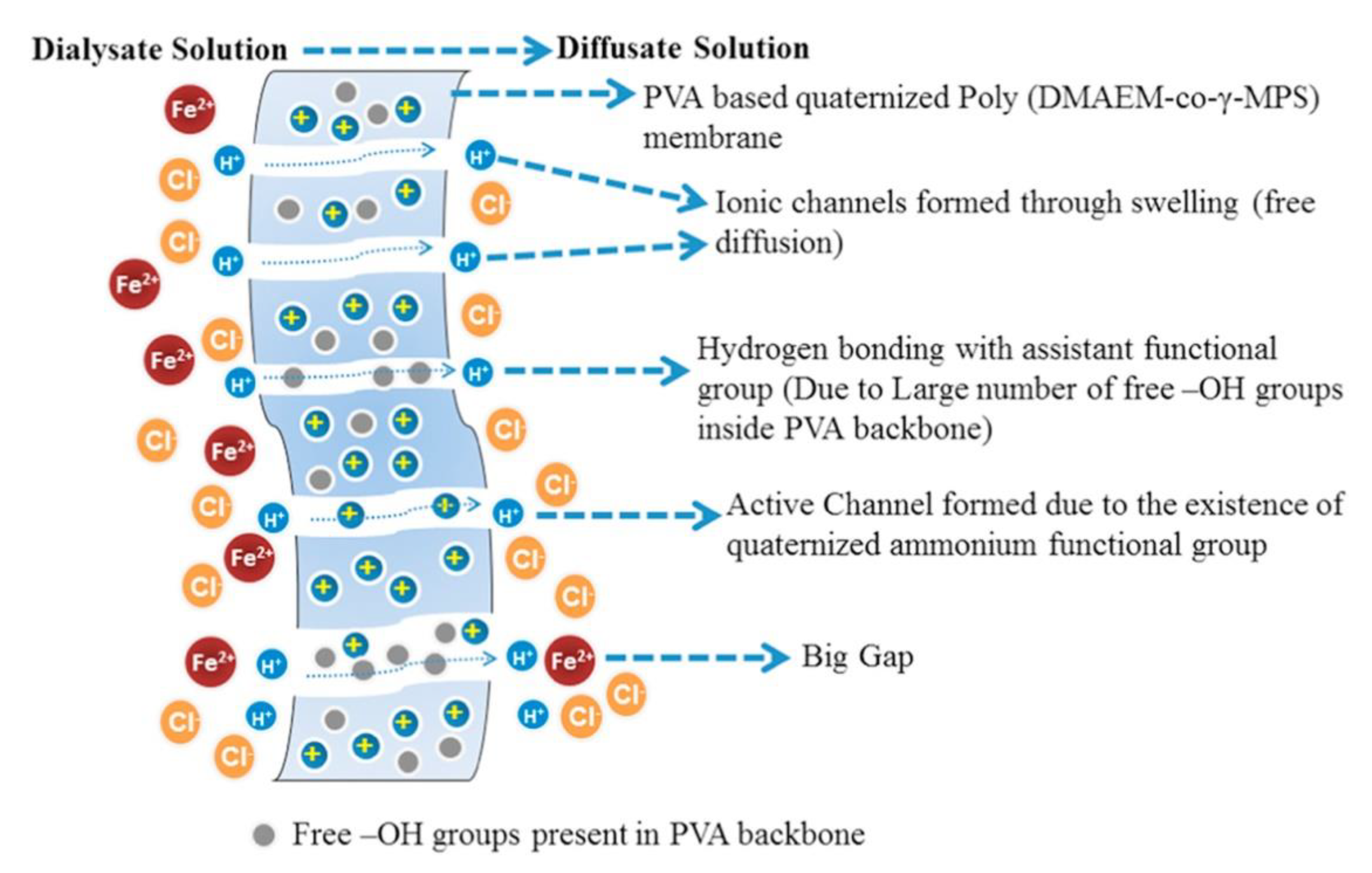




| Polymer Matrix | Si Precursor | Properties | Reference |
|---|---|---|---|
| PEO | N-[3-(trimethoxysilyl)propyl] ethylene diamine | Pore size decreases with the increase in dip-coating sols (0.2–0.6, 0.023–0.12, 0.008–0.033 and 0.002–0.006 μm) | [44] |
| Poly(GMA-co-MPS) | MPS | Pore diameters: 0.006–0.002 μm | [45] |
| Poly(GMA-co-MPS) | MPS APTEOS-I, EPh | APTEOS-I content controls the membrane electrical properties Membrane potential: 11.6–15.8 mV | [47] |
| Poly(MPS-co-GPTMOS) | GPTMOS TEOS; Triethoxysilylpropylammonium | Membrane potential: 14.8–18.8 mV Transport number > 0.92 | [48] |
| BrPPO | APTMOS | Mole ratio Si/PPO in the polymer precursor 0.34, 0.42, 0.62, and 0.91 | [49] |
| PPO-TMA hollow-fibers | TEOS | Maximum water uptake: 1.4 g water/g dry hollow fiber (10% of TEOS) Dimensional change: 13–16% | [50] |
| PPO-TMA | EPh TEOS | Easy diffusion of H+ and Fe2+ (45 °C) at high content of silica: molar ratio (TEOS + EPh)/Br-PPO = 32% | [51] |
| PPO | SiO2 | Proton dialysis coefficient: 0.0703 m/h Separation factor (HCl/FeCl2): 68.0 | [52] |
| PPO-DMAE | IL: 3-methyl-1-(3-(triethoxysilyl)propyl)-1H-imidazolium chloride | Proton dialysis coefficient: 0.020–0.0273 m/h Separation factor (HCl/FeCl2): 38.8–86.5 | [53] |
| PPO-DMAE | IL: 3-methyl-1-(3-(triethoxysilyl)propyl)-1H-imidazol-3-ium chloride | Zwitterionic pores Proton dialysis coefficient: 0.021–0.0386 m/h Separation factor (HCl/FeCl2): 33.9–62.0 | [54] |
| PVA | APTEOS-I TEOS, GPTMOS, EPh; GMA-MPS | Separation factor (HCl/FeCl2): 16–21 | [55] |
| PPO-TMA PVA | TEOS EPh | Proton dialysis coefficient: 0.021–0.049 m/h Separation factor (HCl/FeCl2): 44 | [56] |
| PPO-TMA PVA | TEOS EPh | Proton dialysis coefficient: 0.008–0.011 m/h (15 °C), 0.014–0.018 m/h (55 °C) Separation factor (HCl/FeCl2): 48–68 (15 °C), 40–51 (55 °C) | [57] |
| Poly(PVA-co-GTMA-Cl) | APTEOS | Separation factor (85% ethanol in water): 52–63 (50 °C) Nanofractal blisters on the surface | [58] |
| Poly(VBC-co-MPS) PVA | MPS | Separation factor (HCl/FeCl2): 25–30 (20 °C), 12.1–35.7 (60 °C) | [59] |
