Effect of Immersion in Water or Alkali Solution on the Structures and Properties of Epoxy Resin
Abstract
:1. Introduction
2. Materials and Methods
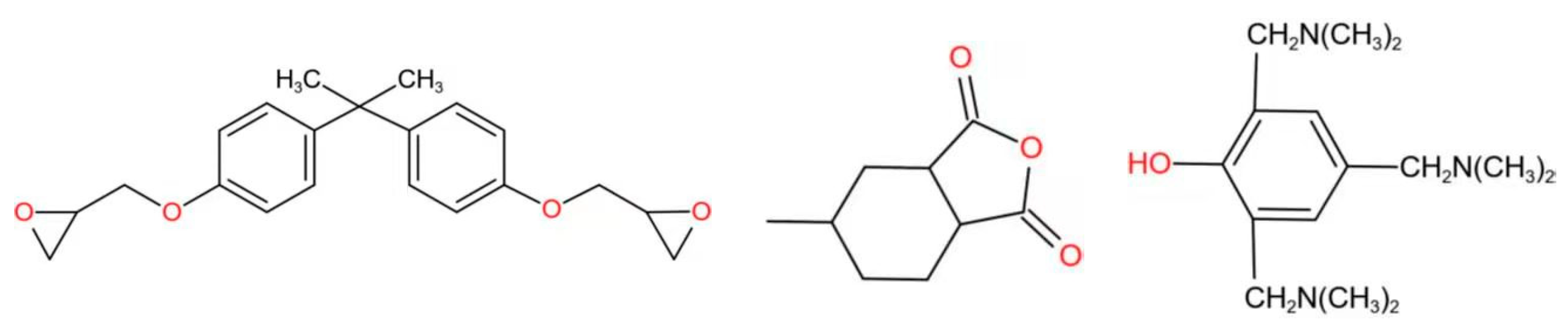
2.1. Raw Materials and Sample Preparation
2.2. Water Uptake Test
2.3. FTIR Test
2.4. DMA Test
2.5. Internal Strain Test
2.6. Positron Annihilation Lifetime Spectra (PALS) Test
2.7. Tensile Test
3. Results and Discussion
3.1. Water Absorption and Diffusion
3.2. Chemical Functional Groups Analysis
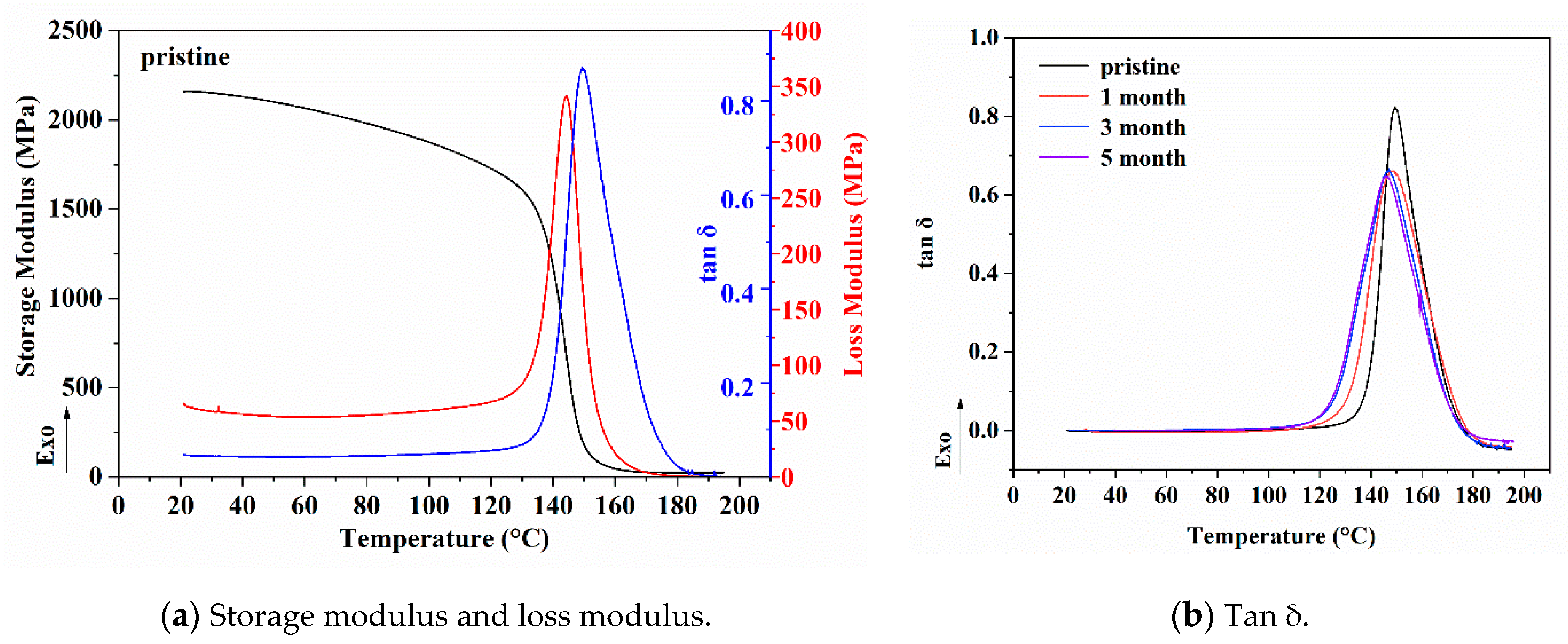
3.3. Viscoelastic Behavior Analysis
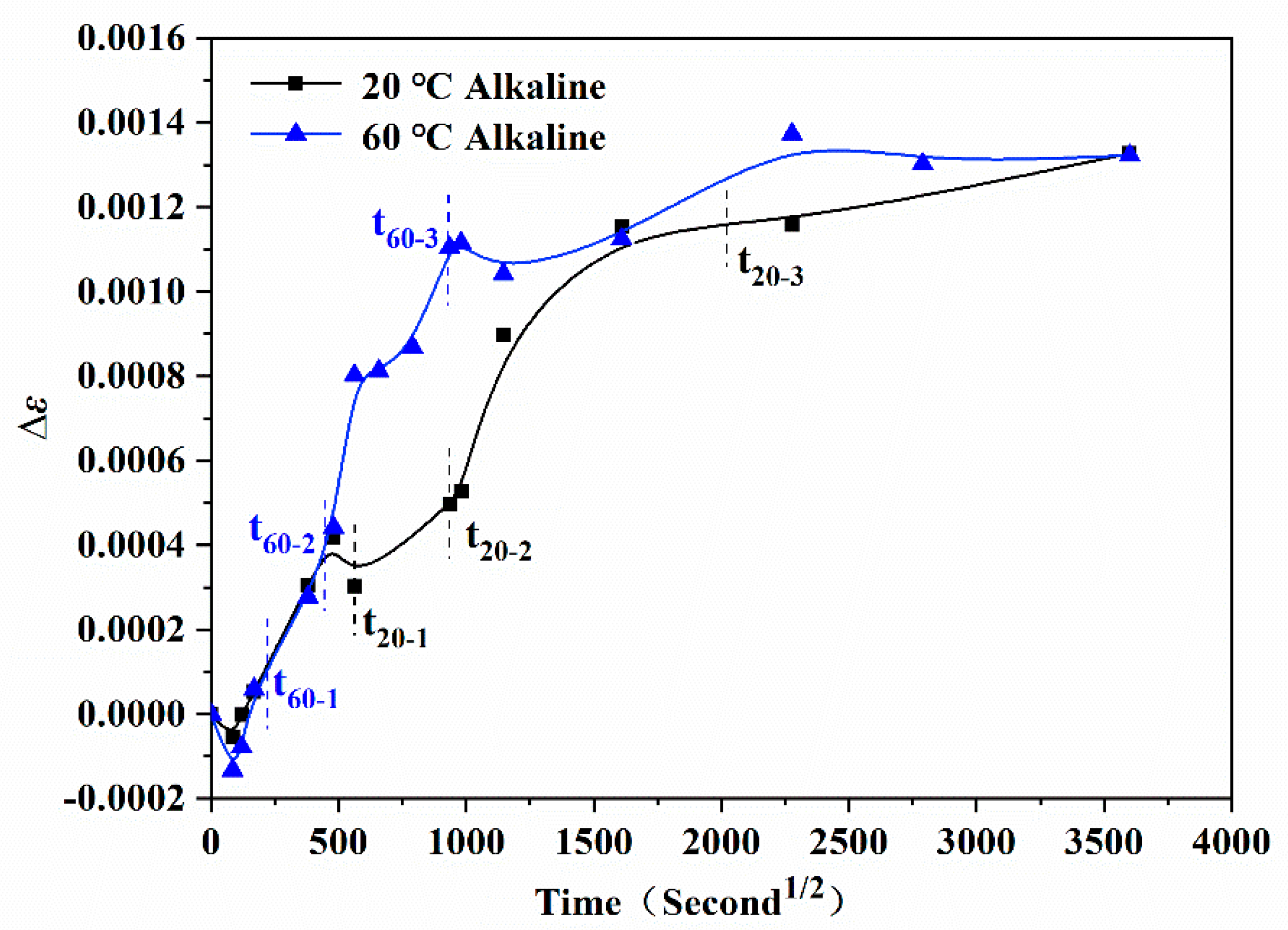
3.4. Internal Strain Monitoring
3.5. Free Volume Variation
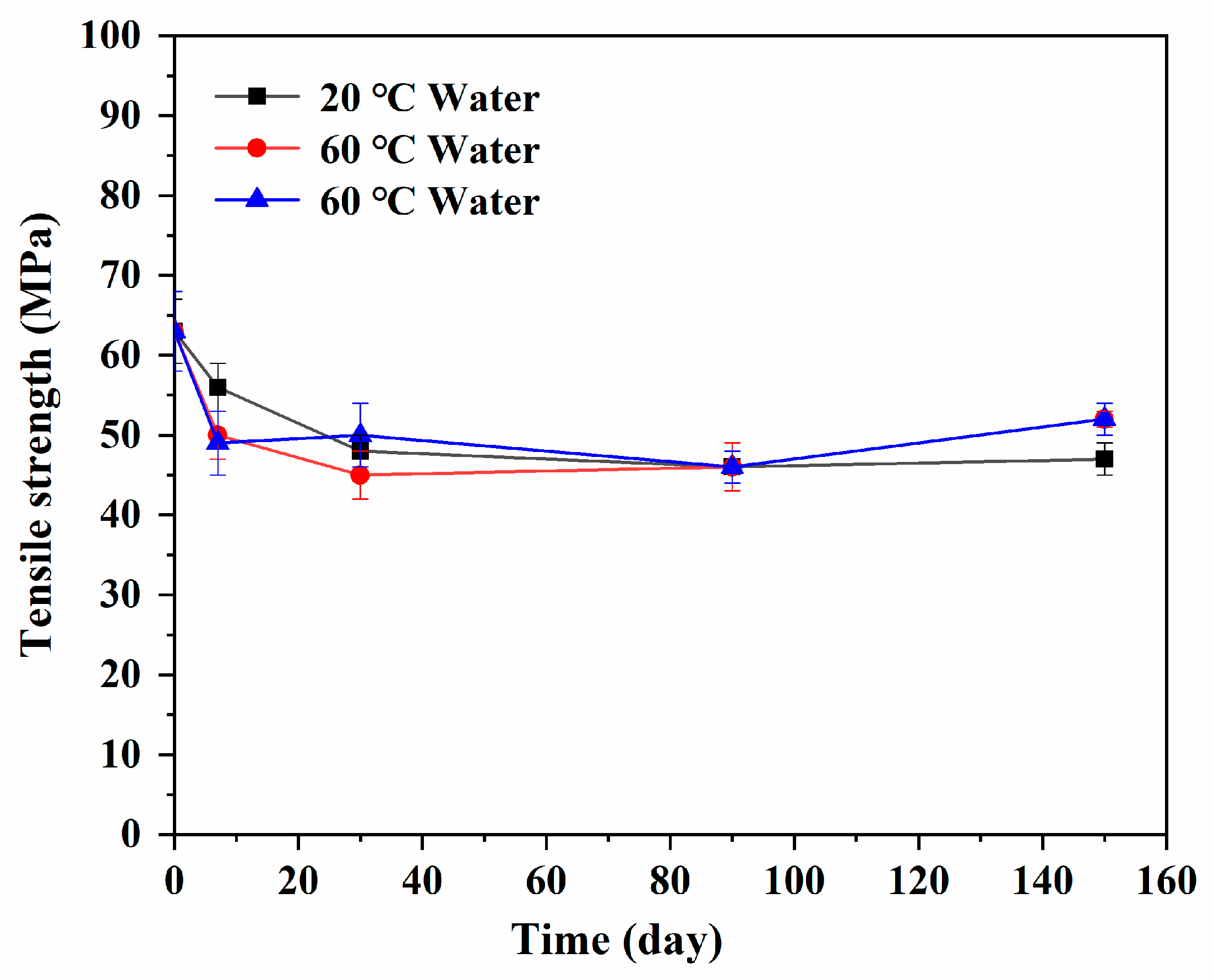
3.6. Tensile Strength Variation
4. Conclusions
- (1)
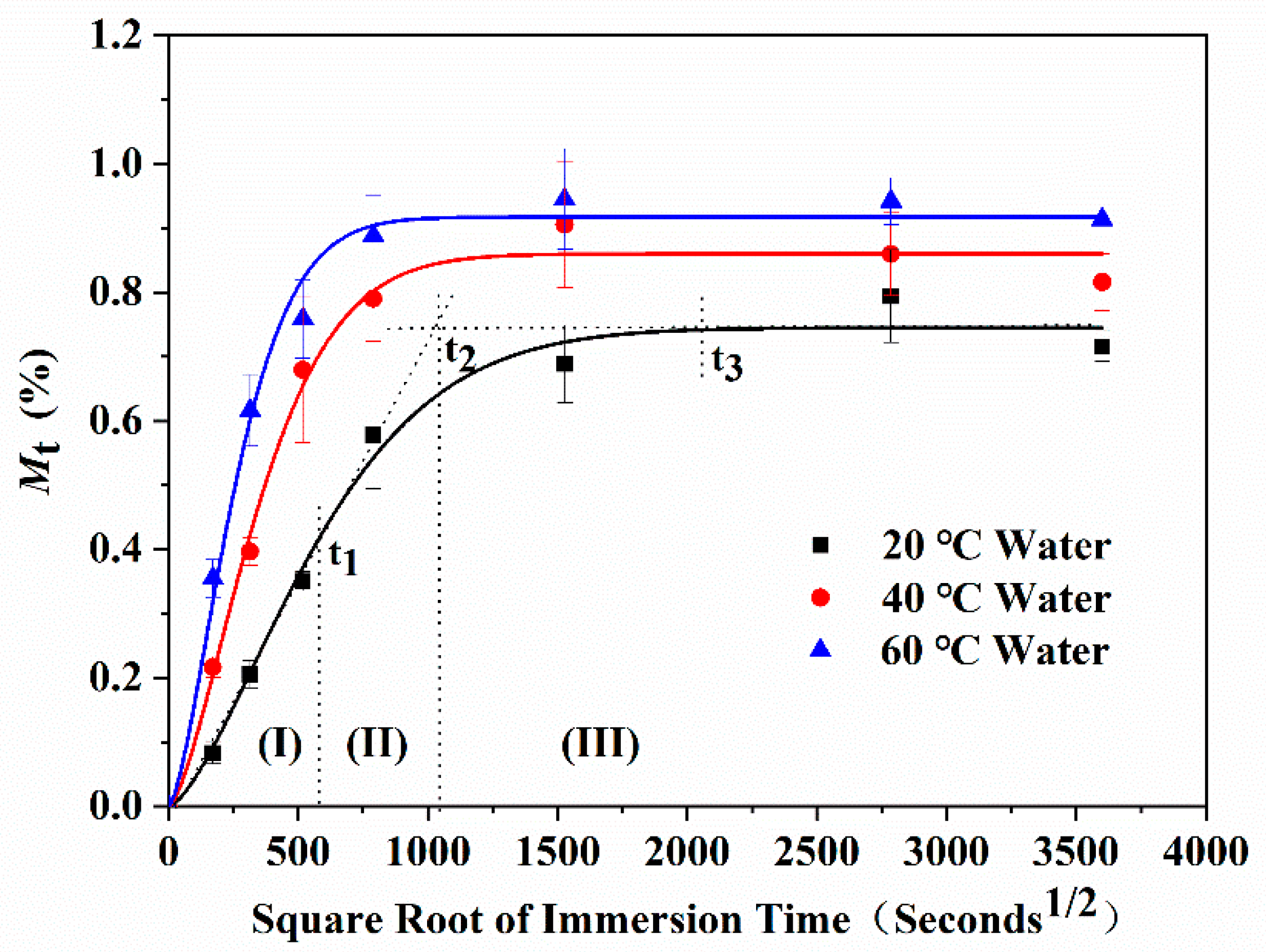
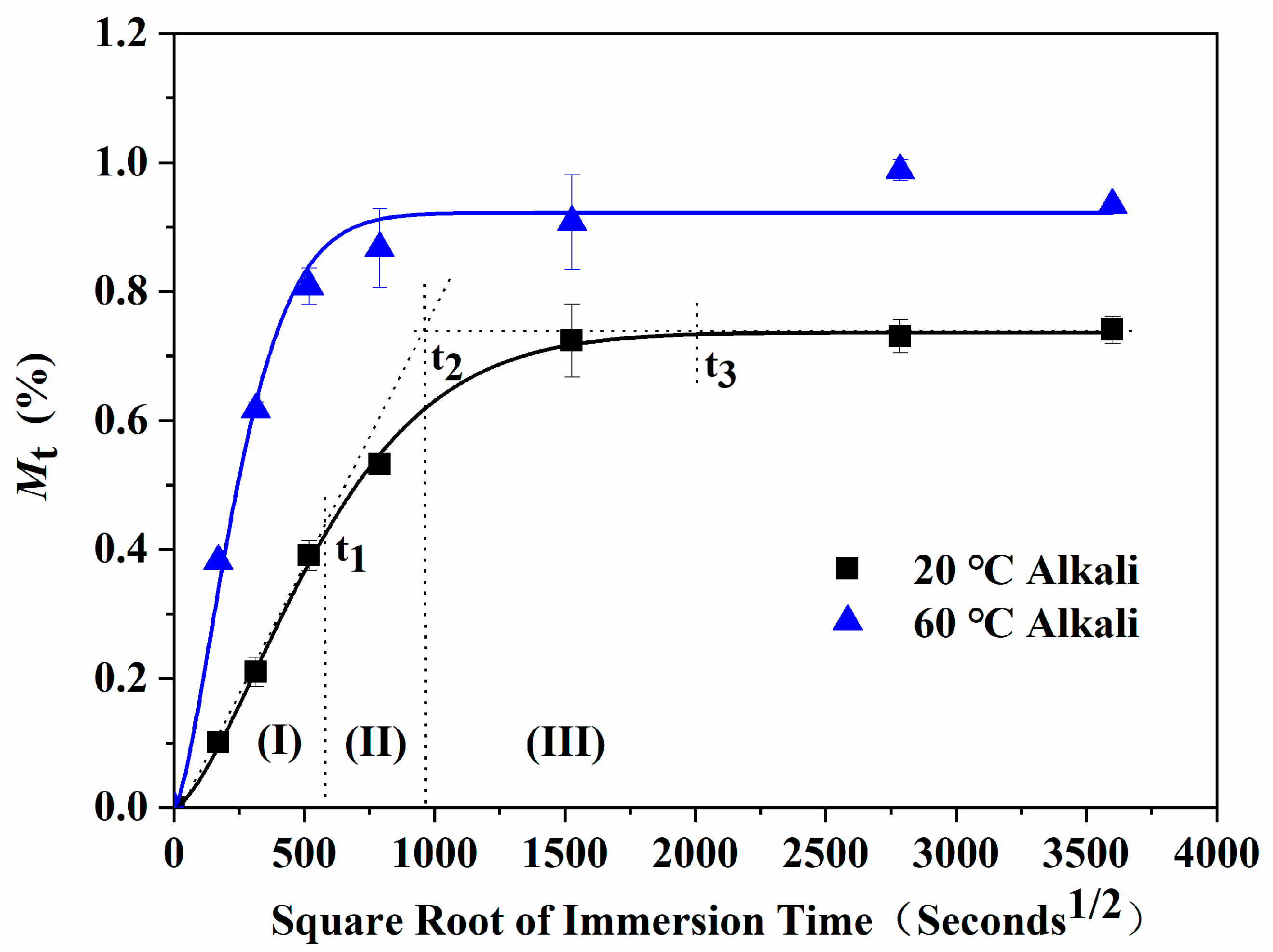
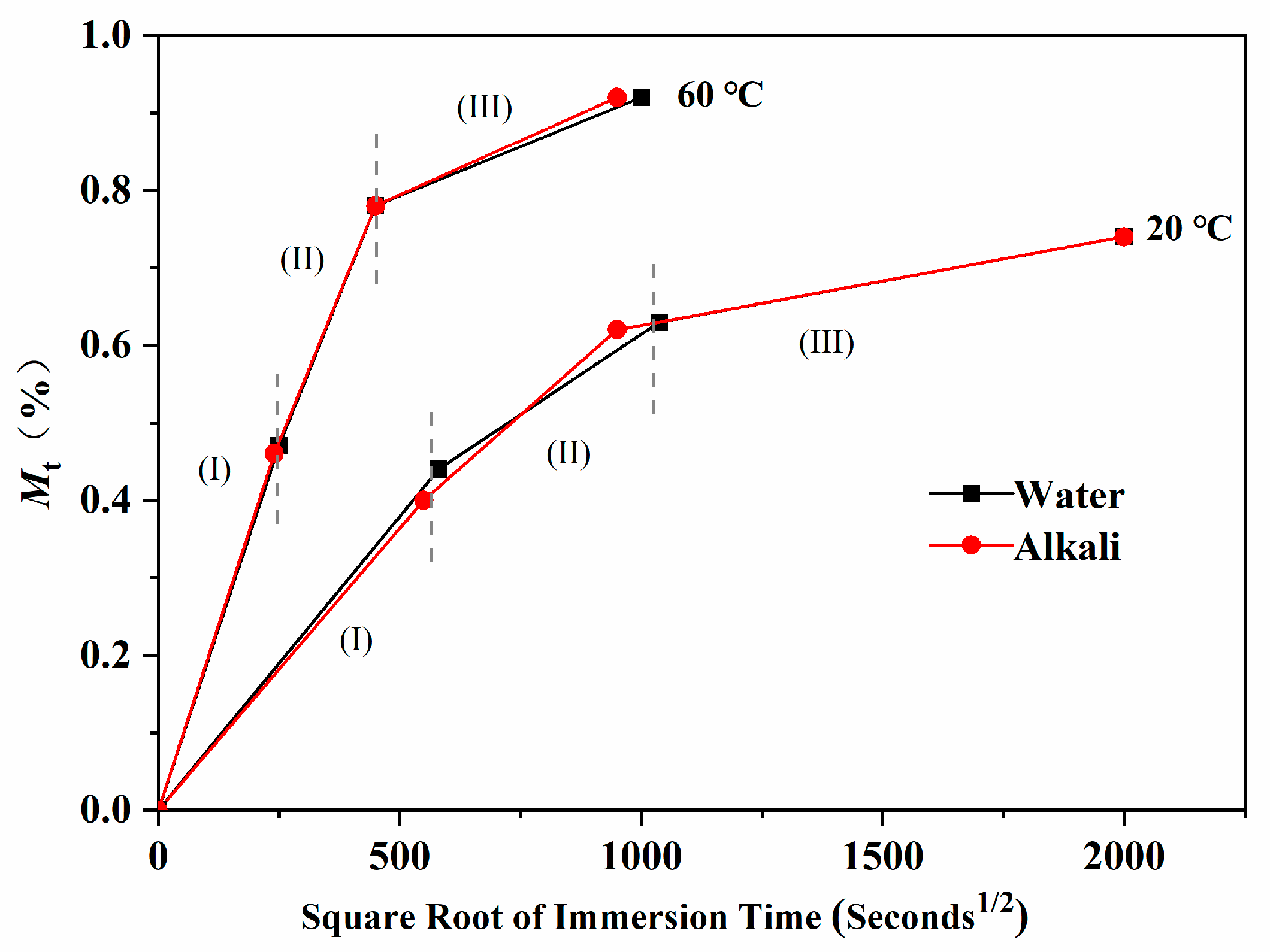
- The water absorption curves of the epoxy resin immersed in water and the alkali solution have good accordance to Fick’s law. Furthermore, the water absorption curves over time presented three characteristic stages, including a rapid water absorption stage, slow growth stage, and saturated stage. Under the coupling effects of the concentration gradient diffusion, hydrolysis reaction, and molecular segment movement, the water absorption showed unique characteristics with the immersion time and temperature.
