Biopolymers for Biological Control of Plant Pathogens: Advances in Microencapsulation of Beneficial Microorganisms
Abstract
:1. Introduction
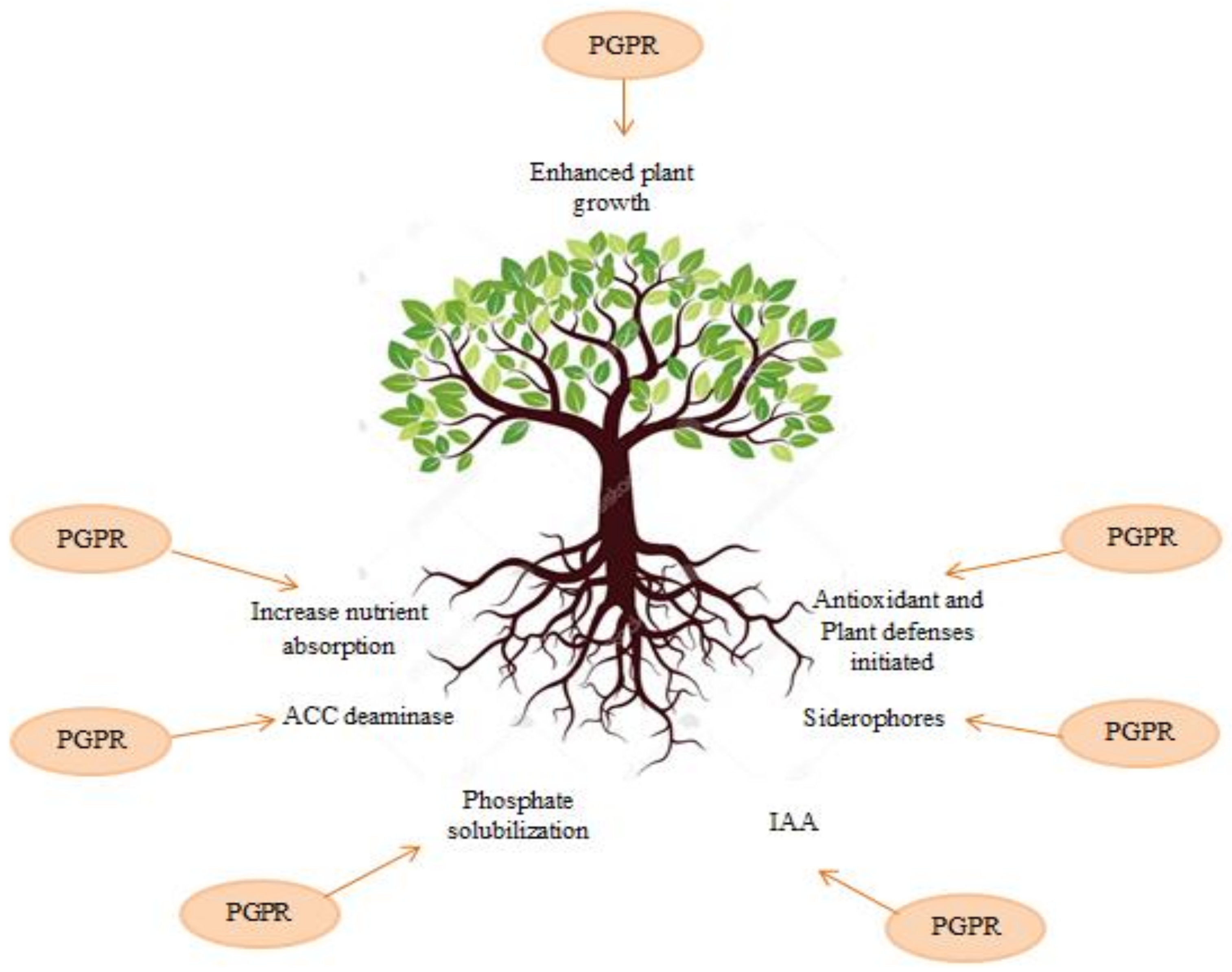
2. Mechanisms of PGPR Bacteria on Biocontrol of Plant Pathogens
3. Encapsulation
3.1. Spray Drying
3.2. Emulsion
3.3. Extrusion
4. Polymeric Materials for Cell Microencapsulation
4.1. Alginate
4.2. Chitosan
4.3. Starch
4.4. Pectin
4.5. Gelatin
4.6. Milk Proteins
4.7. Xanthan and Gellan Gum
4.8. κ-Carrageenan
4.9. Gum Arabic
4.10. Poly-L-Lysine
4.11. Poly l-Glutamic Acid
5. PGPR Encapsulation in Agriculture
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malusa, E.; Vassilev, N. A Contribution to Set a Legal Framework for Biofertilisers. Appl. Microbiol. Biotechnol. 2014, 98, 6599–6607. [Google Scholar] [CrossRef] [Green Version]
- Sanyé-Mengual, E.; Oliver-Solà, J.; Montero, J.I.; Rieradevall, J. An Environmental and Economic Life Cycle Assessment of Rooftop Greenhouse (RTG) Implementation in Barcelona, Spain. Assessing New Forms of Urban Agriculture from the Greenhouse Structure to the Final Product Level. Int. J. Life Cycle Assess. 2015, 20, 350–366. [Google Scholar] [CrossRef] [Green Version]
- Usta, C. Microorganisms in Biological Pest Control -A Review (Bacterial Toxin Application and Effect of Environmental Factors). In Current Progress in Biological Research; IntechOpen: Rijeka, Croatia, 2013; pp. 287–317. [Google Scholar] [CrossRef] [Green Version]
- Van Lenteren, J.C.; Bolckmans, K.; Köhl, J.; Ravensberg, W.J.; Urbaneja, A. Biological Control Using Invertebrates and Microorganisms, Plenty of New Opportunities. BioControl 2018, 63, 39–59. [Google Scholar] [CrossRef] [Green Version]
- Xavier, I.J.; Holloway, G.; Leggett, M. Development of Rhizobial Inoculant Formulations. Philom Bios. Inc. 2004, 3, 1–6. [Google Scholar] [CrossRef]
- Smolińska, U.; Kowalska, B. Biological Control of the Soil-Borne Fungal Pathogen Sclerotinia sclerotiorum––A Review. J. Plant Pathol. 2018, 100, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Saberi-Riseh, R.; Hajieghrari, B.; Rouhani, H.; Sharifi-Tehrani, A. Effects of Inoculum Density and Substrate Type on Saprophytic Survival of Phytophthora Drechsleri, the Causal Agent of Gummosis (crown and Root Rot) on Pistachio in Rafsanjan, Iran. Commun. Agric. Appl. Boil. Sci. 2004, 69, 653–656. [Google Scholar]
- Zeynadini-Riseh, A.; Mahdikhani-Moghadam, E.; Rouhani, H.; Moradi, M.; Saberi-Riseh, R.; Mohammadi, A. Effect of Some Probiotic Bacteria As Biocontrol Agents of Meloidogyne Incognita and Evaluation of Biochemical Changes of Plant Defense Enzymes on Two Cultivars of Pistachio. Jast 2018, 20, 179–191. [Google Scholar]
- Saberi-Riseh, R.; Javan-Nikkhah, M.; Heidarian, R.; Hosseini, S.; Soleimani, P. Detection of Fungal Infectous Agent of Wheat Grains in Store-Pits of Markazi Province, Iran. Commun. Agric. Appl. Boil. Sci. 2004, 69, 541–544. [Google Scholar]
- Shinwari, K.I.; Shah, A.U.; Afridi, M.I.; Zeeshan, M.; Hussain, H.; Hussain, J.; Ahmad, O. Application of plant growth promoting rhizobacteria in bioremediation of heavy metal polluted soil. J. Multidiscip. Stud. 2015, 3, 179–185. [Google Scholar]
- Cunniffe, N.J.; Gilligan, C.A. A Theoretical Framework for Biological Control of Soil-Borne Plant Pathogens: Identifying Effective Strategies. J. Biol. 2011, 278, 32–43. [Google Scholar] [CrossRef] [Green Version]
- Weller, D.M. Biological Control of Soilborne Plant Pathogens in the Rhizosphere with Bacteria. Annu. Rev. Phytopathol. 1988, 26, 379–407. [Google Scholar] [CrossRef]
- Stevens, B.M.; Sonderegger, D.L.; Johnson, N.C. Biotic and Abiotic Factors Predict the Biogeography of Soil Microbes in the Serengeti. BioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Moradi-Pour, M.; Saberi Riseh, R.; Mohammadinejad, R.; Hosseini, A. Biological Control of Phytophthora Drechsleri the Causal Agent of Pistachio Gummosis by Bacillus Subtilis (VRU1 Strain) in Green House Condition. PHJ 2019, 2, 53–61. [Google Scholar] [CrossRef]
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector—Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef] [Green Version]
- Heydari, A.; Pessarakli, M. A Review on Biological Control of Fungal Plant Pathogens Using Microbial Antagonists. J. Biol. Sci. 2010, 10, 273–290. [Google Scholar] [CrossRef] [Green Version]
- Shitan, N. Secondary Metabolites in Plants: Transport and Self-Tolerance Mechanisms. Biosci. Biotechnol. Biochem. 2016, 80, 1283–1293. [Google Scholar] [CrossRef] [Green Version]
- Bashan, Y.; De-Bashan, L.E.; Prabhu, S.R.; Hernandez, J. Advances in Plant Growth-Promoting Bacterial Inoculant Technology: Formulations and Practical Perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Stephens, J.; Rask, H. Inoculant Production and Formulation. Field Crop. Res. 2000, 65, 249–258. [Google Scholar] [CrossRef]
- Lee, J.P.; Lee, S.-W.; Kim, C.S.; Son, J.H.; Song, J.H.; Lee, K.Y.; Kim, H.J.; Jung, S.J.; Moon, B.J. Evaluation of Formulations of Bacillus Licheniformis for the Biological Control of Tomato Gray Mold Caused by Botrytis Cinerea. Biol. Control 2006, 37, 329–337. [Google Scholar] [CrossRef]
