Mechanical Properties and Bioactivity of Polyetheretherketone/Hydroxyapatite/Carbon Fiber Composite Prepared by the Mechanofusion Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
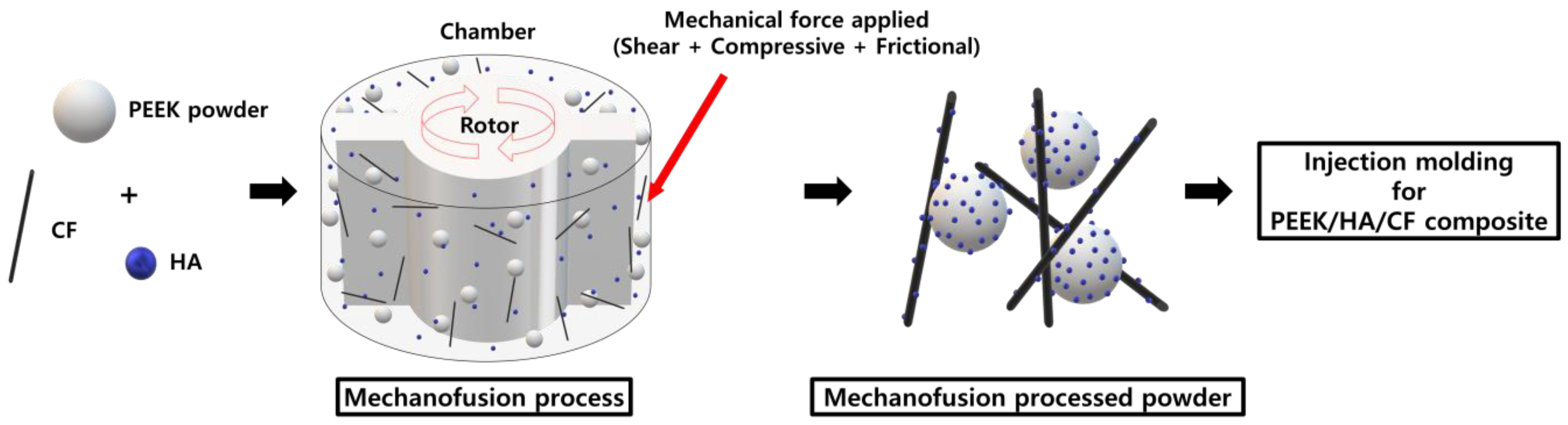
2.2. Preparation of PEEK Composite Powders
2.3. Characterization and Measurement of PEEK Composites
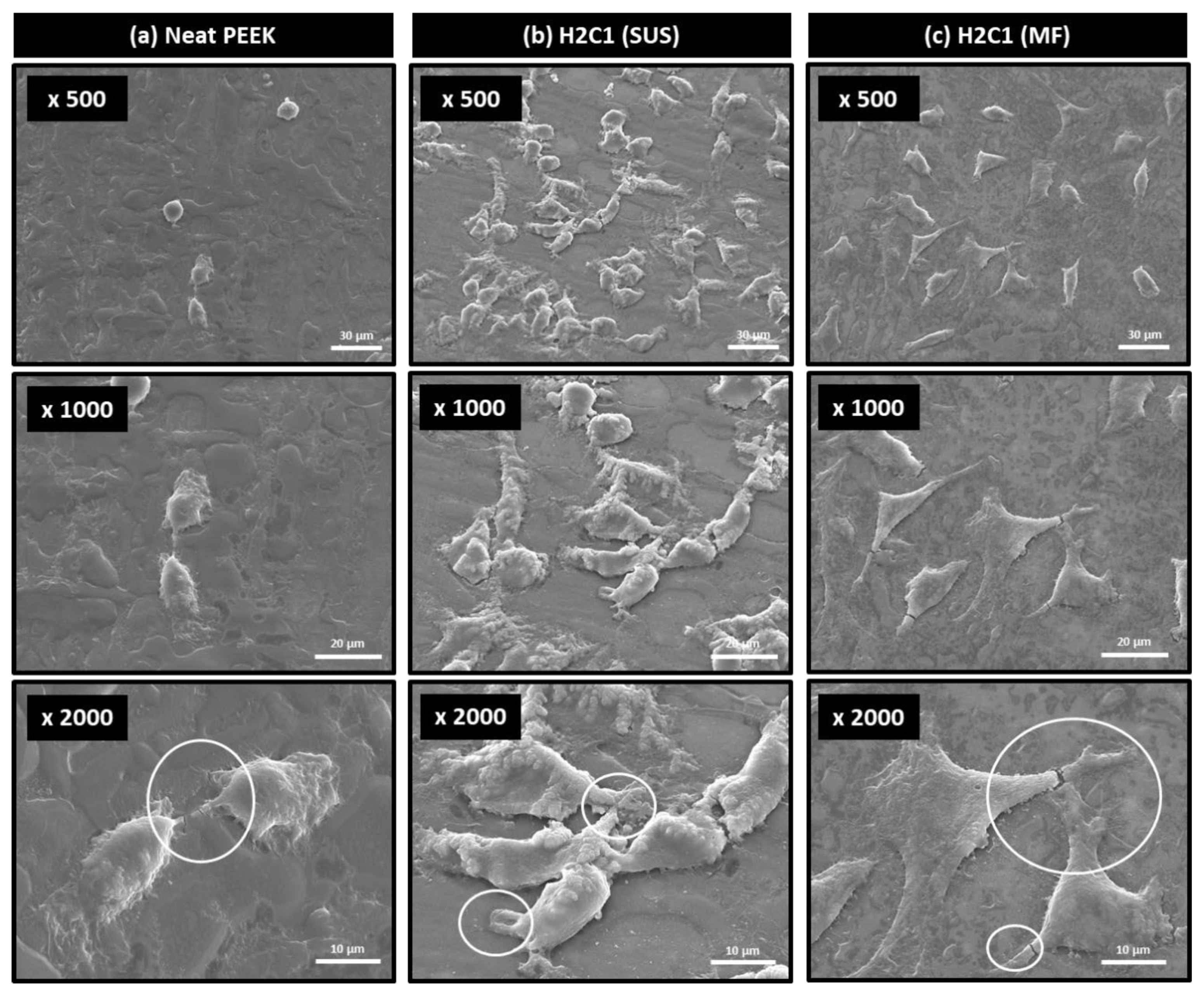
2.4. In Vitro Cell Compatibility Test
2.5. Cell Attachment Test
3. Results and Discussion
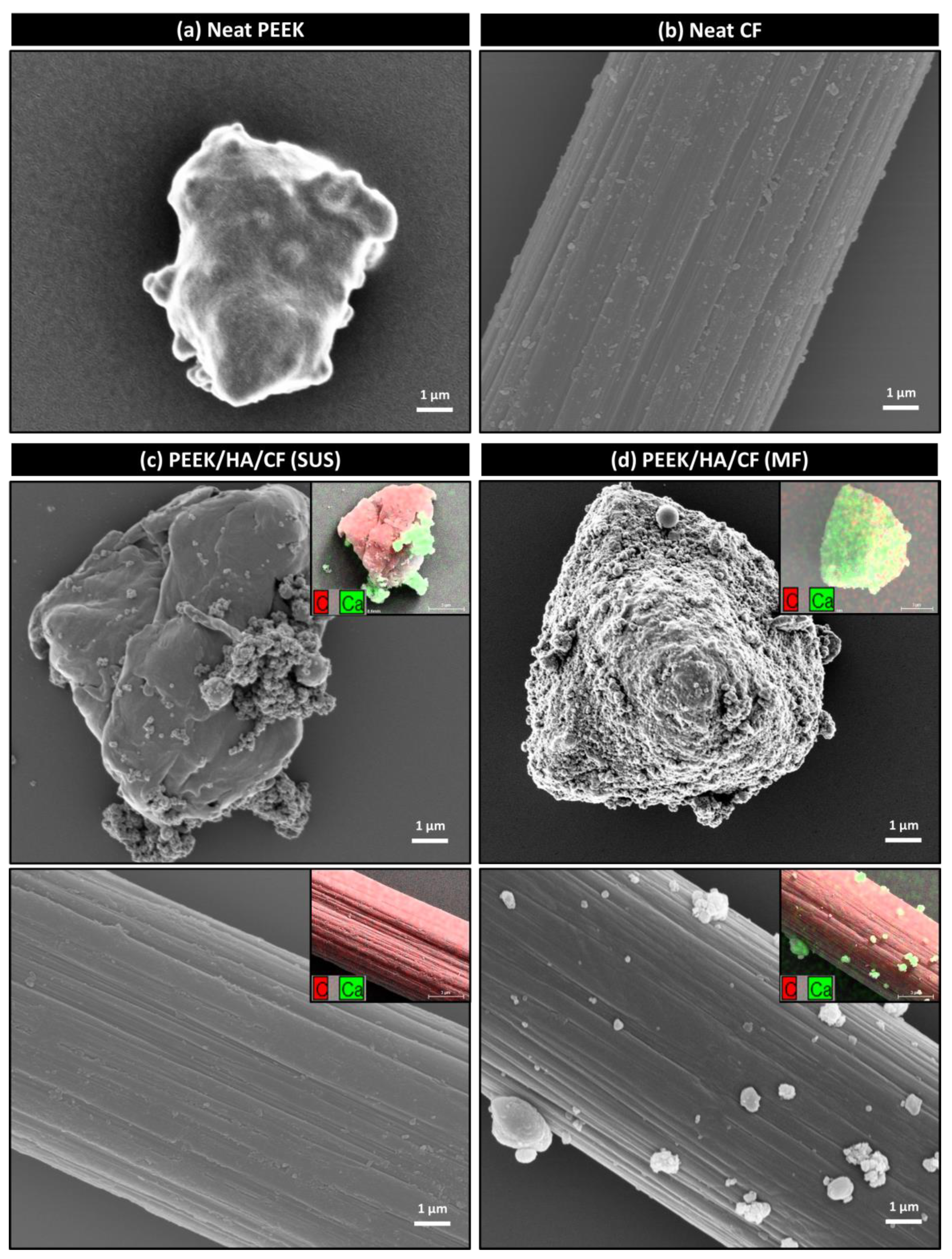
3.1. Morphology of Powders
3.2. Mechanical Properties
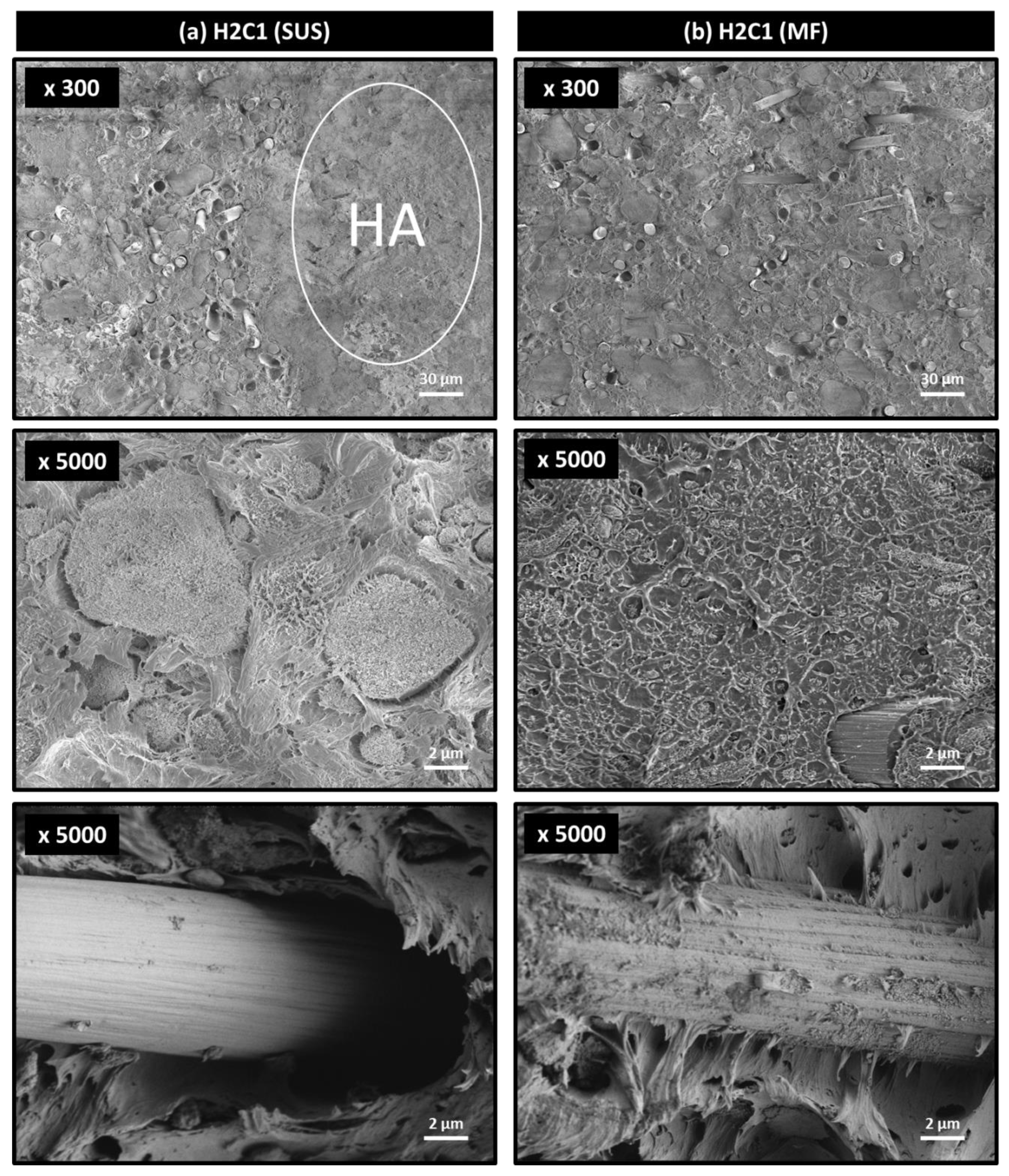
3.3. Morphology of Composites
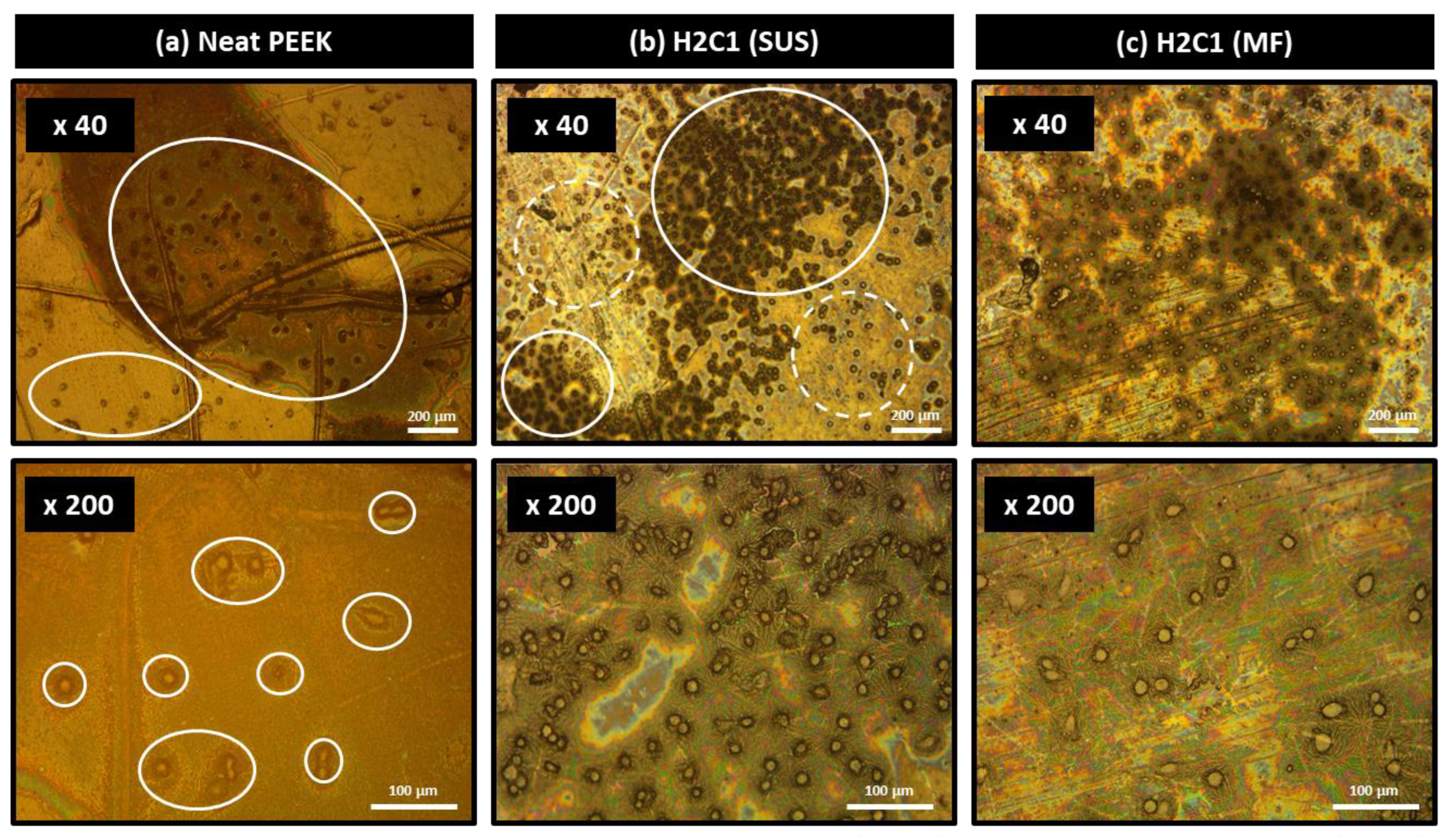
3.4. Bioactivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Warburton, A.; Girdler, S.J.; Mikhail, C.M.; Ahn, A.; Cho, S.K. Biomaterials in Spinal Implants: A Review. Neurospine 2020, 17, 101–110. [Google Scholar] [CrossRef]
- Wang, L.; He, S.; Wu, X.; Liang, S.; Mu, Z.; Wei, J.; Deng, F.; Deng, Y.; Wei, S. Polyetheretherketone/nano-fluorohydroxyapatite composite with antimicrobial activity and osseointegration properties. Biomaterials 2014, 35, 6758–6775. [Google Scholar] [CrossRef]
- Goto, K.; Kuroda, Y.; Kawai, T.; Kawanabe, K.; Matsuda, S. The use of a bioactive bone cement containing apatite-wollastonite glass-ceramic filler and bisphenol-a-glycidyl methacrylate resin for acetabular fixation in total hip arthroplasty: Long-term follow-up results of a clinical trial. Bone Jt. J. 2019, 101, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Vukajlović, Đ.; Bretcanu, O.; Novakovic, K. Comparison between chitosan coated Bioglass 45S5 and apatite-wollastonite scaffolds for tissue engineering applications. In Proceedings of the You-CGMed-Workshop for Young Researchers in Ceramics and Glasses for Medical Applications, Newcastle upon Tyne, UK, 10–11 October 2019; Newcastle University: Newcastle upon Tyne, UK, 2019. [Google Scholar]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [PubMed]