- (2)
- After the immersion in each solution for one month, the water absorption of the resin was determined to be saturated, and the viscoelasticity of the system decreased significantly. When the immersion time was further extended, the water absorption did not change while the relaxation of the molecular segments of the resin was further developed. The original large-scale free space of the resin gradually decreased, and the new small-scale free space began to increase and gradually distributed to the interior of the epoxy resin.
- (3)
- The tensile strength of the resin decreased with the increase in water absorption until the lowest value after the immersion of one month. Compared with the initial sample, the decreased percentages of tensile strength in 20 °C and 60 °C water or the 60 °C alkali solution for one month were 24%, 28%, and 22%, respectively. After that, the tensile strength recovered with the further extension of immersion time, owing to the molecular chain structure of the resin that would be further adjusted to a more stable state at higher temperature and sufficient time. In addition, the effect of the alkali solution and water solution on the tensile strength of the epoxy resin was basically the same.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Graham-Jones, J.; Summerscales, J. Marine Applications of Advanced Fibre-Reinforced Composites; University of Plymouth: Plymouth, UK, 2016. [Google Scholar]
- Hollaway, L.C. A review of the present and future utilisation of FRP composites in the civil infrastructure with reference to their important in-service properties. Constr. Build. Mater. 2010, 24, 2419–2445. [Google Scholar] [CrossRef]
- Mallick, P.K. Fiber-Reinforced Composites: Materials, Manufacturing, and Design; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Rudawska, A. The Impact of the Acidic Environment on the Mechanical Properties of Epoxy Compounds in Different Conditions. Polymers 2020, 12, 2957. [Google Scholar] [CrossRef] [PubMed]
- Gagani, A.I.; Monsas, A.B.; Krauklis, A.E.; Echtermeyer, A.T. The effect of temperature and water immersion on the interlaminar shear fatigue of glass fiber epoxy composites using the I-beam method. Compos. Sci. Technol. 2019, 181, 107703. [Google Scholar] [CrossRef]
- Baltzis, D.; Bekas, D.; Tsirka, K.; Parlamas, A.; Ntaflos, A.; Zafeiropoulos, N.; Lekatou, A.G.; Paipetis, A.S. Multi-scaled carbon epoxy composites underwater immersion: A durability study. Compos. Sci. Technol. 2020, 199, 108373. [Google Scholar] [CrossRef]
- Mourad, A.I.; Idrisi, A.H.; Wrage, M.C.; Abdel-Magid, B.M. Long-term durability of thermoset composites in seawater environment. Compos. Part B 2019, 168, 243–253. [Google Scholar] [CrossRef]
