Effect of Dendritic Side Groups on the Mobility of Modified Poly(epichlorohydrin) Copolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Membrane Preparation
2.2. Physico-Chemical Characterization
2.2.1. Differential Scanning Calorimetry (DSC)
2.2.2. Polarized Optical Microscopy (POM)
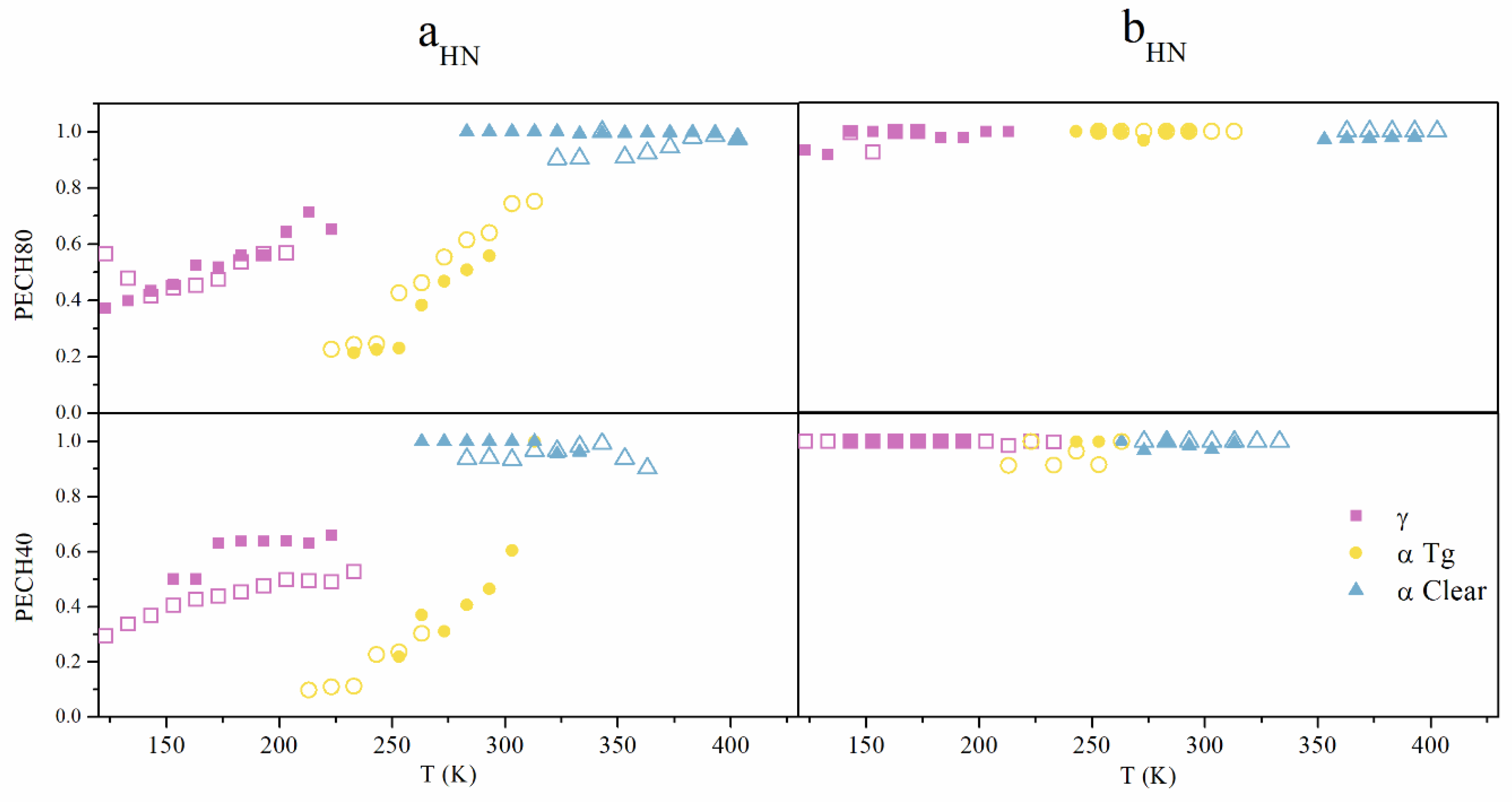
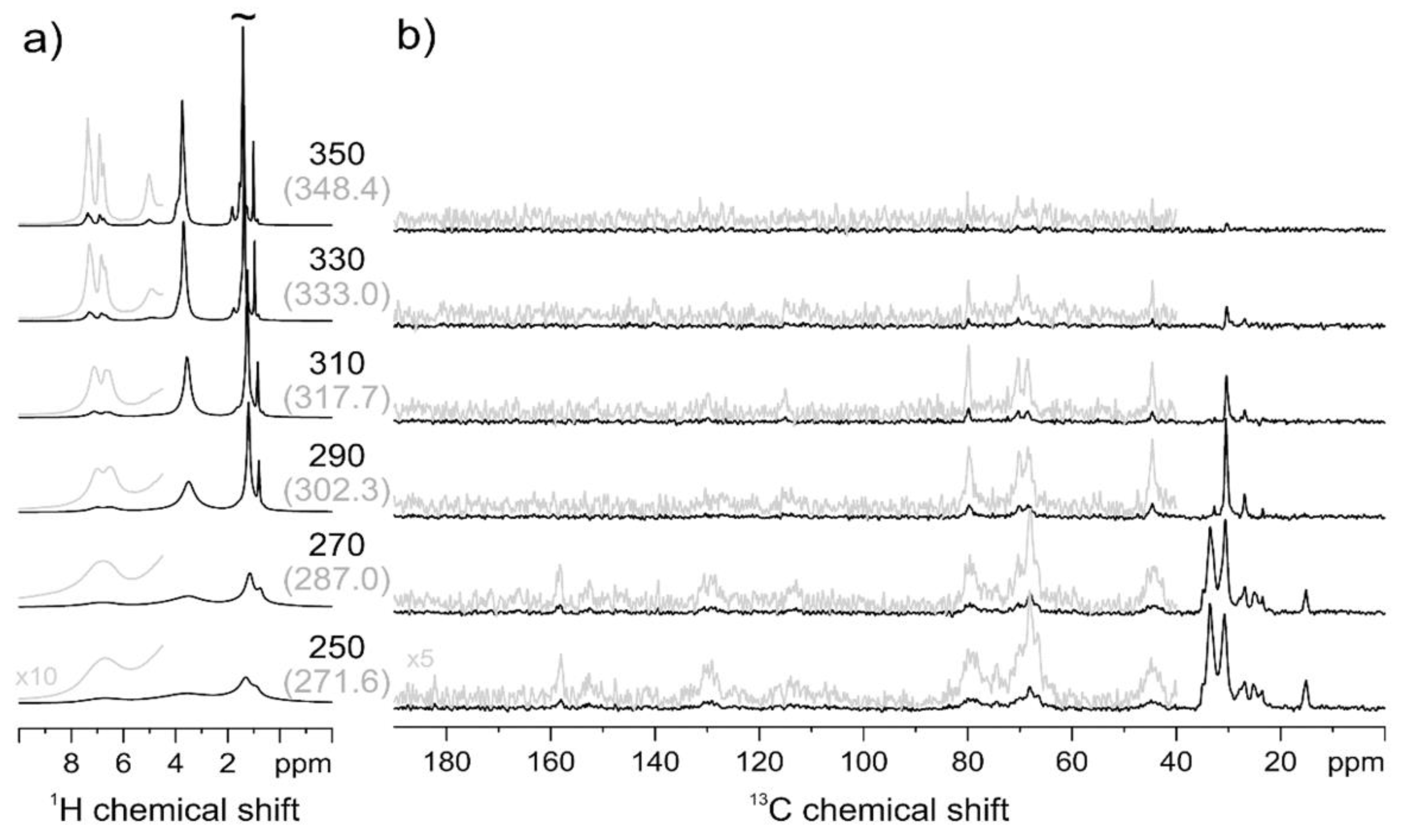
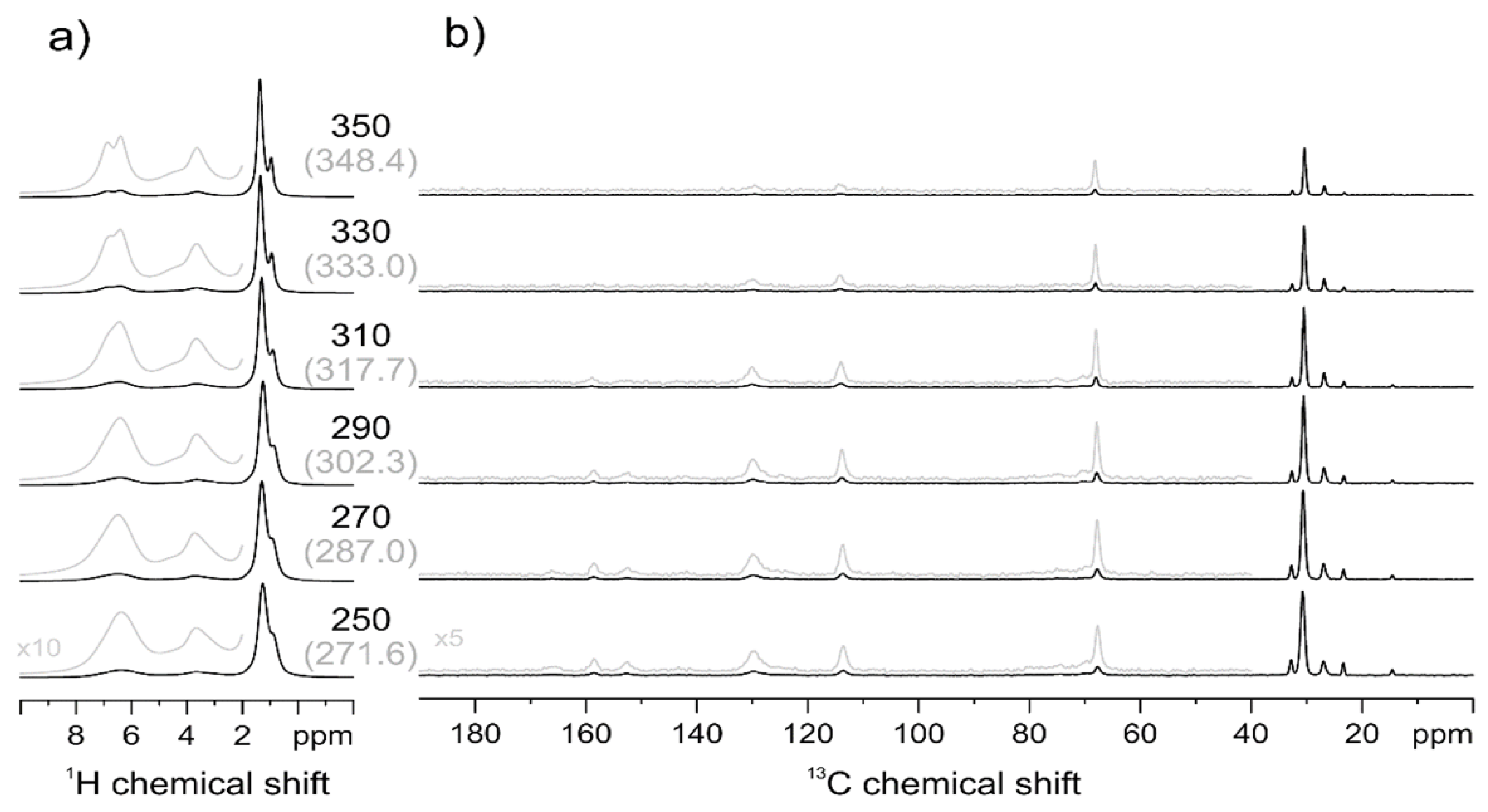
2.2.3. Solid-State Nuclear Magnetic Resonance (NMR) Spectroscopy
2.2.4. X-ray Diffraction (XRD)
2.2.5. Dielectric Thermal Analysis (DETA) Assessment
3. Results
3.1. Morphological and Calorimetric Characterization
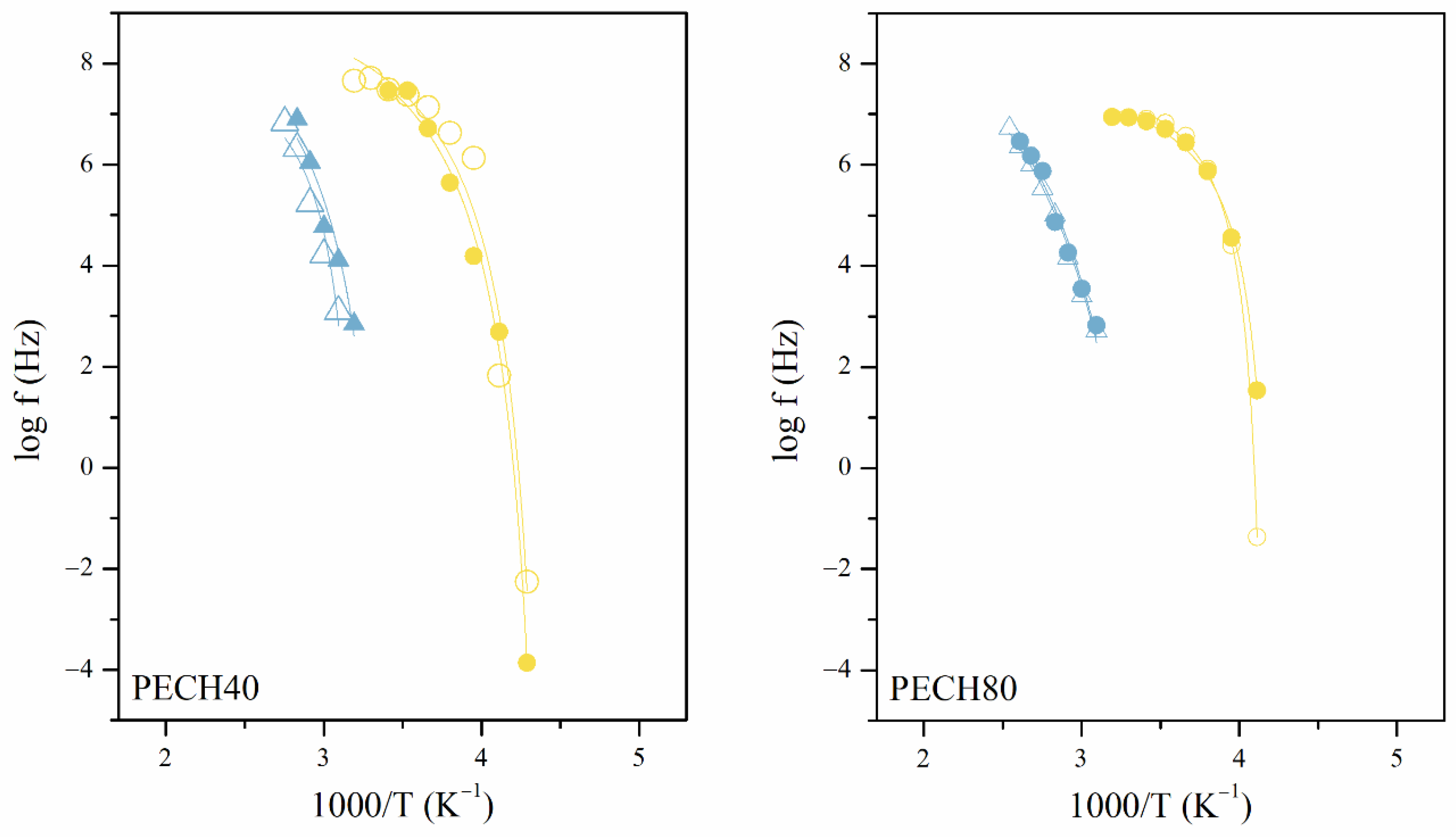
3.2. Analysis of the Dielectric Spectra
3.2.1. Macromolecular Origin and Thermal Activation of the γ Relaxation
3.2.2. Macromolecular Origin and Thermal Activation of the α Relaxation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Šakalyte, A.; Reina, J.A.; Giamberini, M. Liquid crystalline polyamines containing side dendrons: Toward the building of ion channels based on polyamines. Polymer 2013, 54, 5133–5140. [Google Scholar] [CrossRef]
- Ronda, J.C.; Reina, J.A.; Giamberini, M. Self-organized liquid-crystalline polyethers obtained by grafting tapered mesogenic groups onto poly(epichlorohydrin): Toward biomimetic ion channels 2. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 326–340. [Google Scholar] [CrossRef]
- Bogdanowicz, K.A.; Bhosale, S.V.; Li, Y.; Vankelecom, I.F.J.; Garcia-Valls, R.; Reina, J.A.; Giamberini, M. Mimicking nature: Biomimetic ionic channels. J. Membr. Sci. 2016, 509, 10–18. [Google Scholar] [CrossRef]
- Rosen, B.M.; Wilson, C.J.; Wilson, D.A.; Peterca, M.; Imam, M.R.; Percec, V. Dendron-Mediated self-assembly, disassembly, and self-organization of complex systems. Chem. Rev. 2009, 109, 6275–6540. [Google Scholar] [CrossRef]
