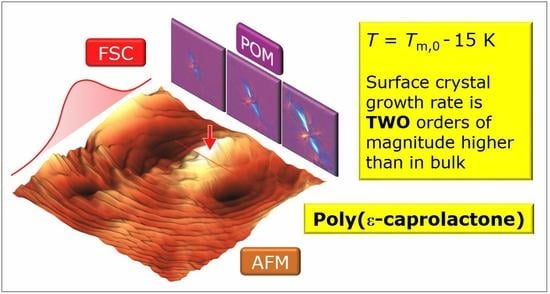
Surface Crystal Nucleation and Growth in Poly (ε-caprolactone): Atomic Force Microscopy Combined with Fast Scanning Chip Calorimetry
Abstract
:1. Introduction
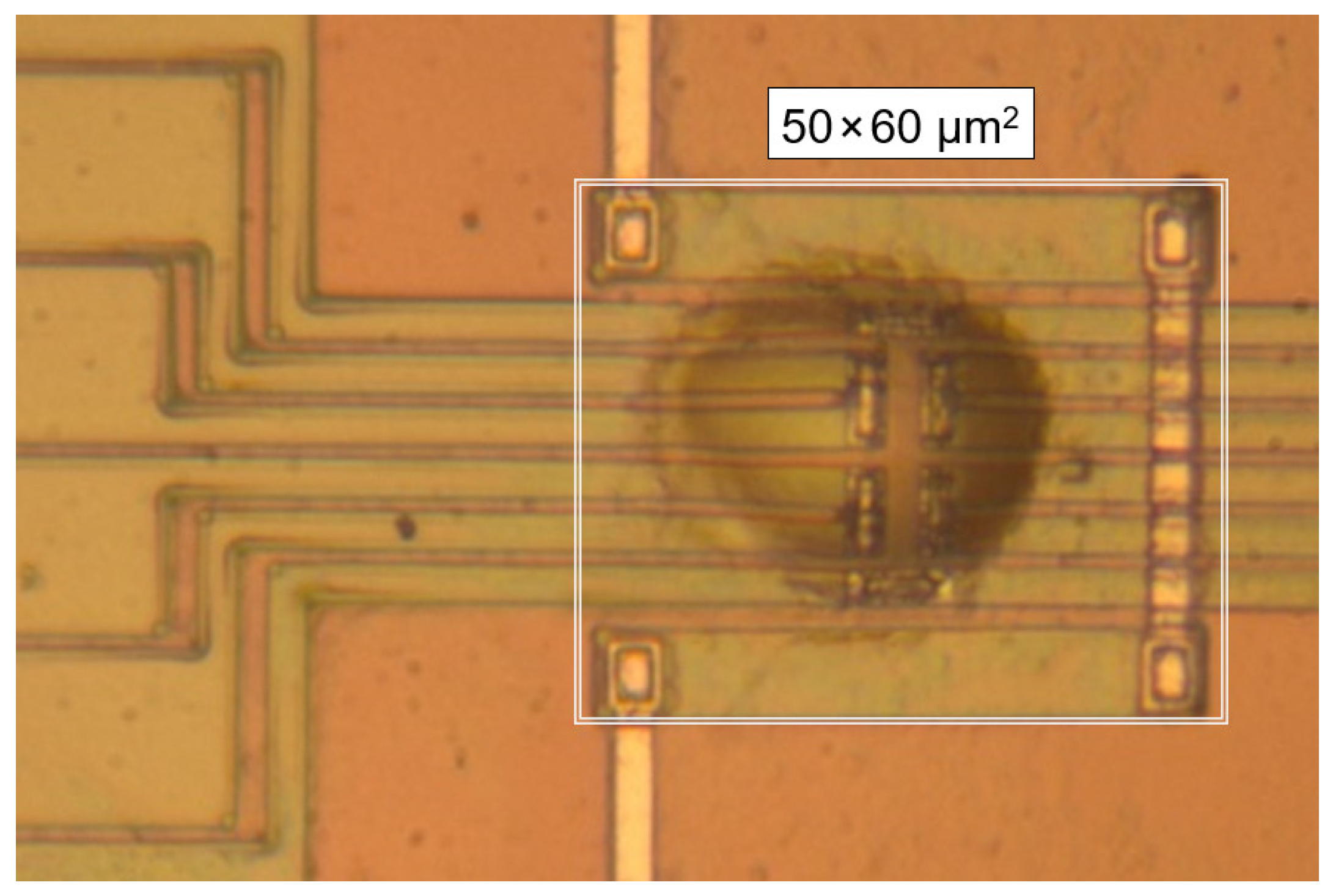