- Ghabezi, P.; Harrison, N. Mechanical behavior and long-term life prediction of carbon/epoxy and glass/epoxy composite laminates under artificial seawater environment. Mater. Lett. 2020, 261, 127091. [Google Scholar] [CrossRef]
- Guen-Geffroy, L.; Le Gac, P.Y.; Habert, B.; Davies, P. Physical ageing of epoxy in a wet environment: Coupling between plasticization and physical ageing. Polym. Degrad. Stab. 2019, 168, 108947. [Google Scholar] [CrossRef]
- Ascione, F.; Granata, L.; Guadagno, L.; Naddeo, C. Hygrothermal durability of epoxy adhesives used in civil structural applications. Compos. Struct. 2021, 265, 113591. [Google Scholar] [CrossRef]
- Nash, N.H.; Portela, A.; Bachour-Sirerol, C.I.; Manolakis, I.; Comer, A.J. Effect of environmental conditioning on the properties of thermosetting-and thermoplastic-matrix composite materials by resin infusion for marine applications. Compos. Part B 2019, 177, 107271. [Google Scholar] [CrossRef]
- Obradovic, V.; Simic, D.; Sejkot, P.; Machalicka, K.V.; Vokacv, M. Moisture absorption characteristics and effects on mechanical properties of Kolon/epoxy composites. Curr. Appl. Phys. 2021, 26, 16–23. [Google Scholar] [CrossRef]