- Percec, V.; Schlueter, D.; Ungar, G.; Cheng, S.Z.D.; Zhang, A. Hierarchical control of internal superstructure, diameter, and stability of supramolecular and macromolecular columns generated from tapered monodendritic building blocks. Macromolecules 1998, 31, 1745–1762. [Google Scholar] [CrossRef]
- Percec, V.; Ahn, C.H.; Ungar, G.; Yeardley, D.J.P.; Möller, M.; Sheiko, S.S. Controlling polymer shape through the self-assembly of dendritic side-groups. Nature 1998, 391, 161–164. [Google Scholar] [CrossRef]
- Percec, V.; Glodde, M.; Bera, T.K.; Miura, Y.; Shiyanovskaya, I.; Singer, K.D.; Balagurusamy, V.S.K.; Heiney, P.A.; Schnell, I.; Rapp, A. Self-organization of supramolecular helical dendrimers into complex electronic materials. Nature 2002, 419, 384. [Google Scholar] [CrossRef] [PubMed]
- Xu, T. Ion exchange membranes: State of their development and perspective. J. Membr. Sci. 2005, 263, 1–29. [Google Scholar] [CrossRef]
- Montané, X.; Bogdanowicz, K.A.; Prats-Reig, J.; Colace, G.; Reina, J.A.; Giamberini, M. Advances in the design of self-supported ion-conducting membranes—New family of columnar liquid crystalline polyamines. Part 2: Ion transport characterisation and comparison to hybrid membranes. Polymer 2016, 105, 234–242. [Google Scholar] [CrossRef]
- Montané, X.; Bogdanowicz, K.A.; Colace, G.; Reina, J.A.; Cerruti, P.; Lederer, A.; Giamberini, M. Advances in the design of self-supported ion-conducting membranes-new family of columnar liquid crystalline polyamines. Part 1: Copolymer synthesis and membrane preparation. Polymer 2016, 105, 298–309. [Google Scholar] [CrossRef]