- Toth, J.M.; Wang, M.; Estes, B.T.; Scifert, J.L.; Seim, H.B., III; Turner, A.S. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials 2006, 27, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Nie, L.; Zhang, L. Posterior lumbar interbody fusion versus posterolateral fusion in spondylolisthesis: A prospective controlled study in the Han nationality. Int. Orthop. 2008, 33, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Glassman, A.H.; Bobyn, J.D.; Tanzer, M. New femoral designs: Do they influence stress shielding? Clin. Orthop. Relat. Res. 2006, 453, 64–74. [Google Scholar] [CrossRef]
- Seal, C.K.; Vince, K.; Hodgson, M.A. Biodegradable surgical implants based on magnesium alloys—A review of current research. IOP Conf. Ser. Mater. Sci. Eng. 2009, 4, 012011. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Rho, J.Y.; Ashman, R.B.; Turner, C.H. Young’s modulus of trabecular and cortical bone material: Ultrasonic and microtensile measurements. J. Biomech. 1993, 26, 111–119. [Google Scholar] [CrossRef]
- Sandler, J.; Werner, P.; Shaffer, M.S.; Demchuk, V.; Altstädt, V.; Windle, A.H. Carbon-nanofibre-reinforced poly (ether ether ketone) composites. Compos. Part A Appl. Sci. Manuf. 2002, 33, 1033–1039. [Google Scholar] [CrossRef]
- Hung, M.-C.; Yuan, S.-Y.; Chang, S.I.; Liao, J.-W.; Ko, T.-H.; Cheng, C.-L. Evaluation of active carbon fibers used in cell bio-compatibility and rat cystitis treatment. Carbon 2014, 68, 628–637. [Google Scholar] [CrossRef]
- Saleem, A.; Frormann, L.; Iqbal, A. High performance thermoplastic composites: Study on the mechanical, thermal, and electrical resistivity properties of carbon fiber-reinforced polyetheretherketone and polyethersulphone. Polym. Compos. 2007, 28, 785–796. [Google Scholar] [CrossRef]