- Colombini, D.; Martinez-Vega, J.J.; Merle, G. Dynamic mechanical investigations of the effects of water sorption and physical ageing on an epoxy resin system. Polymer 2002, 43, 4479–4485. [Google Scholar] [CrossRef]
- Tcharkhtchi, A.; Bronnec, P.Y.; Verdu, J. Water absorption characteristics of diglycidylether of butane diol-3,5-diethyl-2,4-diaminotoluene networks. Polymer 2000, 41, 5777–5785. [Google Scholar] [CrossRef]
- Merdas, I.; Tcharkhtchi, A.; Thominette, F.; Verdu, J.; Dean, K.; Cook, W. Water absorption by uncrosslinked polymers, networks and IPNs having medium to high polarity. Polymer 2002, 43, 4619–4625. [Google Scholar] [CrossRef]
- Merdas, I.; Thominette, F.; Tcharkhtchi, A.; Verdu, J. Factors governing water absorption by composite matrices. Compos. Sci. Technol. 2002, 62, 487–492. [Google Scholar] [CrossRef]
- Karbhari, V.M.; Xian, G. Hygrothermal effects on high VF pultruded unidirectional carbon/epoxy composites: Moisture uptake. Compos. B Eng. 2009, 40, 41–49. [Google Scholar] [CrossRef]
- Marouani, S.; Curtil, L.; Hamelin, P. Ageing of carbon/epoxy and carbon/vinylester composites used in the reinforcement and/or the repair of civil engineering structures. Compos. B Eng. 2012, 43, 2020–2030. [Google Scholar] [CrossRef]
- Wang, C. Structure of New Polypropylene Based FRP Bars and OFBG Sensors and Their Properties; Harbin Institute of Technology: Harbin, China, 2009. (In Chinese) [Google Scholar]
