Transparent, Conductive Hydrogels with High Mechanical Strength and Toughness
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
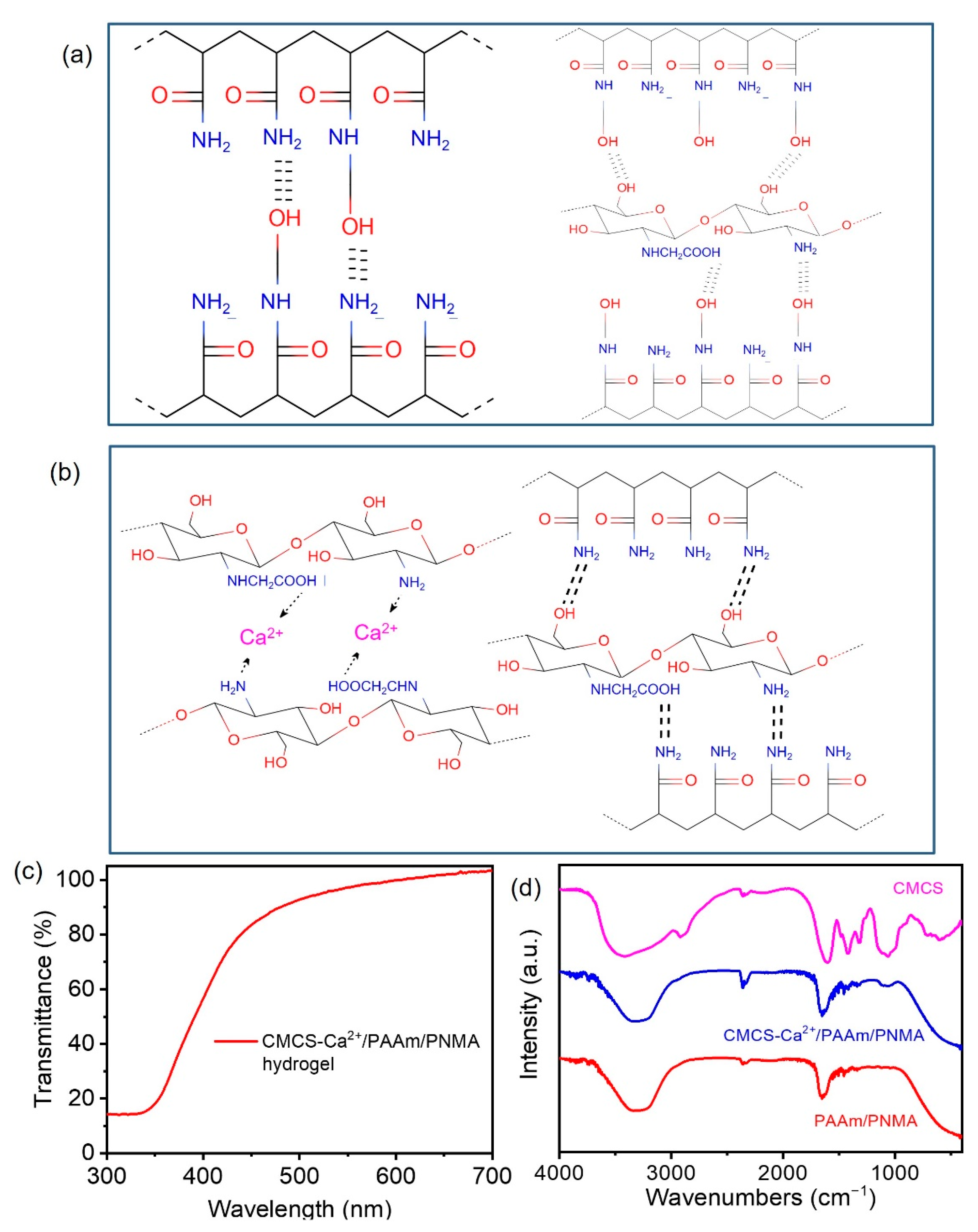
3.1. Characterazation of CMCS-Ca2+/PAAm/PNMA Transparent Conductive Hydrogels
3.2. Mechanical Properties of CMCS-Ca2+/PAAm/PNMA Transparent Conductive Hydrogels
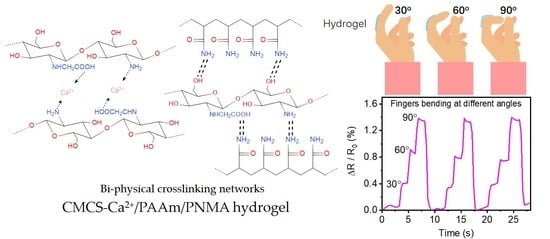
3.3. Electro-Mechanical Properties and Strain-Sensing Performance of CMCS-Ca2+/PAAm/PNMA Transparent Conductive Hydrogels
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liao, M.H.; Wan, P.B.; Wen, J.R.; Gong, M.; Wu, X.X.; Wang, Y.G.; Shi, R.; Zhang, L.Q. Wearable, healable, and adhesive epidermal sensors assembled from mussel-inspired conductive hybrid hydrogel framework. Adv. Funct. Mater. 2017, 27, 1703852. [Google Scholar] [CrossRef]
- Xiao, X.L.; Wu, G.Z.; Zhou, H.T.; Qian, K.; Hu, J.L. Preparation and property evaluation of conductive hydrogel using poly (Vinyl Alcohol)/polyethylene glycol/graphene oxide for human electrocardiogram acquisition. Polymers 2017, 9, 259. [Google Scholar] [CrossRef]
- Liang, Y.P.; Zhao, X.; Hu, T.L.; Chen, B.J.; Yin, Z.H.; Ma, P.X.; Guo, B.L. Adhesive hemostatic conducting injectable composite hydrogels with sustained drug release and photothermal antibacterial activity to promote full-thickness skin regeneration during wound healing. Small 2019, 15, 1900046. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lang, Q.; Yildirimer, L.; Lin, Z.Y.; Cui, W.G.; Annabi, N.; Ng, K.W.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Khademhosseini, A. Photocrosslinkable gelatin hydrogel for epidermal tissue engineering. Adv. Healthc. Mater. 2016, 5, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Lu, B.Y.; Zhao, X.H. Hydrogel bioelectronics. Chem. Soc. Rev. 2019, 48, 1642–1667. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Park, K.; Choi, H.; Son, D.; Shin, M. Self-healing, stretchable, biocompatible, and conductive alginate hydrogels through dynamic covalent bonds for implantable electronics. Polymers 2021, 7, 1133. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Han, S.I.; Song, K.-I.; Seong, D.; Lee, K.; Kim, S.H.; Park, T.; Koo, J.H.; Shin, M.; Baac, H.W.; et al. Durable and fatigue-resistant soft peripheral neuroprosthetics for in vivo bidirectional signaling. Adv. Mater. 2021, 20, 2007346. [Google Scholar] [CrossRef]
- Song, K.I.; Seo, H.; Seong, D.; Kim, S.; Yu, K.J.; Kim, Y.C.; Kim, J.; Kwon, S.J.; Han, H.S.; Youn, I.; et al. Adaptive self-healing electronic epineurium for chronic bidirectional neural interfaces. Nat. Commun. 2020, 11, 114195. [Google Scholar] [CrossRef]