- Li, C.S.; Vannabouathong, C.; Sprague, S.; Bhandari, M. The Use of Carbon-Fiber-Reinforced (CFR) PEEK Material in Orthopedic Implants: A Systematic Review. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2015, 8, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhou, P.; Liu, X.; Wang, L.; Xiong, X.; Tang, Z.; Wei, J.; Wei, S. Preparation, characterization, cellular response and in vivo osseointegration of polyetheretherketone/nano-hydroxyapatite/carbon fiber ternary biocomposite. Colloids Surfaces B Biointerfaces 2015, 136, 64–73. [Google Scholar] [CrossRef]
- Hwang, Y.; Kim, M.; Kim, J. Improvement of the mechanical properties and thermal conductivity of poly(ether-ether-ketone) with the addition of graphene oxide-carbon nanotube hybrid fillers. Compos. Part A Appl. Sci. Manuf. 2013, 55, 195–202. [Google Scholar] [CrossRef]
- Song, H.; Li, N.; Li, Y.; Min, C.; Wang, Z. Preparation and tribological properties of graphene/poly (ether ether ketone) nanocomposites. J. Mater. Sci. 2012, 47, 6436–6443. [Google Scholar] [CrossRef]
- Puértolas, J.A.; Castro, M.; Morris, J.A.; Ríos, A.; Ansón-Casaos, A. Tribological and mechanical properties of graphene nanoplatelet/PEEK composites. Carbon 2019, 141, 107–122. [Google Scholar] [CrossRef]
- Ma, R.; Tang, S.; Tan, H.; Qian, J.; Lin, W.; Wang, Y.; Liu, C.; Wei, J.; Tang, T. Preparation, Characterization, In Vitro Bioactivity, and Cellular Responses to a Polyetheretherketone Bioactive Composite Containing Nanocalcium Silicate for Bone Repair. ACS Appl. Mater. Interfaces 2014, 6, 12214–12225. [Google Scholar] [CrossRef]
- Roeder, R.K.; Converse, G.; Kane, R.J.; Yue, W. Hydroxyapatite-reinforced polymer biocomposites for synthetic bone substitutes. JOM 2008, 60, 38–45. [Google Scholar] [CrossRef]
- Hämmerle, C.H.F.; Olah, A.J.; Schmid, J.; Flückiger, L.; Gogolewski, S.; Winkler, J.R.; Lang, N.P. The biological effect of natural bone mineral on bone neoformation on the rabbit skull. Clin. Oral Implant. Res. 1997, 8, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials 2000, 21, 1803–1810. [Google Scholar] [CrossRef]
- Abu Bakar, M.; Cheang, P.; Khor, K. Mechanical properties of injection molded hydroxyapatite-polyetheretherketone biocomposites. Compos. Sci. Technol. 2003, 63, 421–425. [Google Scholar] [CrossRef]