- Hamielec, A.E.; Eldrup, M.; Mogensen, O. Positron Annihilation Techniques (PAT) in Polymer Science and Engineering. J. Macromol. Sci. Part C Polym. Rev. 1973, 9, 305–337. [Google Scholar] [CrossRef]
- Yang, Y.; Xian, G.; Li, H.; Sui, L. Thermal aging of an anhydride-cured epoxy resin. Polym. Degrad. Stab. 2015, 118, 111–119. [Google Scholar] [CrossRef]
- Kamon, T.; Furukawa, H. Curing mechanisms and mechanical properties of cured epoxy resins. In Epoxy Resins and Composites IV; Springer: Berlin/Heidelberg, Germany, 1986; pp. 173–202. [Google Scholar]
- Zhou, J.; Lucas, J.P. Hygrothermal effects of epoxy resin. Part I: The nature of water in epoxy. Polymer 1999, 40, 5505–5512. [Google Scholar] [CrossRef]
- Zhou, J.; Lucas, J.P. Hygrothermal effects of epoxy resin. Part II: Variations of glass transition temperature. Polymer 1999, 40, 5513–5522. [Google Scholar] [CrossRef]
- Li, Y.; Miranda, J.; Sue, H.J. Hygrothermal diffusion behavior in bismaleimide resin. Polymer 2001, 18, 7791–7799. [Google Scholar] [CrossRef]
- Yin, X.; Liu, Y.; Miao, Y.; Xian, G. Water Absorption, Hydrothermal Expansion, and Thermomechanical Properties of a Vinylester Resin for Fiber-Reinforced Polymer Composites Subjected to Water or Alkaline Solution Immersion. Polymers 2019, 11, 505. [Google Scholar] [CrossRef] [Green Version]