- Cawoy, H.; Bettiol, W.; Fickers, P.; Onge, M. Bacillus-Based Biological Control of Plant Diseases. In Pesticides in the Modern World Pesticides Use and Management; IntechOpen: Rijeka, Croatia, 2011; pp. 273–302. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Castillo, F.; Aguirre-Aguirre, A.; Lira-Saldivar, R.; Guerrero-Rodríguez, E.; Gallegos-Morales, G. Biological efficiency of organic. biological and chemical products against Alternaria dauci Kühn and its effects on carrot crop. Phyton Int. J. Exp. Bot. 2006, 75, 91–101. [Google Scholar]
- Singh, N.; Kumar, S.; Bajpai, V.K.; Dubey, R.; Maheshwari, D.; Kang, S.C. Biological Control of Macrophomina Phaseolina by Chemotactic Fluorescent Pseudomonas Aeruginosa PN1 and Its Plant Growth Promotory Activity in Chir-Pine. Crop. Prot. 2010, 29, 1142–1147. [Google Scholar] [CrossRef]
- Gajbhiye, A.; Rai, A.R.; Meshram, S.U.; Dongre, A.B. Isolation, Evaluation and Characterization of Bacillus Subtilis from Cotton Rhizospheric Soil With Biocontrol Activity Against Fusarium Oxysporum. World J. Microbiol. Biotechnol. 2010, 26, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Maketon, M.; Apisitsantikul, J.; Siriraweekul, C. Greenhouse Evaluation of Bacillus Subtilis AP-01 and Trichoderma Harzianum AP-001 in Controlling Tobacco Diseases. Braz. J. Microbiol. 2008, 39, 296–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in Cancer Therapy and Diagnosis. Adv. Drug Deliv. Rev. 2012, 64, 24–36. [Google Scholar] [CrossRef]
- Vemmer, M.; Patel, A. Review of Encapsulation Methods Suitable for Microbial Biological Control Agents. Biol. Control 2013, 67, 380–389. [Google Scholar] [CrossRef]
- Fazeli, M.; Toliyat, T.; Samadi, N.; Hajjaran, S.; Jamalifar, H. Viability of Lactobacillus Acidophilus in Various Vaginal Tablet Formulations. DARU J. Pharm. Sci. 2006, 14, 172–178. [Google Scholar]
- Hatefi, A.; Amsden, B. Biodegradable Injectable in Situ Forming Drug Delivery Systems. J. Control. Release 2002, 80, 9–28. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Oliveira, J.L.; Fraceto, L.F.; Singh, B. Polysaccharides As Safer Release Systems for Agrochemicals. Agron. Sustain. Dev. 2015, 35, 47–66. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The Hidden World Within Plants: Ecological and Evolutionary Considerations for Defining Functioning of Microbial Endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [Green Version]
- Gray, E.; Smith, D. Intracellular and Extracellular PGPR: Commonalities and Distinctions in the plant–bacterium Signaling Processes. Soil Biol. Biochem. 2005, 37, 395–412. [Google Scholar] [CrossRef]
- Syed-Ab-Rahman, S.F.; Singh, E.; Pieterse, C.M.; Schenk, P.M. Emerging Microbial Biocontrol Strategies for Plant Pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents Against Plant Diseases: Relevance Beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brígido, C.; Glick, B.R.; Oliveira, S. Survey of Plant Growth-Promoting Mechanisms in Native Portuguese Chickpea Meso-Rhizobium Isolates. Microb Ecol. 2017, 73, 900–915. [Google Scholar] [CrossRef] [PubMed]
- Flores-Félix, J.D.; Menéndez, E.; Rivera, L.P.; Marcos-García, M.; Martínez-Hidalgo, P.; Mateos, P.F.; Martínez-Molina, E.; Velázquez, M.d.L.E.; García-Fraile, P.; Rivas, R. Use of Rhizobium Leguminosarum As a Potential Biofertilizer for Lactuca Sativa and Daucus Carota. Crop. Plant Nutr. Soil Sci. 2013, 176, 876–882. [Google Scholar] [CrossRef]
- Kang, S.-M.; Khan, A.L.; Waqas, M.; You, Y.-H.; Hamayun, M.; Joo, G.-J.; Shahzad, R.; Choi, K.-S.; Lee, I.-J. Gibberel-Lin-Producing Serratia Nematodiphila PEJ1011 Ameliorates Low Temperature Stress in Capsicum Annuum L. Eur. J. Soil Biol. 2015, 68, 85–93. [Google Scholar] [CrossRef]
- Ghavami, N.; Alikhani, H.A.; Pourbabaei, A.A.; Besharati, H. Effects of Two New Siderophore-Producing Rhizobacteria on Growth and Iron Content of Maize and Canola Plants. J. Plant Nutr. 2017, 40, 736–746. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.; Trivedi, M.H.; Gobi, A.T. Phosphate Solubilizing Microbes: Sustainable Approach for Managing Phosphorus Deficiency in Agricultural Soils. Springer Plus 2013, 2, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Liddycoat, S.M.; Greenberg, B.M.; Wolyn, D.J. The Effect of Plant Growth-Promoting Rhizobacteria on Asparagus Seedlings and Germinating Seeds Subjected to Water Stress under Greenhouse Conditions. Can. J. Microbiol. 2009, 55, 388–394. [Google Scholar] [CrossRef]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M. Plant Growth-Promoting Rhizobacteria (PGPR): Their Potential As Antagonists and Biocontrol Agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradi-Pour, M.; Saberi-Riseh, R.; Mohammadinejad, R.; Hosseini, A. Investigating the Formulation of Alginate-Gelatin Encapsu-Lated Pseudomonas Fluorescens (VUPF5 and T17-4 Strains) for Controlling Fusarium Solani on Potato. Int. J. Biol. Macromol. 2019, 133, 603–613. [Google Scholar] [CrossRef]
- Lakkis, J.M. Encapsulation and Controlled Release Technologies in Food Systems, 2nd ed.; Blackwell Publishing: Aimes, IO, USA, 2007. [Google Scholar]
- Chen, M.-J.; Chen, K.-N. Applications of Probiotic Encapsulation in Dairy Products. In Encapsulation and Controlled Release Technologies in Food Systems; Blackwell Publishing: Ames, IO, USA, 2007; pp. 83–112. [Google Scholar] [CrossRef]