- Monich, P.R.; Henriques, B.; de Oliveira, A.P.N.; Souza, J.C.; Fredel, M.C. Mechanical and biological behavior of biomedical PEEK matrix composites: A focused review. Mater. Lett. 2016, 185, 593–597. [Google Scholar] [CrossRef]
- Mathieu, L.M.; Bourban, P.-E.; Månson, J.-A.E. Processing of homogeneous ceramic/polymer blends for bioresorbable composites. Compos. Sci. Technol. 2006, 66, 1606–1614. [Google Scholar] [CrossRef]
- Abu Bakar, M.; Cheang, P.; Khor, K. Tensile properties and microstructural analysis of spheroidized hydroxyapatite–poly (etheretherketone) biocomposites. Mater. Sci. Eng. A 2003, 345, 55–63. [Google Scholar] [CrossRef]
- Ma, R.; Li, Q.; Wang, L.; Zhang, X.; Fang, L.; Luo, Z.; Xue, B.; Ma, L. Mechanical properties and in vivo study of modified-hydroxyapatite/polyetheretherketone biocomposites. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 429–439. [Google Scholar] [CrossRef]
- Feng, X.; Sui, G.; Yang, R. Mechanical properties and in vitro bioactivity of carbon fibre-reinforced polyetheretherketone–hydroxyapatite (PEEK-HA) composites for biomedical applications. Chin. J. Mater. Res. 2008, 22, 18–25. [Google Scholar]
- Kaur, I.; Ellis, L.-J.; Romer, I.; Tantra, R.; Carriere, M.; Allard, S.; Hermite, M.M.-L.; Minelli, C.; Unger, W.; Potthoff, A.; et al. Dispersion of Nanomaterials in Aqueous Media: Towards Protocol Optimization. J. Vis. Exp. 2017, 130, e56074. [Google Scholar] [CrossRef]
- Peng, T.; Chang, I. Uniformly dispersion of carbon nanotube in aluminum powders by wet shake-mixing approach. Powder Technol. 2015, 284, 32–39. [Google Scholar] [CrossRef]
- Lee, Y.M.; You, J.; Kim, M.; Kim, T.A.; Lee, S.-S.; Bang, J.; Park, J.H. Highly improved interfacial affinity in carbon fiber-reinforced polymer composites via oxygen and nitrogen plasma-assisted mechanochemistry. Compos. Part B Eng. 2019, 165, 725–732. [Google Scholar] [CrossRef]