- Henmi, M.; Nakatsuji, K.; Ichikawa, T.; Tomioka, H.; Sakamoto, T.; Yoshio, M.; Kato, T. Self-organized liquid-crystalline nanostructured membranes for water treatment: Selective permeation of ions. Adv. Mater. 2012, 24, 2238–2241. [Google Scholar] [CrossRef] [PubMed]
- Komura, M.; Yoshitake, A.; Komiyama, H.; Iyoda, T. Control of air-interface-induced perpendicular nanocylinder orientation in liquid crystal block copolymer films by a surface-covering method. Macromolecules 2015, 48, 672–678. [Google Scholar] [CrossRef]
- Martínez-Felipe, A.; Santonja-Blasco, L.; Badia, J.D.; Imrie, C.T.; Ribes-Greus, A. Characterization of functionalized side-chain liquid crystal methacrylates containing nonmesogenic units by dielectric spectroscopy. Ind. Eng. Chem. Res. 2013, 52, 8722–8731. [Google Scholar] [CrossRef]
- Kremer, F.; Huwe, A.; Schönhals, A.; Różański, S.A. Molecular dynamics in confining space. In Broadband Dielectric Spectroscopy; Springer: Berlin, Germany, 2003; pp. 171–224. [Google Scholar] [CrossRef]
- Teruel-Juanes, R.; Bogdanowicz, K.A.; Badia, J.D.; Sáenz de Juano-Arbona, V.S.d.; Graf, R.; Reina, J.A.; Giamberini, M.; Ribes-Greus, A. Molecular Mobility in Oriented and Unoriented Membranes Based on Poly[2-(Aziridin-1-yl)ethanol]. Polymers 2021, 13, 1060. [Google Scholar] [CrossRef] [PubMed]
- Percec, V.; Heck, J. Liquid Crystalline Polymers Containing Mesogenic Units Based on Half-Disc and Rod-like Moieties. 1. Synthesis and Characterization of 4-(1l-Undecan-l-yloxy)-4′-[3,4,5-Tri(p-n-Dodecan-1-yloxybenzyloxy) benzoate] Biphenyl Side Groups. J. Polym. Sci. Part A 1991, 29, 591–597. [Google Scholar] [CrossRef]
- Bogdanowicz, K.A.; Rapsilber, G.A.; Reina, J.A.; Giamberini, M. Liquid crystalline polymeric wires for selective proton transport, part 1: Wires preparation. Polymer 2016, 92, 50–57. [Google Scholar] [CrossRef]