- Dubey, R. Microencapsulation Technology and Applications. Def. Sci. J. 2009, 59, 82–95. [Google Scholar]
- Garti, N. Delivery and Controlled Release of Bioactives in Foods and Nutraceuticals, 1st ed.; Woodhead Publishing: Cambridge, UK, 2008. [Google Scholar] [CrossRef]
- Shi, L.-E.; Li, Z.-H.; Li, D.-T.; Xu, M.; Chen, H.-Y.; Zhang, Z.-L.; Tang, Z.-X. Encapsulation of Probiotic Lactobacillus Bulgaricus in alginate–milk Microspheres and Evaluation of the Survival in Simulated Gastrointestinal Conditions. J. Food Eng. 2013, 117, 99–104. [Google Scholar] [CrossRef]
- Anal, A.K.; Singh, H. Recent Advances in Microencapsulation of Probiotics for Industrial Applications and Targeted Delivery. Trends Food Sci. Technol. 2007, 18, 240–251. [Google Scholar] [CrossRef]
- Mohamadnia, Z.; Ahmadi, E.; Rafienia, M.; Mirzadeh, H.; Mobedi, H. Erratum: Investigation of Drug Release and 1 H-NMR Analysis of the in Situ Forming Systems Based on poly(lactide-Co-Glycolide). Polym. Adv. Technol. 2009, 20, 507. [Google Scholar] [CrossRef]
- Călinoiu, L.-F.; Ştefănescu, B.E.; Pop, I.D.; Muntean, L.; Vodnar, D.C. Chitosan Coating Applications in Probiotic MicroenCapsulation. Coatings 2019, 9, 194. [Google Scholar] [CrossRef] [Green Version]
- John, R.P.; Tyagi, R.; Brar, S.; Surampalli, R.; Prévost, D. Bio-Encapsulation of Microbial Cells for Targeted Agricultural Delivery. Crit. Rev. Biotechnol. 2010, 31, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, L.; Lesueur, D. Challenges of Formulation and Quality of Biofertilizers for Successful Inoculation. Appl. Microbiol. Biotechnol. 2013, 97, 8859–8873. [Google Scholar] [CrossRef] [PubMed]
- Saberi-Rise, R.; Moradi-Pour, M. The Effect of Bacillus Subtilis Vru1 Encapsulated in Alginate -Bentonite Coating Enriched With Titanium Nanoparticles Against Rhizoctonia Solani on Bean. Int. J. Biol. Macromol. 2020, 152, 1089–1097. [Google Scholar] [CrossRef]
- Schoebitz, M.; López, M.D.; Roldán, A. Bioencapsulation of Microbial Inoculants for Better soil–plant Fertilization. A Review. Agron. Sustain. Dev. 2013, 33, 751–765. [Google Scholar] [CrossRef]
- Moradi-Pour, M.; Saberi-Riseh, R.; Mohammadinejad, R.; Hosseini, A. Nano-Encapsulation of Plant Growth-Promoting Rhizobac-Teria and Their Metabolites Using Alginate-Silica Nanoparticles and Carbon Nanotube Improves UCB1 Pistachio Micropropa-gation. J. Microbiol. Biotechnol. 2019, 29, 1096–1103. [Google Scholar] [CrossRef]
- Boza, Y.; Barbin, D.; Scamparini, A.R.P. Effect of Spray-Drying on the Quality of Encapsulated Cells of Beijerinckia Sp. Process. Biochem. 2004, 39, 1275–1284. [Google Scholar] [CrossRef]
- Maciel, G.; Chaves, K.; Grosso, C.; Gigante, M. Microencapsulation of Lactobacillus Acidophilus La-5 by Spray-Drying Using Sweet Whey and Skim Milk As Encapsulating Materials. J. Dairy Sci. 2014, 97, 1991–1998. [Google Scholar] [CrossRef] [Green Version]
- Picot, A.; Lacroix, C. Encapsulation of Bifidobacteria in Whey Protein-Based Microcapsules and Survival in Simulated Gastrointestinal Conditions and in Yoghurt. Int. Dairy J. 2004, 14, 505–515. [Google Scholar] [CrossRef]
- McGuire, M.R.; Shasha, B.S.; Eastman, C.E.; Oloumi-Sadeghi, H. Starch- and Flour-Based Sprayable Formulations: Effect on Rainfastness and Solar Stability of Bacillus Thuringiensis. J. Econ. Entomol. 1996, 89, 863–869. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Celaya, A.L.; Ortiz-García, M.; Vernon-Carter, E.J.; Jauregui-Rincón, J.; Galindo, E.; Serrano-Carreón, L. Spray-Drying Microencapsulation of Trichoderma Harzianum Conidias in Carbohydrate Polymers Matrices. Carbohydr. Polym. 2012, 88, 1141–1148. [Google Scholar] [CrossRef]
- Saberi-Riseh, R.; Moradi-Pour, M. A Novel Encapsulation of Streptomyces Fulvissimus Uts22 by Spray Drying and Its Biocontrol Efficiency Against Gaeumannomyces Graminis the Causal Agent of take-all Disease in Wheat. Pest. Manag. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Holkem, A.T.; Raddatz, G.C.; Barin, J.S.; Flores, É.M.M.; Muller, E.I.; Codevilla, C.F.; Jacob-Lopes, E.; Grosso, C.R.F.; De Menezes, C.R. Production of Microcapsules Containing Bifidobacterium BB-12 by emulsification/Internal Gelation. LWT 2017, 76, 216–221. [Google Scholar] [CrossRef]
- Chen, K.-N.; Chen, C.-Y.; Lin, Y.-C.; Chen, M.-J. Formulation of a Novel Antagonistic Bacterium Based Biopesticide for Fungal Disease Control Using Microencapsulation Techniques. J. Agric. Sci. 2013, 5, 153. [Google Scholar] [CrossRef]
- Zou, Q.; Zhao, J.; Liu, X.; Tian, F.; Zhang, H.-P.; Zhang, H.; Chen, W. Microencapsulation of Bifidobacterium Bifidum F-35 in Reinforced Alginate Microspheres Prepared by emulsification/Internal Gelation. Int. J. Food Sci. Technol. 2011, 46, 1672–1678. [Google Scholar] [CrossRef]
- Li, X.Y.; Chen, X.G.; Sun, Z.W.; Park, H.J.; Cha, D.-S. Preparation of alginate/chitosan/Carboxymethyl Chitosan Complex Mi-Crocapsules and Application in Lactobacillus Casei ATCC 393. Carbohyd. Polym. 2011, 83, 1479–1485. [Google Scholar] [CrossRef]