- Alonso, M.; Alguacil, F.J. Dry mixing and coating of powders. Rev. Met. 1999, 35, 315–328. [Google Scholar] [CrossRef]
- Yokoyama, T.; Huang, C. Nanoparticle Technology for the Production of Functional Materials. KONA Powder Part. J. 2005, 23, 7–17. [Google Scholar] [CrossRef]
- Koskela, J.; Morton, D.A.; Stewart, P.J.; Juppo, A.M.; Lakio, S. The effect of mechanical dry coating with magnesium stearate on flowability and compactibility of plastically deforming microcrystalline cellulose powders. Int. J. Pharm. 2018, 537, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Posudievsky, O.Y.; Kozarenko, O.A.; Dyadyun, V.S.; Jorgensen, S.W.; Spearot, J.A.; Koshechko, V.G.; Pokhodenko, V.D. Characteristics of mechanochemically prepared host–guest hybrid nanocomposites of vanadium oxide and conducting polymers. J. Power Sources 2011, 196, 3331–3341. [Google Scholar] [CrossRef]
- You, J.; Choi, H.-H.; Cho, J.; Son, J.G.; Park, M.; Lee, S.-S.; Park, J.H. Highly thermally conductive and mechanically robust polyamide/graphite nanoplatelet composites via mechanochemical bonding techniques with plasma treatment. Compos. Sci. Technol. 2018, 160, 245–254. [Google Scholar] [CrossRef]
- Kim, H.S.; Bae, H.S.; Yu, J.; Kim, S.Y. Thermal conductivity of polymer composites with the geometrical characteristics of graphene nanoplatelets. Sci. Rep. 2016, 6, 26825. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.H.; Kim, W.Y.; Lee, H.S.; Kim, S.Y.; Khil, M.-S. Volume control of expanded graphite based on inductively coupled plasma and enhanced thermal conductivity of epoxy composite by formation of the filler network. Carbon 2017, 119, 40–46. [Google Scholar] [CrossRef]
- Pfeffer, R.; Dave, R.; Wei, D.; Ramlakhan, M. Synthesis of engineered particulates with tailored properties using dry particle coating. Powder Technol. 2001, 117, 40–67. [Google Scholar] [CrossRef]