- Pu, X.; Liu, M.M.; Chen, X.Y.; Sun, J.M.; Du, C.H.; Zhang, Y.; Zhai, J.Y.; Hu, W.G.; Wang, Z.L. Ultrastretchable, transparent triboelectric nanogenerator as electronic skin for biomechanical energy harvesting and tactile sensing. Sci. Adv. 2017, 3, e1700015. [Google Scholar] [CrossRef]
- Xu, X.R.; Hu, D.; Yan, L.J.; Fang, S.L.; Shen, C.; Loo, Y.-L.; Lin, Y.; Haines, C.S.; Li, N.; Zakhidov, A.A.; et al. Polar-electrode-bridged electroluminescent displays: 2D sensors remotely communicating optically. Adv. Mater. 2017, 29, 1703552. [Google Scholar] [CrossRef]
- Lin, Z.; Yang, Y.; Liang, Z.; Zeng, L.; Zhang, A. Preparation of chitosan/calcium alginate/bentonite composite hydrogel and its heavy metal ions adsorption properties. Polymers 2021, 13, 1891. [Google Scholar] [CrossRef]
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, structures, and advanced applications of nanocom-posites from biorenewable resources. Chem. Rev. 2020, 120, 9304–9362. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.C.; Yu, S.S. Cationic cellulose nanocrystals-based nanocomposite hydrogels: Achieving 3D printable capacitive sensors with high transparency and mechanical strength. Polymers 2021, 13, 688. [Google Scholar] [CrossRef]
- Verma, A.; Thakur, S.; Mamba, G.; Prateek; Gupta, R.K.; Thakur, P.; Thakur, V.K. Graphite modified sodium alginate hydrogel composite for efficient removal of malachite green dye. Int. J. Biol. Macromol. 2020, 148, 1130–1139. [Google Scholar] [CrossRef]
- Sharma, B.; Thakur, S.; Mamba, G.; Prateek; Gupta, R.K.; Gupta, V.K.; Thakur, V.K. Titania modified gum tragacanth based hydrogel nanocomposite for water remediation. J. Environ. Chem. Eng. 2021, 9, 104608. [Google Scholar] [CrossRef]
- Han, L.; Yan, L.W.; Wang, M.H.; Wang, K.F.; Fang, L.M.; Zhou, J.; Fang, J.; Ren, F.Z.; Lu, X. Transparent, adhesive, and conductive hydrogel for soft bioelectronics based on Light-transmitting polydopamine-doped polypyrrole nanofibrils. Chem. Mater. 2018, 30, 5561–5572. [Google Scholar] [CrossRef]
- Hu, D.; Xu, X.R.; Miao, J.S.; Gidron, O.; Meng, H. A stretchable alternating current electroluminescent fiber. Materials 2018, 11, 184. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Lin, P.; Ma, S.H.; Wang, X.L.; Zhou, F. Molecularly engineered dual-crosslinked hydrogel with ultrahigh mechanical strength, toughness, and good self-recovery. Adv. Mater. 2015, 27, 2054–2059. [Google Scholar] [CrossRef]
- Liu, X.Y.; He, X.; Yang, B.; Lai, L.; Chen, N.X.; Hu, J.H.; Lu, Q.Y. Dual physically cross-linked hydrogels incorporating hydrophobic interactions with promising repairability and ultrahigh elongation. Adv. Funct. Mater. 2021, 31, 2008187. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Dong, C.R.; Xiao, H. Study on synthesis and performance of ST/PVA/PAM superabsorbent resin. Guangzhou Chem. Ind. 2020, 48, 39–41. [Google Scholar]