- Gross, G.W.; Johnson, J. The layered capacitor method for dielectric bridge measurements, data analysis and interpretation of fluoride doped ice. IEEE Trans. Electr. Insul. 1983, EI-18, 485–497. [Google Scholar] [CrossRef]
- Lu, Z.; Evangelos, M.; Digby, D.M. Dielectric Relaxation Spectroscopy Studies on Water Saturated Nafion 117 Membranes. In Proceedings of the Electrochemical Society 204th National Meeting, Orlando, FL, USA, 12–16 October 2003. [Google Scholar]
- Angell, C.A. Perspective on the glass transition. J. Phys. Chem. Solids 1988, 49, 863–871. [Google Scholar] [CrossRef]
- Vogel, H. The temperature dependence law of the viscosity of fluids. Phys. Z. 1921, 22, 645–646. [Google Scholar]
- Fulcher, G.S. Analysis of recent measurements of the viscosity of glasses. J. Am. Ceram. Soc. 1992, 75, 1043–1055. [Google Scholar] [CrossRef]
- Angell, C.A. Relaxation in liquids, polymers and plastic crystals—Strong/fragile patterns and problems. J. Non-Cryst. Solids 1991, 131–133, 13–31. [Google Scholar] [CrossRef]
- Pascual-Jose, B.; Badia, J.D.; Múgica, A.; Addiego, F.; Müller, A.J.; Ribes-Greus, A. Analysis of plasticization and reprocessing effects on the segmental cooperativity of polylactide by dielectric thermal spectroscopy. Polymer 2021, 223, 123701. [Google Scholar] [CrossRef]
- Badia, J.D.; Monreal, L.; Sáenz De Juano-Arbona, V.; Ribes-Greus, A. Dielectric spectroscopy of recycled polylactide. Polym. Degrad. Stab. 2014, 107, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Sakalyte, A. Proton Transport Membranes Based on Liquid Crystalline Polymers Having Columnar Mesophases. Ph.D. Thesis, Universitat Rovira i Virgili, Tarragona, Spain, 2013. [Google Scholar]