- Zhou, Q.T.; Qu, L.; Larson, I.; Stewart, P.J.; Morton, D.A. Effect of mechanical dry particle coating on the improvement of powder flowability for lactose monohydrate: A model cohesive pharmaceutical powder. Powder Technol. 2011, 207, 414–421. [Google Scholar] [CrossRef]
- Jang, J.; Yang, H. The effect of surface treatment on the performance improvement of carbon fiber/polybenzoxazine composites. J. Mater. Sci. 2000, 35, 2297–2303. [Google Scholar] [CrossRef]
- Bhatt, P.; Goel, A. Carbon Fibres: Production, Properties and Potential Use. Mater. Sci. Res. India 2017, 14, 52–57. [Google Scholar] [CrossRef]
- Caeiro, J.R.; González, P.; Guede, D. Biomechanics and bone (& II): Trials in different hierarchical levels of bone and alternative tools for the determination of bone strength. Rev. Osteoporos. Metab. Miner. 2013, 5, 99–108. [Google Scholar]
- Wang, M.; Porter, D.; Bonfield, W. Processing, characterization, and evaluation of hydroxyapatite reinforced polyethylene composites. Br. Ceram. Trans. 1994, 93, 91–95. [Google Scholar]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Fabrication, Properties and Applications of Dense Hydroxyapatite: A Review. J. Funct. Biomater. 2015, 6, 1099–1140. [Google Scholar] [CrossRef]
- Lawson, A.C.; Czernuszka, J.T. Collagen-calcium phosphate composites. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1998, 212, 413–425. [Google Scholar] [CrossRef]
- You, J.; Choi, H.-H.; Lee, Y.M.; Cho, J.; Park, M.; Lee, S.-S.; Park, J.H. Plasma-assisted mechanochemistry to produce polyamide/boron nitride nanocomposites with high thermal conductivities and mechanical properties. Compos. Part B Eng. 2019, 164, 710–719. [Google Scholar] [CrossRef]
- You, J.; Choi, H.-H.; Kim, T.A.; Park, M.; Ha, J.S.; Lee, S.-S.; Park, J.H. High-performance polyketone nanocomposites achieved via plasma-assisted mechanochemistry. Compos. Sci. Technol. 2019, 183, 107800. [Google Scholar] [CrossRef]
- Kong, L.; Gao, Y.; Lu, G.; Gong, Y.; Zhao, N.; Zhang, X. A study on the bioactivity of chitosan/nano-hydroxyapatite composite scaffolds for bone tissue engineering. Eur. Polym. J. 2006, 42, 3171–3179. [Google Scholar] [CrossRef]