| Poly(VBC-co-MPS) (high and low molecular weight) PVA | MPS | Proton dialysis coefficient (CH3COOH): 0.009 m/h; Proton Dialysis coefficient (HCl): 0.01–0.029 m/h) Separation factor (HCl/FeCl2): 28–39 | [60] |
| PVA | TEOS 1-methylimidazole-AESP | Proton dialysis coefficient 0.0315–0.0483 m/h Separation factor (HCl/FeCl2): 28.6–52.5 | [61] |
| PPO-triethylamine | 1-vinylimidazole-APTEOS | Power consumption: 0.98–1.17 kWh/Kg Current efficiency: 74.02–89.73% | [62] |
| PVA-2-(dimethylaminomethyl)pyridine | TEOS | Proton dialysis coefficient: 0.009–0.022 m/h Fe2+ dialysis coefficient: 0.00017–0.00055 m/h Separation factor (HCl/FeCl2): 42–54 | [63] |
| PVA-DABCO | GPTMOS | Proton dialysis coefficient: 0.03–0.045 m/h Fe2+ dialysis coefficient: 0.0009–0.0015 m/h Separation factor (HCl/FeCl2): 20.9–32.3 | [64] |
| PVA-1,5-diaminonaphthalene-GTMA-Cl | TEOS | Proton dialysis coefficient: 0.0225 m/h (HCl-NaCl); 0.025 m/h (HCl-ZnCl2); 0.0275 m/h (HCl-FeCl2); 0.026 m/h (HCl-AlCl3) | [65] |
| Poly(DMAEM-co-MPS) PVA | MPS | Proton dialysis coefficient: 0.016–0.029 m/h Separation factor (HCl/FeCl2): 23.3–87.7 | [66] |
| PVA Anion exchange resin particles (Indoin) | TEOS | Counter-ion transport numbers: 0.910–0.916 (Cl−); 0.785–0.838 (Br−); 0.712–0.786 (F−) Permselectivity: 0.775–0.790 (Cl−); 0.462–0.594 (Br−); 0.387–0.545 (F−) | [67] |
| PVA-4-vinylpyridine | TEOS | Electroosmotic permeability: 0.41–2.17 × 10−4 cm3/C Counterion transport number: 0.92 | [68] |
| PVA GTMA-Cl | APTEOS | Counter ion transport number: 0.91–0.96 Permselectivity (OH−): 0.86–0.94 OH− ion conductivity: 5.9–7.6 mS/cm | [11] |
| PVA GTMA-Cl | APTEOS | Transport number: 0.79–0.92 OH− ion conductivity: 34.8–75.7 mS/cm | [69] |
| DMAEM-VTMS PVA | VTMS | Permselectivity (Cl−): 0.76–0.90 Cl− ion conductivity: 7.2 mS/cm | [70] |
| PVA | APTEOS -GTMA-Cl | Tensile strength: 55–69 MPa Elongation at break: 45–80% IEC: 0.25–0.73 meq/g | [71] |
| PVA-allyltrimethylammonium chloride | TEOS | Proton dialysis coefficient: 0.015–0.060 m/h Fe2+ dialysis coefficient: 0.013–0.020 m/h Separation factor (HCl/FeCl2): 7–22 | [72] |
| SMPEI, PVA | GPTMOS | Permselectivity (Cl−): 0.79 | [73] |