- Vogel, H. The law of the relation between the viscosity of liquids and the temperature. Phys. Z. 1921, 22, 645–646. [Google Scholar]
- Charlesworth, J.M. Deconvolution of overlapping relaxations in dynamic mechanical spectra. J. Mater. Sci. 1993, 28, 399–404. [Google Scholar] [CrossRef]
- Havriliak, S.; Negami, S. A complex plane representation of dielectric and mechanical relaxation processes in some polymers. Polymer 1967, 8, 161–210. [Google Scholar] [CrossRef]
- Havriliak, S.; Negami, S. A complex plane analysis of α-dispersions in some polymer systems. J. Polym. Sci. Part C Polym. Symp. 1966, 14, 99–117. [Google Scholar] [CrossRef]
- Rapp, A.; Schnell, I.; Sebastiani, D.; Brown, S.P.; Percec, V.; Spiess, H.W. Supramolecular Assembly of Dendritic Polymers Elucidated by 1H and 13C Solid-State MAS NMR Spectroscopy. J. Am. Chem. Soc. 2003, 125, 13284–13297. [Google Scholar] [CrossRef] [PubMed]
- Sperling, L.H. Introduction to Physical Polymer Science; John Wiley & Sons: Hoboken, NJ, USA, 2005; ISBN 047170606X. [Google Scholar]
- Ferry, J.D. Viscoelastic Properties of Polymers; Wiley: New York, NY, USA, 1980; ISBN1 0471048941. ISBN2 9780471048947. [Google Scholar]
- Jordan, E.F.; Artymyshyn, B.; Speca, A.; Wrigley, A. Wournal of Polymer Science Part A-1. Polym. Chem. 1971, 9, 1835–1851. [Google Scholar]
- Flory, P.J.; Vrij, A. Melting Points of Linear-Chain Homologs. The Normal Paraffin Hydrocarbons. J. Am. Chem. Soc. 1963, 85, 3548–3553. [Google Scholar] [CrossRef]
- Silva, M.A.; De Paoli, M.A.; Felisberti, M.I. Flory-Huggins interaction parameter of poly(ethylene oxide)/poly(epichlorohydrin) and poly(ethylene oxide)/poly(epichlorohydrin-co-ethylene oxide) blends. Polymer 1998, 39, 2551–2556. [Google Scholar] [CrossRef]