- Desmond, C.; Ross, R.P.; O’Callaghan, E.; Fitzgerald, G.; Stanton, C. Improved Survival of Lactobacillus Paracasei NFBC 338 in Spray-Dried Powders Containing Gum Acacia. J. Appl. Microbiol. 2002, 93, 1003–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nami, Y.; Haghshenas, B.; Khosroushahi, A.Y. Effect of Psyllium and Gum Arabic Biopolymers on the Survival Rate and Storage Stability in Yogurt Of Enterococcus Durans IW3 Encapsulated in Alginate. Food Sci. Nutr. 2016, 5, 554–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Guo, L.; Qin, S.; Li, C. Encapsulation of R. Planticola Rs-2 from Alginate-Starch-Bentonite and Its Controlled Release and Swelling Behavior under Simulated Soil Conditions. J. Ind. Microbiol. Biotechnol. 2012, 39, 317–327. [Google Scholar] [CrossRef]
- Rekha, P.D.; Lai, W.A.; Arun, A.B.; Young, C.C. Effect of Free and Encapsulated Pseudomonas Putida CC-FR2-4 and Bacillus Subtilis CC-pg104 on Plant Growth under Gnotobiotic Conditions. Bioresour. Technol. 2007, 98, 447–451. [Google Scholar] [CrossRef]
- Burgain, J.; Gaiani, C.; Linder, M.; Scher, J. Encapsulation of Probiotic Living Cells: From Laboratory Scale to Industrial Ap-Plications. J. Food Eng. 2011, 104, 467–483. [Google Scholar] [CrossRef]
- Menshutina, N.V.; Gordienko, M.G.; Voinovskiy, A.A.; Zbicinski, I. Spray Drying of Probiotics: Process Development and Scale-Up. Dry. Technol. 2010, 28, 1170–1177. [Google Scholar] [CrossRef]
- Meng, X.; Stanton, C.; Fitzgerald, G.; Daly, C.; Ross, R. Anhydrobiotics: The Challenges of Drying Probiotic Cultures. Food Chem. 2008, 106, 1406–1416. [Google Scholar] [CrossRef]
- de Vos, P.; Faas, M.M.; Spasojevic, M.; Sikkema, J. Encapsulation for Preservation of Functionality and Targeted Delivery of Bioactive Food Components. Int. Dairy J. 2010, 20, 292–302. [Google Scholar] [CrossRef]
- Gardiner, E.G.; Bouchier, P.; O’Sullivan, E.; Kelly, J.; Collins, J.K.; Fitzgerald, G.; Ross, R.P.; Stanton, C. A Spray-Dried Culture for Probiotic Cheddar Cheese Manufacture. Int. Dairy J. 2002, 12, 749–756. [Google Scholar] [CrossRef]
- Ma, X.; Wang, X.; Cheng, J.; Nie, X.; Yu, X.; Zhao, Y.; Wang, W. Microencapsulation of Bacillus Subtilis B99-2 and Its Biocontrol Efficiency Against Rhizoctonia Solani in Tomato. Biol. Control. 2015, 90, 34–41. [Google Scholar] [CrossRef]
- Guerin, J.; Petit, J.; Burgain, J.; Borges, F.; Bhandari, B.; Perroud, C.; Desobry, S.; Scher, J.; Gaiani, C. Lactobacillus Rhamnosus GG Encapsulation by Spray-Drying: Milk Proteins Clotting Control to Produce Innovative Matrices. J. Food Eng. 2017, 193, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Jantzen, M.; Göpel, A.; Beermann, C. Direct Spray Drying and Microencapsulation of Probiotic Lactobacillus Reuteri from Slurry Fermentation With Whey. J. Appl. Microbiol. 2013, 115, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Tamez-Guerra, P.; McGuire, M.R.; Medrano-Roldan, H.; Galan-Wong, L.J.; Shasha, B.S.; Vega, F.E. Sprayable Granule Formu-Lations for Bacillus Thuringiensis. J. Econ. Entom. 1996, 89, 1424–1430. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. Evaluation of Encapsulation Techniques of Probiotics for Yoghurt. Int. Dairy J. 2003, 13, 3–13. [Google Scholar] [CrossRef]
- Kailasapathy, K. Encapsulation Technologies for Functional Foods and Nutraceutical Product Development. Cab Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2009, 4, 1–19. [Google Scholar] [CrossRef]
- Kavitake, D.; Kandasamy, S.; Devi, P.B.; Shetty, P.H. Recent Developments on Encapsulation of Lactic Acid Bacteria As Potential Starter Culture in Fermented Foods - A Review. Food Biosci. 2018, 21, 34–44. [Google Scholar] [CrossRef]
- Mortazavian, A.; Razavi, S.H.; Ehsani, M.R.; Sohrabvandi, S. Principles and Methods of Microencapsulation of Probiotic Mi-Croorganisms. Iran. J. Biotechnol. 2007, 5, 1–18. [Google Scholar]
- Krisanti, E.A.; Naziha, G.M.; Amany, N.S.; Mulia, K.; Handayani, N.A. Effect of Biopolymers Composition on Release Profile of Iron(II) Fumarate from Chitosan-Alginate Microparticles. Iop Conf. Ser. Mater. Sci. Eng. 2019, 509, 012100. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant starch–A Review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 1–17. [Google Scholar] [CrossRef]
- Thakur, S.; Sharma, B.; Verma, A.; Chaudhary, J.; Tamulevicius, S.; Thakur, V.K. Recent Progress in Sodium Alginate Based Sustainable Hydrogels for Environmental Applications. J. Clean. Prod. 2018, 198, 143–159. [Google Scholar] [CrossRef] [Green Version]
- Gomez, C.G.; Lambrecht, M.V.P.; Lozano, J.E.; Rinaudo, M.; Villar, M.A. Influence of the extraction–purification Conditions on Final Properties of Alginates Obtained from Brown Algae (Macrocystis Pyrifera). Int. J. Biol. Macromol. 2009, 44, 365–371. [Google Scholar] [CrossRef]
- Urtuvia, V.; Maturana, N.; Acevedo, F.; Peña, C.; Díaz-Barrera, A. Bacterial Alginate Production: An Overview of Its Biosynthesis and Potential Industrial Production. World J. Microbiol. Biotechnol. 2017, 33, 198. [Google Scholar] [CrossRef]
- Gouin, S. Microencapsulation: Industrial Appraisal of Existing Technologies and Trends. Trends Food Sci. Tech. 2004, 15, 330–347. [Google Scholar] [CrossRef]
- Wohlgemuth, S.; Loh, G.; Blaut, M. Recent Developments and Perspectives in the Investigation of Probiotic Effects. Int. J. Med. Microbiol. 2010, 300, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Bashan, Y. Inoculants of Plant Growth-Promoting Bacteria for Use in Agriculture. Biotechnol. Adv. 1998, 16, 729–770. [Google Scholar] [CrossRef]