| 4-butanedioldiglycidyl ether-methylamine | Silica Gel APTEOS | Retention factor: 2.08–4.10 (Cl−); 3.59–7.01 (Br−); 1.02–1.88 (F−); 4.42–8.57 (NO3−); 2.10–5.64 (NO2−) | [74] |
| PEO | EPh TEOS | Tensile Strength: 1.0–20.5 MPa Elongation at break: 33–120% OH− ion conductivity: 3 mS/cm | [75] |
| PPO-triethylamine | EPh TEOS | IEC (Br− form) 1.27–2.05 mmol/g OH− ion conductivity: 1.0–8.5 mS/cm | [76] |
| Poly(VBC-co-MPS) | MPS EPh, TEOS | IEC (Cl− form) 1.70–2.20 mmol/g OH− ion conductivity: 0.2–0.4 mS/cm | [77] |
| PPO-triethylamine | EPh TEOS | OH- ion conductivity: 8–11 mS/cm (RT); 35 mS/cm (90 °C) Peak power density: 32 mW/cm (fuel cell test) | [78] |
| Poly(VBC-co-MPS) BrPPO | MPS | IEC (Cl− form): 2.20–2.25 mmol/g OH− ion conductivity: 12 mS/cm | [79] |
| Cardo poly(aryl ether sulfone ketone | 3-Chloropropyltrimethoxysilane TEOS | Tensile Strength: 20.0–40.3 MPa Young’s Modulus: 196–1166 MPa Elongation at break: 36–70% | [80] |
| PSU | ODGBS | OH− ion conductivity: 20–26 mS/cm (60 °C) | [81] |
| PNB | APTMOS | Methanol permeability: 1.54–2.75 × 10−7 cm2/s OH− ion conductivity 6.3–41.0 mS/cm Peak power density: 43 mW/cm (fuel cell test) | [82] |
| PNB | TMSP | Methanol permeability: 1.34–2.89 × 10−7 cm2/s OH− ion conductivity (80 °C): 6.8–9.3 mS/cm Peak power density: 32 mW/cm2;(fuel cell test) | [83] |
| PPO-1,2-dimethylimidazole | GPTMOS TEOS | IEC: 2.19–2.63 mmol/g OH− ion conductivity: 10–22 mS/cm (25 °C); 26–36 mS/cm (80 °C) | [84] |
| 5PA | silica and APTEOS | IEC: 1.29 mmol/g | [85] |
| PPO-N-methyldiethanolamine | 2-(3,4-epoxycyclohexyl) ethyltrimethoxysilane | OH− ion conductivity: 21 mS/cm Peak power density: 14.2 mW/cm2 (40 °C); 16.9 mW/cm2 (60 °C) (single cell test) | [86] |
| PPO-triethylamine | GPTMOS | OH− ion conductivity: 46.0 mS/cm (80 °C) | [87] |
| PSU-DEA GGO | APTMOS | IEC: 0.48–0.90 mmol/g OH− ion conductivity: 6–11 mS/cm (RT); 12–20 mS/cm (70 °C) | [88] |
| CS | 3-(Methacryloxy) propyl-trimethoxysilane TEOS | IEC: 0.37–0.46 mmol/g OH− ion conductivity: 1–3 mS/cm (20 °C); 6–13 mS/cm (90 °C) | [89] |
| PSU-TMA | TMSP AEAPS | IEC: 1.3–1.4 mmol/g Cl− ion conductivity: 0.8–1.3 mS/cm (RT); 3.4–3.9 mS/cm (80 °C) | [90] |