- Tammann, G.; Hesse, W. Die Abhängigkeit der Viscosität von der Temperatur bie unterkühlten Flüssigkeiten. Z. Anorg. Allg. Chem. 1926, 156, 245–257. [Google Scholar] [CrossRef]
- Heijboer, J. Molecular origin of relaxations in polymers. Ann. N. Y. Acad. Sci. 1976, 279, 104–116. [Google Scholar] [CrossRef]
- Ribes-Greus, A.; Calleja, R.D. Dielectric relaxations of high and low density irradiated polyethylenes. J. Appl. Polym. Sci. 1989, 38, 1127–1143. [Google Scholar] [CrossRef]
- Mandelkern, L. Crystallization of Polymers: Volume 2, Kinetics and Mechanisms; Cambridge University Press: Cambridge, UK, 2004; ISBN 1139453505. [Google Scholar]
- Boyd, R.H. Relaxation processes in crystalline polymers: Experimental behaviour—A review. Polymer 1985, 26, 323–347. [Google Scholar] [CrossRef]
- Brás, A.R.; Viciosa, M.T.; Wang, Y.; Dionísio, M.; Mano, J.F. Crystallization of poly(L-lactic acid) probed with dielectric relaxation spectroscopy. Macromolecules 2006, 39, 6513–6520. [Google Scholar] [CrossRef]
- Percec, V.; Heck, J.; Lee, M.; Ungar, G.; Alvarez-Castillo, A. Poly{2-vinyloxyethyl 3,4,5-tris[4-(n-dodecanyloxy) benzyloxy] benzoate}: A self-assembled supramolecular polymer similar to tobacco mosaic virus. J. Mater. Chem. 1992, 2, 1033–1039. [Google Scholar] [CrossRef]
- Bogdanowicz, K.A.; Sistat, P.; Reina, J.A.; Giamberini, M. Liquid crystalline polymeric wires for selective proton transport, part 2: Ion transport in solid-state. Polymer 2016, 92, 58–65. [Google Scholar] [CrossRef]
- Qin, Q.; McKenna, G.B. Correlation between dynamic fragility and glass transition temperature for different classes of glass forming liquids. J. Non-Cryst. Solids 2006, 352, 2977–2985. [Google Scholar] [CrossRef]