- Wang, J.S.; Peng, F.; Yan, F.F.; Fang, B. Preparation and drug releasing property of poly(N-isopropylacrylaimde-co-N-hdroxymethyl acrylamide)/chitosan hydrogel. J. Chem. Eng. Chin. Univ. 2014, 28, 1119–1125. [Google Scholar]
- Rasoulzadeh, M.; Namazi, H. Carboxymethyl cellulose/graphene oxide bio-nanocomposite hydrogel beads as anticancer drug carrier agent. Carbohydr. Polym. 2017, 168, 320–326. [Google Scholar] [CrossRef]
- Wang, Q.F.; Li, S.M.; Wang, Z.Y.; Liu, H.Z.; Li, C.J. Preparation and characterization of a positive thermoresponsive hydrogel for drug loading and release. J. Appl. Polym. Sci. 2009, 111, 1417–1425. [Google Scholar] [CrossRef]
- Morelle, X.P.; Illeperuma, W.R.; Tian, K.; Bai, R.B.; Suo, Z.G.; Vlassak, J.J. Highly stretchable and tough hydrogels below water freezing temperature. Adv. Mater. 2018, 30, 1801541. [Google Scholar] [CrossRef]
- Lei, H.; Dong, L.; Li, Y.; Zhang, J.S.; Chen, H.Y.; Wu, J.H.; Zhang, Y.; Fan, Q.Y.; Xue, B.; Qin, M.; et al. Stretchable hydrogels with low hysteresis and anti-fatigue fracture based on polyprotein cross-linkers. Nat. Commun. 2020, 11, 4032. [Google Scholar] [CrossRef]
- Li, D.J.; Gao, H.C.; Li, M.S.; Chen, G.X.; Guan, L.Y.; He, M.H.; Tian, J.F.; Cao, R. Nanochitin/metal ion dual reinforcement in synthetic polyacrylamide network-based nanocomposite hydrogels. Carbohydr. Polym. 2020, 236, 116061. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Lopez, D.; Grande, C.; Gomez, C.M. Reversible stress softening and stress recovery of cellulose networks. Soft Matter 2009, 5, 4185–4190. [Google Scholar] [CrossRef]
- Yang, J.; Ma, M.G.; Zhang, X.M.; Xu, F. Elucidating dynamics of precoordinated ionic bridges as sacrificial bonds in interpenetrating network hydrogels. Macromolecules 2016, 49, 4340–4348. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Lin, Y.-J.; Enriquez, E.; Peng, X.-F.; Turng, L.-S. Highly stretchable and biocompatible strain sensors based on mussel-inspired super-adhesive self-healing hydrogels for human motion monitoring. ACS Appl. Mater. Interfaces 2018, 10, 20897–20909. [Google Scholar] [CrossRef]
- Sun, X.; Qin, Z.H.; Ye, L.; Zhang, H.T.; Yu, Q.Y.; Wu, X.J.; Li, J.J.; Yao, F.L. Carbon nanotubes reinforced hydrogel as flexible strain sensor with high stretchability and mechanically toughness. Chem. Eng. J. 2020, 382, 122832. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, Y.; Chen, Y.; Han, X.; Jiang, F. Cellulose nanofibrils enhanced, strong, stretchable, freezing-tolerant ionic conductive organohydrogel for multi-functional sensors. Adv. Funct. Mater. 2020, 30, 2003430. [Google Scholar] [CrossRef]