- Iyer, C.; Kailasapathy, K. Effect of Co-Encapsulation of Probiotics With Prebiotics on Increasing the Viability of Encapsulated Bacteria under In Vitro Acidic and Bile Salt Conditions and in Yogurt. J. Food Sci. 2005, 70, M18–M23. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Khutoryanskiy, V.V.; Charalampopoulos, D. Layer-by-Layer Coating of Alginate Matrices With Chi-tosan–alginate for the Improved Survival and Targeted Delivery of Probiotic Bacteria After Oral Administration. J. Mater. Chem. B 2013, 1, 52–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, V.K.; Voicu, S.I. Recent Advances in Cellulose and Chitosan Based Membranes for Water Purification: A Concise Review. Carbohydr. Polym. 2016, 146, 148–165. [Google Scholar] [CrossRef]
- Khairul, W.M.; Daud, A.I.; Ismail, N. Understanding the Properties of Chitosan Aryl Substituted Thioureas in Their Role and Potential as Antibacterial Agents. In 2nd biomedical engineering’ recent progress in drug development and, and medical devices. In Proceedings of the International Symposium of Biomedical Engineering (ISBE) 2017; AIP Publishing: Melville, NY, USA, 2018; Volume 1933. [Google Scholar] [CrossRef]
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, Structures, and Advanced Applications of Nanocomposites from Biorenewable Resources. Chem. Rev. 2020, 120, 9304–9362. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Recent Advances in Graft Copolymerization and Applications of Chitosan: A Review. Acs Sustain. Chem. Eng. 2014, 2, 2637–2652. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Kotla, N.G.; Afshar, E.G.; Samarghandian, S.; Mandegary, A.; Pardakhty, A.; Mohammadinejad, R.; Sethi, G. Nanoparticles Targeting STATs in Cancer Therapy. Cells 2019, 8, 1158. [Google Scholar] [CrossRef] [Green Version]
- Dehshahri, A.; Kumar, A.; Madamsetty, V.; Uzieliene, I.; Tavakol, S.; Azedi, F.; Fekri, H.; Zarrabi, A.; Mohammadinejad, R.; Thakur, V. New Horizons in Hydrogels for Methotrexate Delivery. Gels 2020, 7, 2. [Google Scholar] [CrossRef]
- Chavarri, M.; Maranon, I.; Carmen, M. Encapsulation Technology to Protect Probiotic Bacteria. In Probiotics; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef] [Green Version]
- Dumitriu, S.; Chornet, E. Inclusion and Release of Proteins from Polysaccharide-Based Polyion Complexes. Adv. Drug Deliv. Rev. 1998, 31, 223–246. [Google Scholar] [CrossRef]
- Illum, L.; Farraj, N.F.; Davis, S.S. Chitosan As a Novel Nasal Delivery System for Peptide Drugs. Pharm. Res. 1994, 11, 1186–1189. [Google Scholar] [CrossRef] [PubMed]
- Schipper, N.G.M.; Olsson, S.; Hoogstraate, J.A.; DeBoer, A.G.; Vårum, K.M.; Artursson, P. Chitosans As Absorption Enhancers for Poorly Absorbable Drugs 2: Mechanism of Absorption Enhancement. Pharm. Res. 1997, 923–929. [Google Scholar] [CrossRef] [PubMed]
- De Alvarenga, E.S. Characterization and Properties of Chitosan. Biotechnol. Biopolym. 2011, 91, 48–53. [Google Scholar]
- Peniche, C.; Argüelles-Monal, W.; Peniche, H.; Acosta, N. Chitosan: An Attractive Biocompatible Polymer for Microencap-Sulation. Macromol. Biosci. 2003, 3, 511–520. [Google Scholar] [CrossRef]
- Elhussiny, A.; Faisal, M.; D’angelo, G.; Everitt, N.M.; Fahim, I.S. Experimental Investigation of Chitosan Film Reinforced by Chitin Fibers and Chitin Whiskers Extracted from Shrimp Shell Waste. J. Eng. Sci. Technol. 2020, 15, 2730–2745. [Google Scholar]
- Zhu, F. Encapsulation and Delivery of Food Ingredients Using Starch Based Systems. Food Chem. 2017, 229, 542–552. [Google Scholar] [CrossRef]
- Balmayor, E.R.; Baran, E.; Azevedo, H.; Reis, R.L. Injectable Biodegradable starch/Chitosan Delivery System for the Sustained Release of Gentamicin to Treat Bone Infections. Carbohydr. Polym. 2012, 87, 32–39. [Google Scholar] [CrossRef]
- Miculescu, F.; Maidaniuc, A.; Voicu, S.I.; Thakur, V.K.; Stan, G.E.; Ciocan, L.T. Progress in Hydroxyapatite–Starch Based Sustainable Biomaterials for Biomedical Bone Substitution Applications. Acs Sustain. Chem. Eng. 2017, 5, 8491–8512. [Google Scholar] [CrossRef] [Green Version]
- Madhumitha, G.; Fowsiya, J.; Roopan, S.M.; Thakur, V.K. Recent Advances in Starch–Clay Nanocomposites. Int. J. Polym. Anal. Charact. 2018, 23, 331–345. [Google Scholar] [CrossRef]
- Wijaya, M.; Small, D.M.; Bui, L. Microencapsulation of Ascorbic Acid for Enhanced Long-Term Retention During Storage; Technical Report; Defense Science and Technology Organization: Melbourne, Australia, 2011. [Google Scholar]
- Lian, W.-C.; Hsiao, H.-C.; Chou, C.-C. Viability of Microencapsulated Bifidobacteria in Simulated Gastric Juice and Bile so-Lution. Int. J. Food Microbiol. 2003, 86, 293–301. [Google Scholar] [CrossRef]
- Saurav, K.; Majhi, M.R.; Singh, V.K. Preperation of porous magenesia by decomposing an ex -potato known as starch soluble (C6H10O5)N. J. Sci. Ind. Res. 2013, 5, 120–125. [Google Scholar] [CrossRef]
- Thakur, S.; Chaudhary, J.; Kumar, V.; Thakur, V.K. Progress in Pectin Based Hydrogels for Water Purification: Trends and Challenges. J. Environ. Manag. 2019, 238, 210–223. [Google Scholar] [CrossRef] [Green Version]