| PVA- GTMA-Cl | TEOS | Methanol permeability: 8.4–11.6 × 10−7 cm2/s. OH− ion conductivity: 3.1–6.8 mS/cm (30 °C); 14 mS/cm (60 °C) | [91] |
| PVA- pyridine | AAPTEOS | Peak power density: 53 mW/cm2 (80 °C) (fuel cell test) Alkaline stability: remaining conductivity 90% (360 h in 6M NaOH at 80 °C) | [92] |
| Fumasep FAP | TEOS | IEC: 1.07–1.13 mmol/g VO2+ permeability: 5.48 × 10−7 cm2/min | [93] |
| CS | GPTMOS | IEC: 0.34–0.71 mmol/g VO2+ permeability: 3.13–8.17 × 10−6 cm2/min SO42- ion conductivity: 7.6–11.3 mS/cm | [94] |
| PVA CS-TMA | AAPTMS TEOS | IEC: 0.82–1.29 mmol/g Chloride ion transport number: 0.86–0.94 | [95] |
| (3-acrylamidopropyl)-trimethylammonium, Polyethylene | Siloxane resins | IEC: 1.67–2.26 mmol/g Resistance: 0.23–0.32 Ω/cm2 | [96] |
| poly(QVBC-co-triethoxyvinylsilane) PVA, Graphene nano-ribbons | Triethoxyvinylsilane | IEC: 1.92–2.09 meq/g Energy consumption: 1.36 kWh/kg | [97] |
| PPO-DEA | AIPA | Zwitterionic membrane Permselectivity: 8 (Li+/Mg2+); 24.8 (K+/Mg2+); 41.3 (Na+/Mg2+); 261.7 (H+/Fe2+) | [98] |
| PSU | AEAPS 3-cyanopropyltrichlorosilane | Zwitterionic membrane 0.071 mS/cm (acidic), 0.051 mS/cm (basic), 0.0065–0.0088 mS/cm (zwitterionic) (80 °C) | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sgreccia, E.; Narducci, R.; Knauth, P.; Di Vona, M.L. Silica Containing Composite Anion Exchange Membranes by Sol–Gel Synthesis: A Short Review. Polymers 2021, 13, 1874. https://doi.org/10.3390/polym13111874
Sgreccia E, Narducci R, Knauth P, Di Vona ML. Silica Containing Composite Anion Exchange Membranes by Sol–Gel Synthesis: A Short Review. Polymers. 2021; 13(11):1874. https://doi.org/10.3390/polym13111874
Chicago/Turabian StyleSgreccia, Emanuela, Riccardo Narducci, Philippe Knauth, and Maria Luisa Di Vona. 2021. "Silica Containing Composite Anion Exchange Membranes by Sol–Gel Synthesis: A Short Review" Polymers 13, no. 11: 1874. https://doi.org/10.3390/polym13111874
APA StyleSgreccia, E., Narducci, R., Knauth, P., & Di Vona, M. L. (2021). Silica Containing Composite Anion Exchange Membranes by Sol–Gel Synthesis: A Short Review. Polymers, 13(11), 1874. https://doi.org/10.3390/polym13111874