- Sun, X.; Yao, F.; Wang, C.; Qin, Z.; Zhang, H.; Yu, Q.; Zhang, H.; Dong, X.; Wei, Y.; Li, J. Ionically conductive hydrogel with fast self-recovery and low residual strain as strain and pressure sensors. Macromol. Rapid Commun. 2020, 41, 2000185. [Google Scholar] [CrossRef]
- Han, S.; Liu, C.; Lin, X.; Zheng, J.; Wu, J.; Liu, C. Dual conductive network hydrogel for a highly conductive, self-healing, anti-Freezing, and non-drying strain sensor. ACS Appl. Polym. Mater. 2020, 2, 996–1005. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, X.; Lin, C.; Ma, X.; Cao, S.; Ni, Y. Ultrafast gelling using sulfonated lignin-Fe3+ chelates to produce dynamic crosslinked hydrogel/coating with charming stretchable, conductive, self-healing, and ultraviolet-blocking properties. Chem. Eng. J. 2020, 396, 125341. [Google Scholar] [CrossRef]
- Chen, H.; Ren, X.; Gao, G. Skin-inspired gels with toughness, antifreezing, conductivity, and remoldability. ACS Appl. Mater. Interfaces 2019, 11, 28336–28344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, X.; Duan, L.J.; Gao, G.H. A DNA-inspired hydrogel mechanoreceptor with skin-like mechanical behavior. J. Mater. Chem. A 2021, 9, 1835. [Google Scholar] [CrossRef]




| Hydrogels | Transparency | Fracture Strain | Tensile Strength | Toughness | Conductivity |
|---|---|---|---|---|---|
| (%) | (%) | (MPa) | (MJ/m3) | (S/m) | |
| PVA/cellulose nanofibrils/DMSO/H2O [33] | 90% | 660 | 2.1 | 5.25 | 3.2 |
| PAAm/GE/Na3Cit [34] | non-transparent (milky white) | 849 | 1.66 | 4.37 | 1.5 |
| Cellulose/benzyltrimethyl ammonium hydroxide/epichlorohydrin [35] | non-transparent (black) | 219 | 2 | 1.8 | 2.37 |
| Fe3+/SL/PAA [36] | 82% (red) | 1680 | 0.052 | 0.59 | 7.0 × 10−2 |
| PVA/GE/GL/NaCl [37] | Semi-transparent (N/A) | 715 | 1.044 | 3.605 | 0.4 |
| Adenosine monophosphate/quaternized chitosan/NaCl/PAAm [38] | 92% | 1731 | 0.347 | 2.8 | 1.45 |
| This work | 90% | 707 | 2.65 | 10.72 | 2.688 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; He, C.; Luo, F.; Wang, H.; Peng, Z. Transparent, Conductive Hydrogels with High Mechanical Strength and Toughness. Polymers 2021, 13, 2004. https://doi.org/10.3390/polym13122004
Xu X, He C, Luo F, Wang H, Peng Z. Transparent, Conductive Hydrogels with High Mechanical Strength and Toughness. Polymers. 2021; 13(12):2004. https://doi.org/10.3390/polym13122004
Chicago/Turabian StyleXu, Xiuru, Chubin He, Feng Luo, Hao Wang, and Zhengchun Peng. 2021. "Transparent, Conductive Hydrogels with High Mechanical Strength and Toughness" Polymers 13, no. 12: 2004. https://doi.org/10.3390/polym13122004
APA StyleXu, X., He, C., Luo, F., Wang, H., & Peng, Z. (2021). Transparent, Conductive Hydrogels with High Mechanical Strength and Toughness. Polymers, 13(12), 2004. https://doi.org/10.3390/polym13122004