- Adam, G.; Gibbs, J.H. On the temperature dependence of cooperative relaxation properties in glass-forming liquids. J. Chem. Phys. 1965, 43, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Turnbull, D.; Cohen, M.H. Free-volume model of the amorphous phase: Glass transition. J. Chem. Phys. 1961, 34, 120–125. [Google Scholar] [CrossRef]

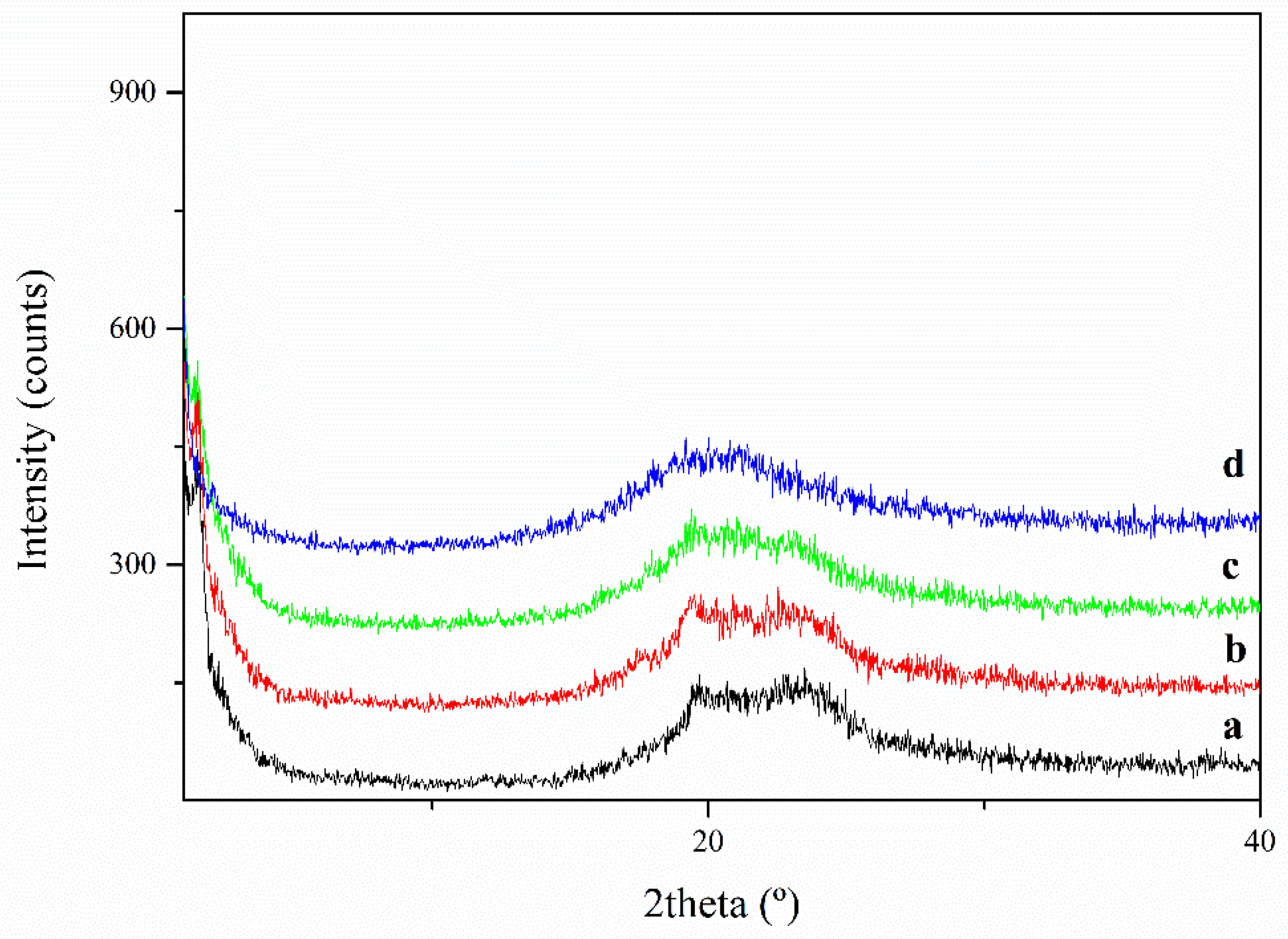

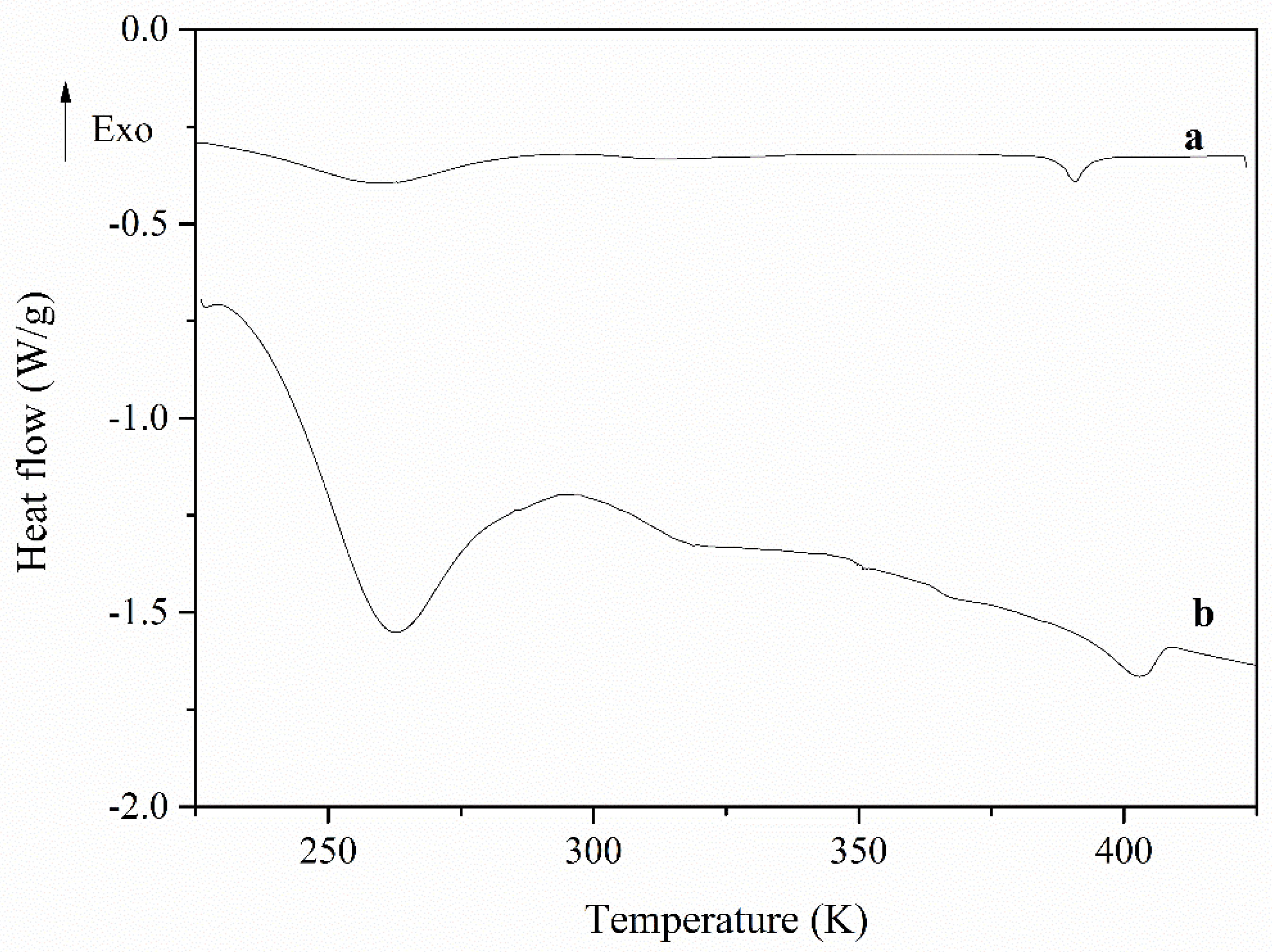

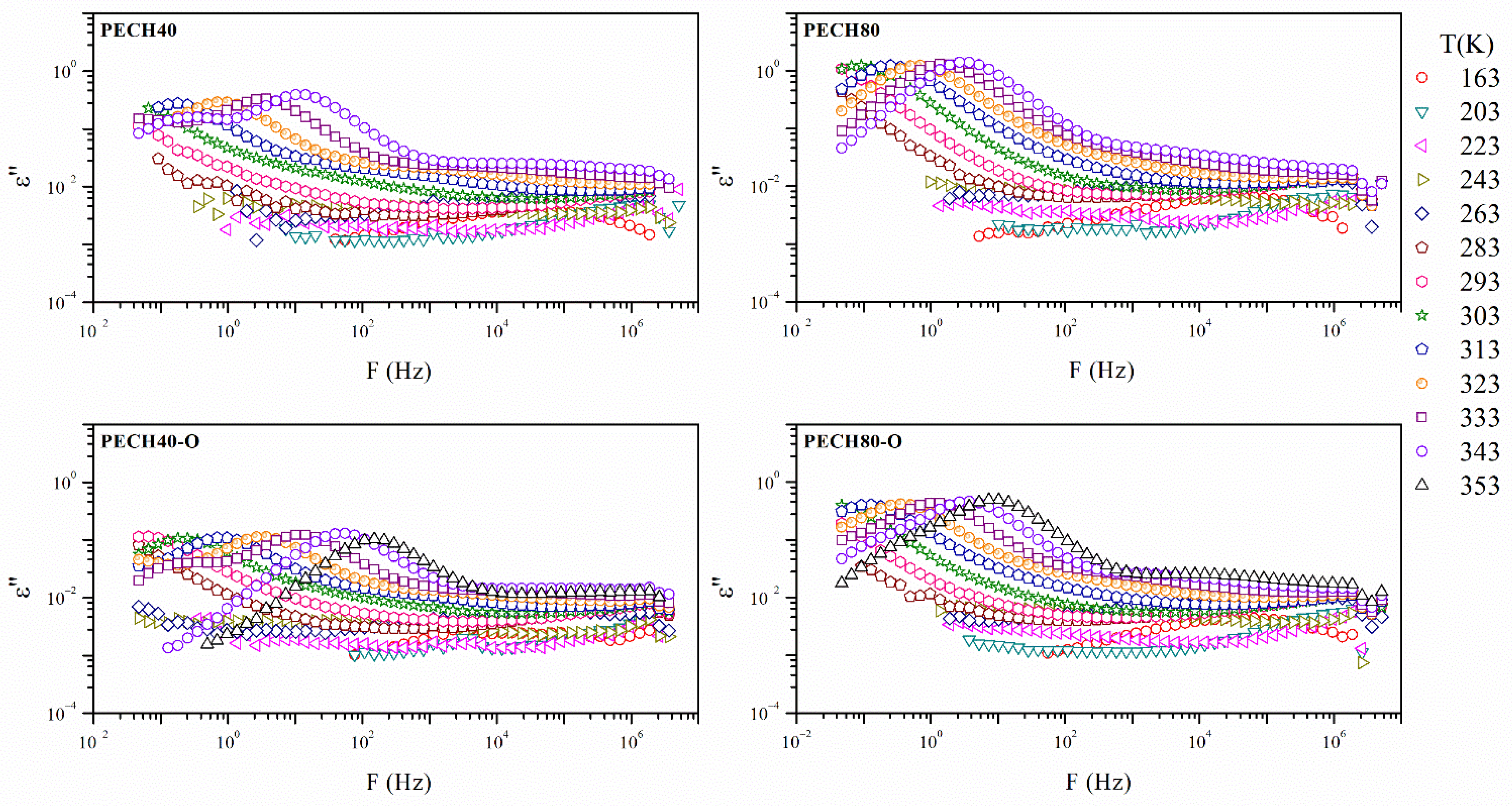
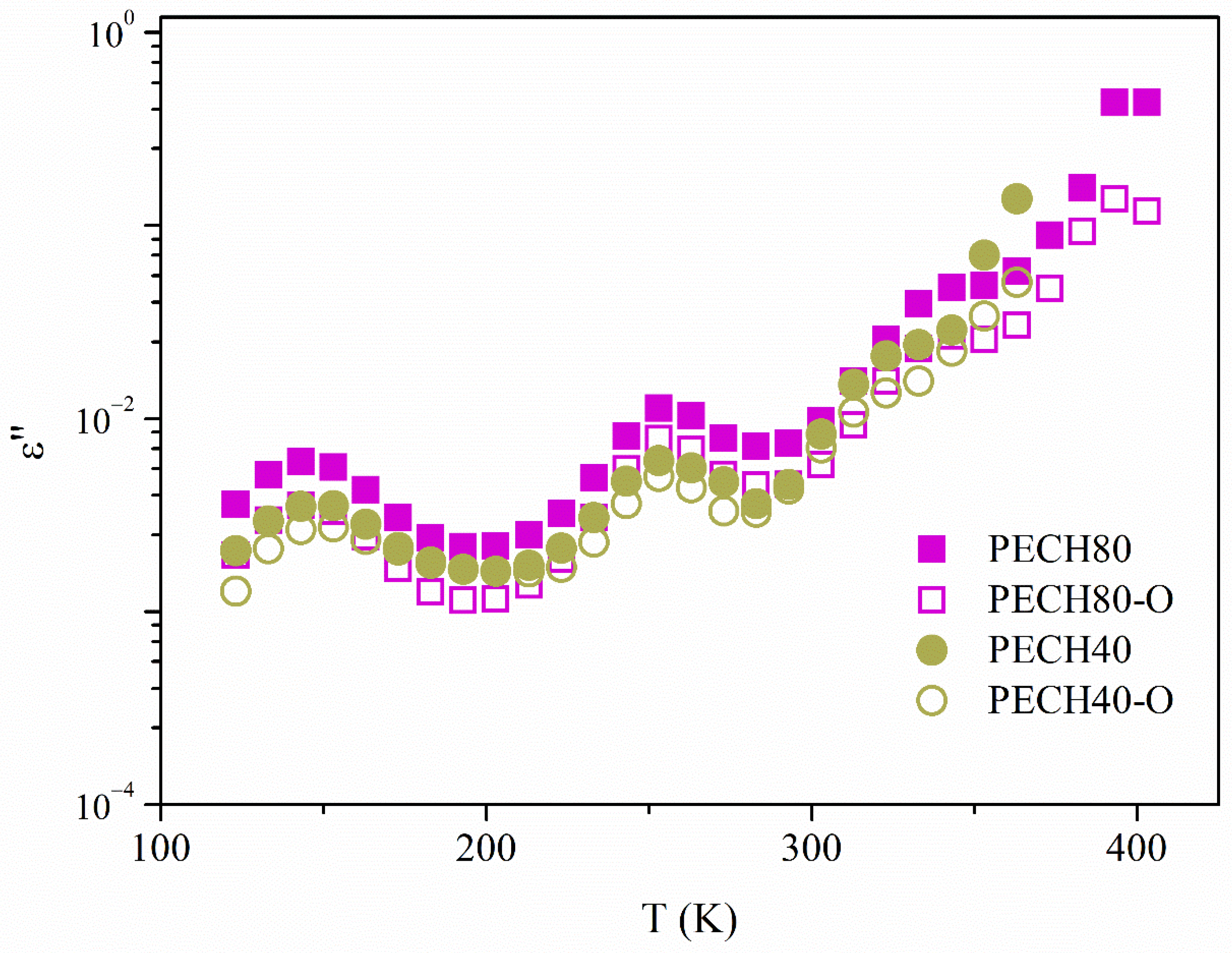

| Sample | Tm (K) | Tg (K) | Tc (K) | ΔHm (J/g) | ΔHc (J/g) |
|---|---|---|---|---|---|
| PECH40 | 230–280 | 303 | 353 | 11.6 | 1.0 |
| PECH80 | 225–290 | 306 | 403 | 17.3 | 1.9 |
| Δ ε″ | PECH80 | |||
| T (K) | 153 | 163 | 173 | |
| γ | 0.030 | 0.032 | 0.028 | |
| γ-O | 0.022 | 0.023 | 0.024 | |
| T (K) | 223 | 233 | 243 | |
| αTg | 0.094 | 0.149 | 0.245 | |
| αTg-O | 0.050 | 0.059 | 0.080 | |
| T (K) | 343 | 353 | 363 | |
| αClear | 0.218 | 0.232 | 0.246 | |
| αClear-O | 0.079 | 0.112 | 0.127 | |
| Δ ε″ | PECH40 | |||
| T (K) | 153 | 163 | 173 | |
| γ | 0.014 | 0.014 | 0.016 | |
| γ-O | 0.015 | 0.021 | 0.027 | |
| T (K) | 243 | 253 | 263 | |
| αTg | 0.623 | 0.055 | 0.070 | |
| αTg-O | 0.031 | 0.041 | 0.045 | |
| T (K) | 313 | 323 | 333 | |
| αClear | 0.163 | 0.196 | 0.226 | |
| αClear-O | 0.091 | 0.168 | 0.193 | |
| Copolymer | logf0 (Hz) | Ea (kJ·mol−1) | Tmax 1 kHz (K) | R2 |
|---|---|---|---|---|
| PECH80 | 13.7 | 26.5 | 129.1 | 0.99 |
| PECH80-O | 15.4 | 31.1 | 130.6 | 0.99 |
| PECH40 | 14.9 | 30.2 | 132.7 | 0.99 |
| PECH40-O | 16.4 | 34.5 | 135.1 | 0.98 |
| Tg (K) | τ0 (s) | D | TVFTH (K) | ΦTg | αTg 104 (K−1) | R2 | |
|---|---|---|---|---|---|---|---|