- Rodsamran, P.; Sothornvit, R. Lime Peel Pectin Integrated With Coconut Water and Lime Peel Extract As a New Bioactive Film Sachet to Retard Soybean Oil Oxidation. Food Hydrocoll. 2019, 97, 105173. [Google Scholar] [CrossRef]
- Bekhit, M.; Sánchez-González, L.; Messaoud, G.B.; Desobry, S. Encapsulation of Lactococcus Lactis Subsp. Lactis on Alginate/Pectin Composite Microbeads: Effect of Matrix Composition on Bacterial Survival and Nisin Release. J. Food Eng. 2016, 180, 1–9. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, F.; Han, D.; Zhao, Y.; Liu, Z.; Lei, H.; Song, Y.; Huang, X.; Li, X.; Ma, A. Preparation and Optimization of Soy Protein isolate–high Methoxy Pectin Microcapsules Loaded With L Actobacillus Delbrueckii. Int. J. Food Sci. Technol. 2014, 49, 1287–1293. [Google Scholar] [CrossRef]
- Pavláth, A.E.; Voisin, A.; Robertson, G.H. Pectin-Based Biodegradable Water Insoluble Films. Macromol. Symp. 1999, 140, 107–113. [Google Scholar] [CrossRef]
- Islan, G.A.; De Verti, I.P.; Marchetti, S.G.; Castro, G.R. Studies of Ciprofloxacin Encapsulation on Alginate/Pectin Matrixes and Its Relationship With Biodisponibility. Appl. Biochem. Biotechnol. 2012, 167, 1408–1420. [Google Scholar] [CrossRef] [PubMed]
- Aburto, J.; Morana, M.; Galano, A.; Torres-García, E. Non-isothermal pyrolysis of pectin: A thermochemical and kinetic approach. J. Anal. Appl. Pyrolysis 2015, 112, 94–104. [Google Scholar] [CrossRef]
- He, W.; Hosseinkhani, H.; Mohammadinejad, R.; Roveimiab, Z.; Hueng, D.-Y.; Ou, K.-L.; Domb, A.J. Polymeric Nanoparticles for Therapy and Imaging. Polym. Adv. Technol. 2014, 25, 1216–1225. [Google Scholar] [CrossRef]
- Sun, W.; Griffiths, M.W. Survival of Bifidobacteria in Yogurt and Simulated Gastric Juice Following Immobilization in gellan–xanthan Beads. Int. J. Food Microbiol. 2000, 61, 17–25. [Google Scholar] [CrossRef]
- Zuidam, N.J.; Shimoni, E. Overview of Microencapsulates for Use in Food Products or Processes and Methods to Make Them. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Springer: New York, NY, USA, 2009; pp. 3–29. [Google Scholar] [CrossRef]
- Singh, P.; Medronho, B.; Miguel, M.G.; Esquena, J. On the Encapsulation and Viability of Probiotic Bacteria in Edible Car-Boxymethyl Cellulose-Gelatin Water-in-Water, Emulsions. Food Hydrocoll. 2018, 75, 41–50. [Google Scholar] [CrossRef]
- Thakur, S.; Govender, P.P.; Mamo, M.A.; Tamulevicius, S.; Thakur, V.K. Recent Progress in Gelatin Hydrogel Nanocomposites for Water Purification and Beyond. Vacuum 2017, 146, 396–408. [Google Scholar] [CrossRef] [Green Version]
- Shakeri, S.; Ashrafizadeh, M.; Zarrabi, A.; Roghanian, R.; Afshar, E.G.; Pardakhty, A.; Mohammadinejad, R.; Kumar, A.; Thakur, V.K. Multifunctional Polymeric Nanoplatforms for Brain Diseases Diagnosis, Therapy and Theranostics. Biomedicines 2020, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Kommareddy, S.; Shenoy, D.B.; Amiji, M.M. Gelatin Nanoparticles and Their Biofunctionalization. In Nanotechnologies for the Life Sciences Vol. 1. Biofunctionalization of Nanomaterials; Challa, S.S.R., Ed.; Wiley-VCH Verlag: Weinheim, Germany, 2005. [Google Scholar]
- Rebouillat, S.; Ortega-Requena, S. Potential Applications of Milk Fractions and Valorization of Dairy By-Products: A Review of the State-of-the-Art Available Data, Outlining the Innovation Potential from a Bigger Data Standpoint. J. Biomater. Nanobiotechnol. 2015, 6, 176–203. [Google Scholar] [CrossRef] [Green Version]
- Sultana, K.; Godward, G.; Reynolds, N.; Arumugaswamy, R.; Peiris, P.; Kailasapathy, K. Encapsulation of Probiotic Bacteria With alginate–starch and Evaluation of Survival in Simulated Gastrointestinal Conditions and in Yoghurt. Int. J. Food Microbiol. 2000, 62, 47–55. [Google Scholar] [CrossRef]
- Chiriac, A.P.; Ghilan, A.; Neamtu, I.; Nita, L.E.; Rusu, A.G.; Chiriac, V.M. Advancement in the Biomedical Applications of the (Nano)gel Structures Based on Particular Polysaccharides. Macromol. Biosci. 2019, 1900187. [Google Scholar] [CrossRef]
- Petri, D.F.S. Xanthan gum: A versatile biopolymer for biomedical and technological applications. J. Appl. Polym. Sci. 2015, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Shukla, P.S.; Borza, T.; Critchley, A.T.; Prithiviraj, B. Carrageenans from Red Seaweeds As Promoters of Growth and Elicitors of Defense Response in Plants. Front. Mar. Sci. 2016, 3, 81. [Google Scholar] [CrossRef]
- McHugh, D.J. A Guide to the Seaweed Industry; FAO Fisheries Technical Paper 441; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003. [Google Scholar]
- Pascalău, V.; Popescu, V.A.; Popescu, G.; Dudescu, M.C.; Borodi, G.; Dinescu, A.M.; Perhaiţa, I.; Paul, M.C. The alginate/K-Carrageenan ratio’s Influence on the Properties of the Cross-Linked Composite Films. J. Alloy. Compd. 2012, 536, S418–S423. [Google Scholar] [CrossRef]
- Mampho, C.; Pandey, S.; Ramontja, J.; Fosso-Kankeu, E.; Waanders, F. Synthesis and Characterization of Superabsorbent Hydrogels Based on Natural Polymers: Kappa Carrageenan. In Proceedings of the International Conference on Advances in Science, Engineering, Technology and Natural Resources (ICASETNR-16), Parys, South Africa, 24–25 November 2016. [Google Scholar]