- Ogata, K.; Imazato, S.; Ehara, A.; Ebisu, S.; Kinomoto, Y.; Nakano, T.; Umakoshi, Y. Comparison of osteoblast responses to hydroxyapatite and hydroxyapatite/soluble calcium phosphate composites. J. Biomed. Mater. Res. Part A 2004, 72, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.; Schäfer, C. Filopodial focal complexes direct adhesion and force generation towards filopodia outgrowth. Cell Adhes. Migr. 2010, 4, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Lee, K.-B.; Jeon, H.-S.; Park, H.-K. Effects of Surface Nano-Topography on Human Osteoblast Filopodia. Anal. Sci. 2011, 27, 369. [Google Scholar] [CrossRef] [PubMed]


| PEEK | HA | CF | |
|---|---|---|---|
| Product name | Victrex 450PF | Nano HAP04 | PX 35 |
| Diameter/Length | 50 μm | 20 nm/150 nm | 7.2 μm/150 μm |
| Tensile strength | 98 MPa | − | 4137 MPa |
| Tensile modulus | 4 GPa | − | 242 GPa |
| Flexural strength | 165 MPa | − | − |
| Flexural modulus | 3.8 GPa | − | − |
| Compressive strength | 125 MPa | − | − |
| Density | 1.3 g/cm3 | − | 1.81 g/cm3 |
| Sample Code | PEEK Content (wt%) | HA Content (wt%) | CF Content (wt%) |
|---|---|---|---|
| PEEK | 100 | 0 | 0 |
| C1 | 90 | 0 | 0 |
| C2 | 80 | 0 | 20 |
| C3 | 70 | 0 | 30 |
| H1C1 | 80 | 10 | 10 |
| H1C2 | 70 | 10 | 20 |
| H1C3 | 60 | 10 | 30 |
| H2C1 | 70 | 20 | 10 |
| H2C2 | 60 | 20 | 20 |
| Flexural Modulus (GPa) | Flexural Strength (MPa) | Flexural Strain at Break(%) | Compressive Modulus (GPa) | Compressive Strength (MPa) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SUS | MF | SUS | MF | SUS | MF | SUS | MF | SUS | MF | |
| PEEK | 3.7 ± 0.2 | 155 ± 3 | − | 2.7 ± 0.1 | 120 ± 2 | |||||
| C1 | 5.6 ± 0.1 | 5.7 ± 0.3 | 182 ± 3 | 185 ± 3 | 5.5 ± 0.3 | 5.4 ± 0.2 | 3.5 ± 0.1 | 3.6 ± 0.1 | 134 ± 1 | 135 ± 3 |
| C2 | 8.7 ± 0.2 | 8.9 ± 0.3 | 219 ± 2 | 221 ± 2 | 3.8 ± 0.3 | 3.9 ± 0.1 | 4.0 ± 0.1 | 3.9 ± 0.1 | 151 ± 3 | 153 ± 4 |