| PECH80 | 287.0 | 8.1 ± 0.1 | 0.6 ± 0.1 | 237 ± 1 | 0.377 | 75.4 | 0.99 |
| PECH80-O | 283.3 | 7.9 ± 0.1 | 0.6 ± 0.1 | 233 ± 1 | 0.344 | 68.9 | 0.99 |
| PECH40 | 270.8 | 9.7 ± 0.8 | 1.6 ± 0.5 | 220 ± 3 | 0.145 | 28.9 | 0.95 |
| PECH40-O | 270.8 | 9.7 ± 0.6 | 1.8 ± 0.3 | 221 ± 2 | 0.129 | 25.7 | 0.99 |
| αClear | τ0 (s) | D | TVFTH (K) | Φg/B | αf × 104 (K−1) | R2 |
|---|---|---|---|---|---|---|
| PECH80 | 10.4 ± 0.2 | 4.4 ± 0.18 | 260 | 0.044 | 8.7 | 0.98 |
| PECH80-O | 10.5 ± 0.4 | 4.4 ± 0.3 | 260 | 0.044 | 8.7 | 0.97 |
| PECH40 | 8.5 ± 0.5 | 0.89 ± 0.12 | --- | --- | --- | 0.92 |
| PECH40-O | 9.8 ± 0.7 | 1.9 ± 0.3 | 280 | --- | --- | 0.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teruel-Juanes, R.; Pascual-Jose, B.; Graf, R.; Reina, J.A.; Giamberini, M.; Ribes-Greus, A. Effect of Dendritic Side Groups on the Mobility of Modified Poly(epichlorohydrin) Copolymers. Polymers 2021, 13, 1961. https://doi.org/10.3390/polym13121961
Teruel-Juanes R, Pascual-Jose B, Graf R, Reina JA, Giamberini M, Ribes-Greus A. Effect of Dendritic Side Groups on the Mobility of Modified Poly(epichlorohydrin) Copolymers. Polymers. 2021; 13(12):1961. https://doi.org/10.3390/polym13121961
Chicago/Turabian StyleTeruel-Juanes, R., B. Pascual-Jose, R. Graf, J. A. Reina, M. Giamberini, and A. Ribes-Greus. 2021. "Effect of Dendritic Side Groups on the Mobility of Modified Poly(epichlorohydrin) Copolymers" Polymers 13, no. 12: 1961. https://doi.org/10.3390/polym13121961
APA StyleTeruel-Juanes, R., Pascual-Jose, B., Graf, R., Reina, J. A., Giamberini, M., & Ribes-Greus, A. (2021). Effect of Dendritic Side Groups on the Mobility of Modified Poly(epichlorohydrin) Copolymers. Polymers, 13(12), 1961. https://doi.org/10.3390/polym13121961