- Hong, B.; Xian, G.; Li, H. Effects of water or alkali solution immersion on the water uptake and physicomechanical properties of polyurethane. Polym. Eng. Sci. 2018, 58, 2276–2287. [Google Scholar] [CrossRef]
- Nogueira, P.; Ramarez, C.; Torres, A.; Abad, M.J.; Cano, J.; Lopez, J.; Lopez-Bueno, I.; Barral, L. Effect of water sorption on the structure and mechanical properties of an epoxy resin system. J. Appl. Polym. Sci. 2001, 80, 71–80. [Google Scholar] [CrossRef]
- Weng, S.; Xu, Y. Fourier Transform Infrared Spectroscopy Analysis; Chemical Industry Press: Beijing, China, 2016. (In Chinese) [Google Scholar]


| Immersion Temperature (°C) | Stage I | Stage II | Stage III | |||
|---|---|---|---|---|---|---|
| t1 (s1/2) | (%) | t2 (s1/2) | (%) | t3 (s1/2) | (%) | |
| 20 | 580 | 0.44 | 1038 | 0.63 | 2000 | 0.74 |
| 40 | 300 | 0.45 | 625 | 0.72 | 1250 | 0.86 |
| 60 | 250 | 0.47 | 450 | 0.78 | 1000 | 0.92 |
| Peak (cm−1) | Characteristic Functional Groups | Peak (cm−1) | Characteristic Functional Groups |
|---|---|---|---|
| 3450 | –OH stretching | 1430–1400 | OH bending of saturated alcohol |
| 3026 | Hydrocarbon CH stretching | 1300 | C–O cable |
| 2964 | CH stretching (antisymmetric) | 1200–1100 | CO cable of alcohol |
| 1739 | C=O stretching | 1046 | Stretching of phenyl ether bond |
| 1608 | Benzene skeleton | 830 | Bending between adjacent hydrogens on benzene ring |