- Mohammadinejad, R.; Kumar, A.; Ranjbar-Mohammadi, M.; Ashrafizadeh, M.; Han, S.S.; Khang, G.; Roveimiab, Z. Recent Advances in Natural Gum-Based Biomaterials for Tissue Engineering and Regenerative Medicine: A Review. Polymers 2020, 12, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashemabadi, M.; Sasan, H.; Amandadi, M.; Mohammadinejad, R.; Farnoosh, G.; Azimzadeh, M.; Taheri, R.A. Natural Gum As Bio-Reductant to Green Synthesize Silver Nanoparticles: Assessing the Apoptotic Efficacy on MCF-7 and SH-SY5Y Cell Lines and Their Antimicrobial Potential. Polym. Bull. 2021, 78, 2867–2886. [Google Scholar] [CrossRef]
- Almuslet, N.; Hassan, E.A.; Al-Sherbini, A.; Muhgoub, M.A. Diode Laser (532 Nm) Induced Grafting of Polyacrylamide onto Gum Arabic. J. Physic. Sci. 2012, 23, 43–53. [Google Scholar]
- Nussinovitch, A. Hydrocolloids in Flavor Encapsulation. In Water-Soluble Polymer Applications in Foods; Wiley-Blackwell: Hoboken, NJ, USA, 2007. [Google Scholar]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of Spray-Drying in Microencapsulation of Food Ingredients: An Overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Frascareli, E.; Silva, V.; Tonon, R.; Hubinger, M. Effect of Process Conditions on the Microencapsulation of Coffee Oil by Spray Drying. Food Bioprod. Process. 2012, 90, 413–424. [Google Scholar] [CrossRef]
- Jahandideh, A.; Ashkani, M.; Moini, N. Chapter 8—Biopolymers in textile industries. In Biopolymers and Their Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 193–218. [Google Scholar] [CrossRef]
- Shih, I.-L.; Shen, M.-H.; Van, Y.-T. Microbial Synthesis of poly(ε-Lysine) and Its Various Applications. Bioresour. Technol. 2006, 97, 1148–1159. [Google Scholar] [CrossRef]
- Zhang, L.; Li, R.; Dong, F.; Tian, A.; Li, Z.; Dai, Y. Physical, mechanical and antimicrobial properties of starch films incorporated with ε-poly-l-lysine. Food Chem. 2015, 166, 107–114. [Google Scholar] [CrossRef]
- Pan, H.M.; Subramanian, A.; Ochs, C.J.; Dewavrin, J.-Y.; Beyer, S.; Trau, D.W. Edible Polyelectrolyte Microcapsules With Wa-Ter-Soluble Cargo Assembled in Organic Phase. Rsc Adv. 2014, 4, 35163–35166. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Yan, S.; Gong, L.; Wang, J.; Chen, X.; Cui, L.; Yin, J. Repair of an Articular Cartilage Defect Using Adi-Pose-Derived Stem Cells Loaded on a Polyelectrolyte Complex Scaffold Based on Poly (l-Glutamic Acid) and Chitosan. Acta Biomater. 2013, 9, 7276–7288. [Google Scholar] [CrossRef] [PubMed]
- Li, C. Poly (L-Glutamic acid)–anticancer Drug Conjugates. Adv. Drug Deliv. Rev. 2002, 54, 695–713. [Google Scholar] [CrossRef]
- Markland, P.; Amidon, G.L.; Yang, V.C. Modified Polypeptides Containing γ-Benzyl Glutamic Acid As Drug Delivery Platforms. Int. J.Pharm. 1999, 178, 183–192. [Google Scholar] [CrossRef]
- Zhao, N.; Yang, C.; Wang, Y.; Zhao, B.; Bian, F.; Li, X.; Wang, J. Probing the Surface Microstructure of Layer-by-Layer Self-Assembly chitosan/Poly( L -Glutamic Acid) Multilayers: A Grazing-Incidence Small-Angle X-Ray Scattering Study. Mater. Sci. Eng. C 2016, 58, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Yazdi, F.T.; Mortazavi, S.A.; Shahabi-Ghahfarrokhi, I.; Chamani, J. Development of Active Antimicrobial Poly (l-Glutamic) Acid-Poly (l-Lysine) Packaging Material to Protect Probiotic Bacterium. Polym. Test. 2020, 83, 106338. [Google Scholar] [CrossRef]
- Gagné-Bourque, F.; Mayer, B.F.; Charron, J.-B.; Vali, H.; Bertrand, A.; Jabaji, S. Accelerated Growth Rate and Increased Drought Stress Resilience of the Model Grass Brachypodium Distachyon Colonized by Bacillus Subtilis B26. PLoS ONE 2015, 10, e0130456. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Aguirre, J.; Ruiz-Sanchez, E.; Reyes-Ramírez, A.; Cristóbal-Alejo, J.; Tun-Suárez, J.; Borges-Gómez, L. Polymer-Based Encapsulation of Bacillus Subtilis and Its Effect on Meloidogyne Incognita in Tomato. Phyton Int. J. Exper. Bot. 2016, 85, 1–6. [Google Scholar]
- He, Y.; Wu, Z.; Tu, L.; Shan, C. Effect of Encapsulated Pseudomonas Putida Rs-198 Strain on Alleviating Salt Stress of Cotton. J. Plant Nutr. 2017, 40, 1180–1189. [Google Scholar] [CrossRef]
- Slusarenko, A.J.; Patel, A.; Portz, D. Control of Plant Diseases by Natural Products: Allicin from Garlic As a Case Study. Eur. J. Plant Pathol. 2008, 121, 313–322. [Google Scholar] [CrossRef]
- Wiwattanapatapee, R.; Chumthong, A.; Pengnoo, A.; Kanjanamaneesathian, M. Preparation and Evaluation of Bacillus Megaterium-Alginate Microcapsules for Control of Rice Sheath Blight Disease. World J. Microbiol. Biotechnol. 2013, 29, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-Y.; Pusey, P.L.; Zhao, Y.; Korban, S.S.; Choi, H.; Kim, K.K. Controlled Release of Pantoea Agglomerans E325 for Biocontrol of Fire Blight Disease of Apple. J. Control Release 2012, 161, 109–115. [Google Scholar] [CrossRef]
- Ma, Y.; Feng, Q. Alginate Hydrogel-Mediated Crystallization of Calcium Carbonate. J. Solid State Chem. 2011, 184, 1008–1015. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, Y.-j.; Liu, B.; Shi, H.; Su, M.-X. Microcapsule Manufacture for Bio-Controlling Strain ANTI-8098A (Bacillus Cereus) for Bacterial Wilt Disease. Fujian J. Agric. Sci. 2005, 20, 26–29. [Google Scholar]