| C3 | 13.5 ± 0.4 | 13.5 ± 0.4 | 252 ± 5 | 254 ± 4 | 2.5 ± 0.1 | 2.4 ± 0.1 | 4.5 ± 0.1 | 4.4 ± 0.1 | 168 ± 2 | 170 ± 3 |
| H1C1 | 7.7 ± 0.1 | 7.3 ± 0.1 | 165 ± 4 | 178 ± 1 | 2.4 ± 0.1 | 4.0 ± 0.1 | 2.3 ± 0.1 | 2.4 ± 0.1 | 139 ± 2 | 149 ± 2 |
| H1C2 | 11.0 ± 0.2 | 10.9 ± 0.2 | 198 ± 3 | 215 ± 1 | 2.1 ± 0.1 | 3.2 ± 0.1 | 4.1 ± 0.2 | 2.6 ± 0.1 | 158 ± 2 | 170 ± 3 |
| H1C3 | 18.2 ± 0.9 | 17.7 ± 0.3 | 228 ± 7 | 246 ± 2 | 1.5 ± 0.1 | 1.9 ± 0.1 | 4.6 ± 0.3 | 3.2 ± 0.2 | 178 ± 2 | 198 ± 3 |
| H2C1 | 8.8 ± 0.1 | 8.9 ± 0.4 | 132 ± 3 | 172 ± 2 | 1.6 ± 0.1 | 2.4 ± 0.1 | 3.8 ± 0.1 | 3.2 ± 0.2 | 151 ± 5 | 167 ± 3 |
| H2C2 | 13.3 ± 0.2 | 13.5 ± 0.1 | 163 ± 13 | 206 ± 3 | 1.4 ± 0.1 | 1.8 ± 0.1 | 4.5 ± 0.1 | 3.4 ± 0.1 | 170 ± 6 | 192 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, I.S.; Lee, M.H.; Choi, H.-H.; Lee, S.; Chon, J.W.; Chung, D.J.; Park, J.H.; Jho, J.Y. Mechanical Properties and Bioactivity of Polyetheretherketone/Hydroxyapatite/Carbon Fiber Composite Prepared by the Mechanofusion Process. Polymers 2021, 13, 1978. https://doi.org/10.3390/polym13121978
Jeon IS, Lee MH, Choi H-H, Lee S, Chon JW, Chung DJ, Park JH, Jho JY. Mechanical Properties and Bioactivity of Polyetheretherketone/Hydroxyapatite/Carbon Fiber Composite Prepared by the Mechanofusion Process. Polymers. 2021; 13(12):1978. https://doi.org/10.3390/polym13121978
Chicago/Turabian StyleJeon, In Sung, Moon Hyun Lee, Han-Hyeong Choi, Sangwoon Lee, Joon Woo Chon, Dong June Chung, Jong Hyuk Park, and Jae Young Jho. 2021. "Mechanical Properties and Bioactivity of Polyetheretherketone/Hydroxyapatite/Carbon Fiber Composite Prepared by the Mechanofusion Process" Polymers 13, no. 12: 1978. https://doi.org/10.3390/polym13121978
APA StyleJeon, I. S., Lee, M. H., Choi, H.-H., Lee, S., Chon, J. W., Chung, D. J., Park, J. H., & Jho, J. Y. (2021). Mechanical Properties and Bioactivity of Polyetheretherketone/Hydroxyapatite/Carbon Fiber Composite Prepared by the Mechanofusion Process. Polymers, 13(12), 1978. https://doi.org/10.3390/polym13121978