| 1510 | Benzene skeleton |
| Immersion Solution | (I) Stage (0~T1) | (II) Stage (T1~T2) | (III) Stage (T2~T3) | After Stage | ||||
|---|---|---|---|---|---|---|---|---|
| 20 °C alkali | 0.40 | 0.03 | 0.22 | 0.02 | 0.12 | 0.06 | 0.01 | 0.02 |
| 60 °C alkali | 0.46 | 0.01 | 0.32 | 0.03 | 0.14 | 0.07 | 0.01 | 0.02 |
| Parameter | Initial Sample | W-20 | W-60 | W-20 (Drying) | W-40 (Polished) |
|---|---|---|---|---|---|
| τ3 (ns) | 1.8807 | 1.8523 | 1.8279 | 1.8692 | 1.8411 |
| R (nm) | 0.274 | 0.271 | 0.269 | 0.273 | 0.270 |
| I3 (%) | 26.338 | 26.4492 | 26.6104 | 26.7063 | 27.0773 |
| fapp (nm) | 2.269 | 2.205 | 2.169 | 2.276 | 2.232 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Li, D.; Xian, G.; Li, C. Effect of Immersion in Water or Alkali Solution on the Structures and Properties of Epoxy Resin. Polymers 2021, 13, 1902. https://doi.org/10.3390/polym13121902
Wang B, Li D, Xian G, Li C. Effect of Immersion in Water or Alkali Solution on the Structures and Properties of Epoxy Resin. Polymers. 2021; 13(12):1902. https://doi.org/10.3390/polym13121902
Chicago/Turabian StyleWang, Bin, Dihui Li, Guijun Xian, and Chenggao Li. 2021. "Effect of Immersion in Water or Alkali Solution on the Structures and Properties of Epoxy Resin" Polymers 13, no. 12: 1902. https://doi.org/10.3390/polym13121902