- Alabouvette, C.; Olivain, C.; Steinberg, C. Biological Control of Plant Diseases The European Situation. Eur. J. Plant Pathol. 2006, 114, 329–341. [Google Scholar] [CrossRef]
- Trenkel, M.E. Slow-and Controlled-Release and Stabilized Fertilizers: An Option for Enhancing Nutrient Use Efficiency in Agriculture; IFA, International Fertilizer Industry Association: Paris, France, 2010. [Google Scholar]
- Shaviv, A.; Mikkelsen, R.L. Controlled-Release Fertilizers to Increase Efficiency of Nutrient Use and Minimize Environmental Degradation -A Review. Nutr. Cycl. Agroecosys. 1993, 35, 1–12. [Google Scholar] [CrossRef]
- Nakkeeran, S.; Fernando, W.D.; Siddiqui, Z.A. Plant Growth Promoting Rhizobacteria Formulations and Its Scope in Commercialization for the Management of Pests and Diseases. In PGPR: Biocontrol and Biofertilization; Springer: New York, NY, USA, 2006. [Google Scholar]
- Byrne, M.E.; Park, K.; Peppas, N.A. Molecular Imprinting Within Hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 149–161. [Google Scholar] [CrossRef]
- Gnanamanickam, S.S. Biological Control of Rice Diseases; Springer: New York, NY, USA, 2009. [Google Scholar]
- Young, C.-C.; Rekha, P.; Lai, W.-A.; Arun, A. Encapsulation of Plant Growth-Promoting Bacteria in Alginate Beads Enriched With Humic Acid. Biotechnol. Bioeng. 2006, 95, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Júnior, P.I.F.; Rohr, T.G.; De Oliveira, P.J.; Xavier, G.R.; Rumjanek, N.G. Polymers As Carriers for Rhizobial Inoculant Formulations. Pesqui. Agropecu. Bras. 2009, 44, 1184–1190. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, M.F.; Antônio, C.D.S.; De Oliveira, P.J.; Xavier, G.R.; Rumjanek, N.G.; Soares, L.H.D.B.; Reis, V.M. Survival of Endophytic Bacteria in Polymer-Based Inoculants and Efficiency of Their Application to Sugarcane. Plant Soil 2012, 356, 231–243. [Google Scholar] [CrossRef]
- Tu, L.; He, Y.; Yang, H.; Wu, Z.; Yi, L. Preparation and Characterization of alginate–gelatin Microencapsulated Bacillus Subtilis SL-13 by emulsification/Internal Gelation. J. Biomater. Sci. Polym. Ed. 2015, 26, 735–749. [Google Scholar] [CrossRef]













| Carrier | Method | Microorganism | References |
|---|---|---|---|
| Malt dextrin | Spray drying | Beijerinckia sp. | [56] |
| Whey and skim milk | Spray drying | Lactobacillus acidophilus | [57] |
| Whey protein | Spray drying | Bifidobacterium breve | [58] |
| Corn flour | Spray drying | Bacillus thuringiensis | [59] |
| Gum Arabic | Spray drying | Trichoderma harzianum | [60] |
| Chitosan-gellan gum | Spray drying | Streptomyces fulvissimus Uts22 | [61] |
| Alginate-Gelatin | Emulsion | Pseudomonasfluorescens VUPF5 | [42] |
| Alginate | Emulsion | Bifidobacterium BB-12 | [62] |
| Alginate-bentonite | Extrusion | Bacillus subtilis Vru1 | [53] |
| Maltodextrin-gum arabic | Spray Drying | Bacillus cereus C1L | [63] |
| Starch-pectin | Emulsion | Bifidobacterium bifidum F-35 | [64] |
| Alginate, chitosan | Extrusion | Lactobacillus casei ATCC 393 | [65] |
| Arabic gum | Spray drying | Lactobacillus paracasei NFBC 338 | [66] |
| Psyllium-gum Arabic | Extrusion | Enterococcus durans IW3 | [67] |
| Alginate-starch-bentonite | Extrusion | R. planticola Rs-2 | [68] |
| Alginate | Extrusion | Pseudomonas putida CC-FR2-4 and Bacillus subtilis CC-pg104 | [69] |
| Formulation | Additives or Treatment | Microorganism | Plant Species or Substrate | References |
|---|---|---|---|---|
| Alginate | Humic acid | - | - | [164] |
| Carboxymethyl cellulose/corn starch | Magnesium oxide | Azospirillum brasilense, Burkholderia tropica | Cowpea, Sugarcane | [165,166] |
| Alginate | Starch | Raoultella terrigena, Azospirillum brasilene | ||
| Alginate-Bentonite- Starch | - | Raoultella planticola | - | [68] |
| Alginate- gelatin | - | Bacillus subtilis | - | [167] |
| Alginate- gelatin | Carbon Nanotubes and Silicon dioxide nanoparticle | Pseudomonas fluorescens, Bacillus subtilis | Potato | [42] |
| Alginate-Bentonite- Starch | titanium dioxide nano particle | Bacillus subtilis | Bean | [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saberi-Riseh, R.; Moradi-Pour, M.; Mohammadinejad, R.; Thakur, V.K. Biopolymers for Biological Control of Plant Pathogens: Advances in Microencapsulation of Beneficial Microorganisms. Polymers 2021, 13, 1938. https://doi.org/10.3390/polym13121938
Saberi-Riseh R, Moradi-Pour M, Mohammadinejad R, Thakur VK. Biopolymers for Biological Control of Plant Pathogens: Advances in Microencapsulation of Beneficial Microorganisms. Polymers. 2021; 13(12):1938. https://doi.org/10.3390/polym13121938
Chicago/Turabian StyleSaberi-Riseh, Roohallah, Mojde Moradi-Pour, Reza Mohammadinejad, and Vijay Kumar Thakur. 2021. "Biopolymers for Biological Control of Plant Pathogens: Advances in Microencapsulation of Beneficial Microorganisms" Polymers 13, no. 12: 1938. https://doi.org/10.3390/polym13121938
APA StyleSaberi-Riseh, R., Moradi-Pour, M., Mohammadinejad, R., & Thakur, V. K. (2021). Biopolymers for Biological Control of Plant Pathogens: Advances in Microencapsulation of Beneficial Microorganisms. Polymers, 13(12), 1938. https://doi.org/10.3390/polym13121938