2. Materials and Methods
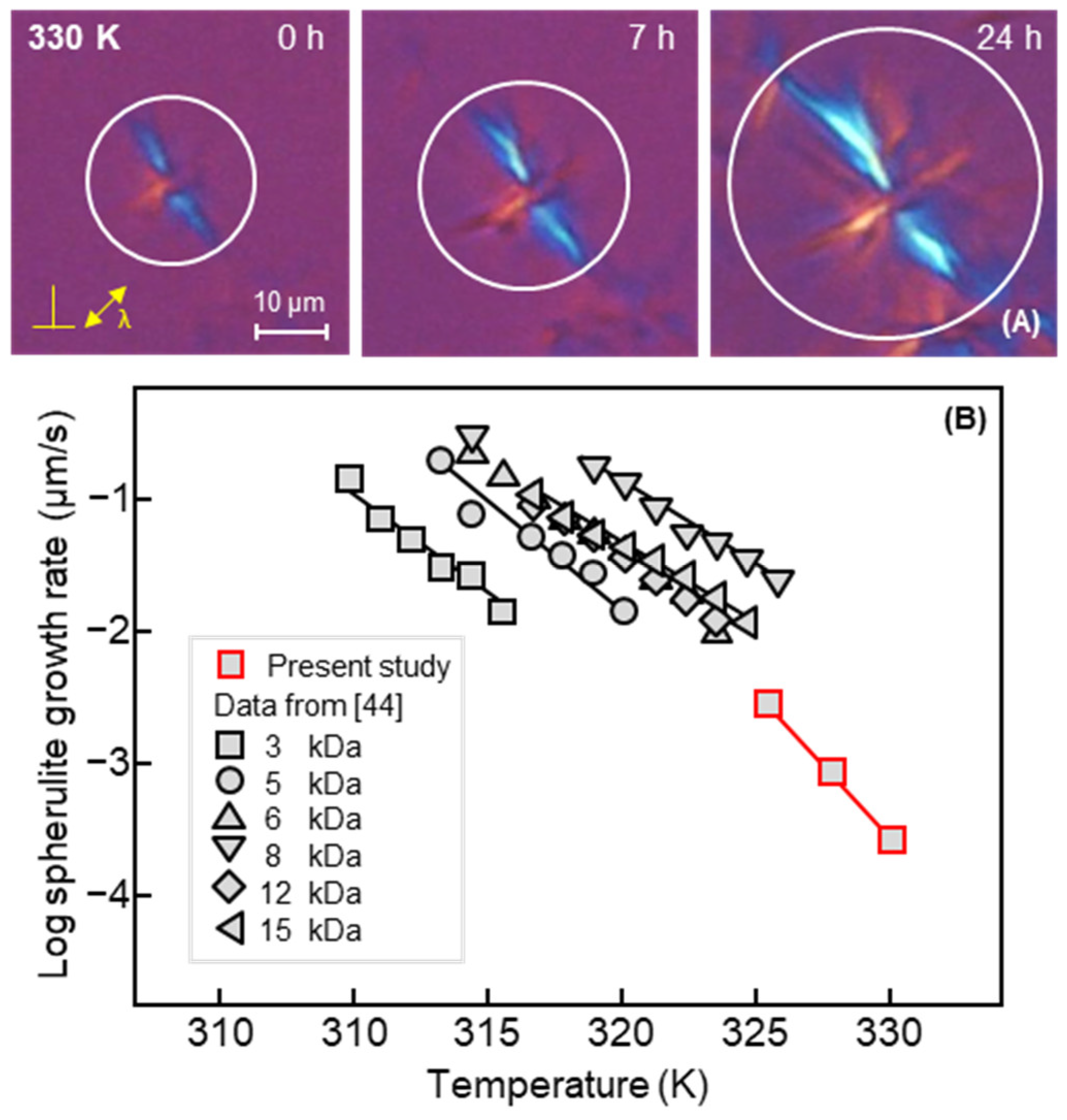
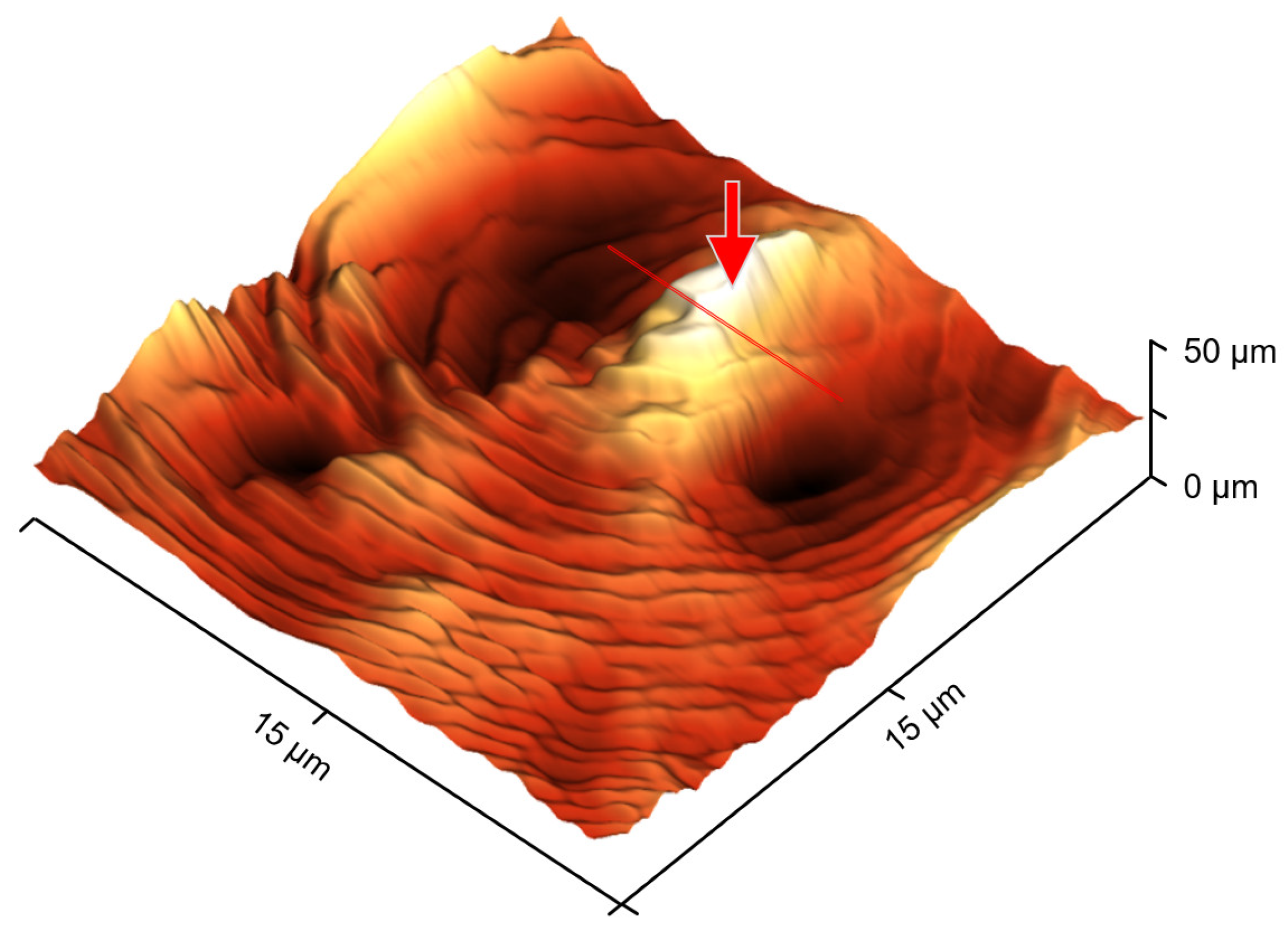
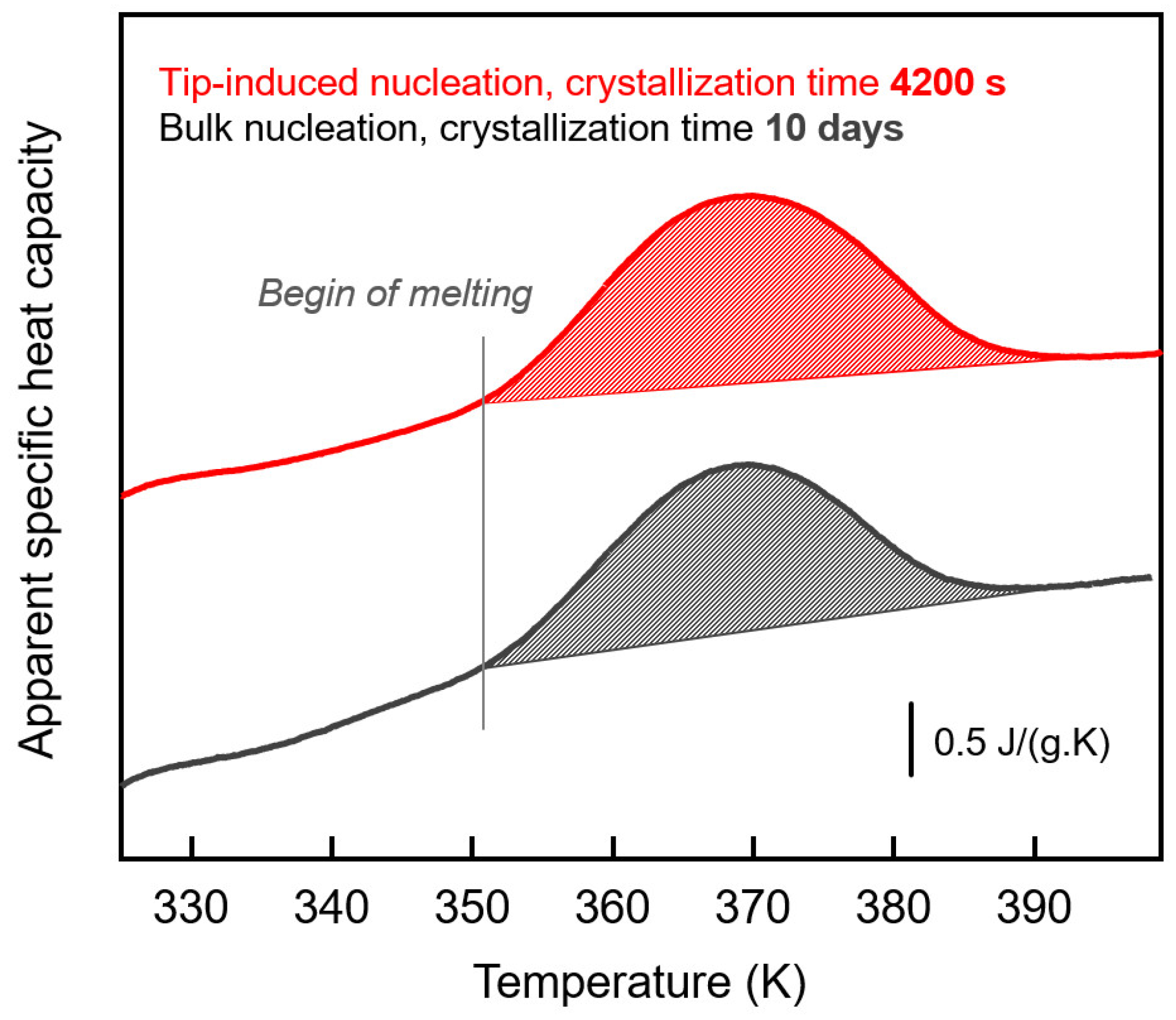
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, R.; Zhuravlev, E.; Androsch, R.; Schick, C. Visualization of Polymer Crystallization by In Situ Combination of Atomic Force Microscopy and Fast Scanning Calorimetry. Polymers 2019, 11, 890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Androsch, R.; Rhoades, A.M.; Stolte, I.; Schick, C. Density of heterogeneous and homogeneous crystal nuclei in poly (butylene terephthalate). Eur. Polym. J. 2015, 66, 180–189. [Google Scholar] [CrossRef]
- Mileva, D.; Androsch, R.; Zhuravlev, E.; Schick, C. Morphology of mesophase and crystals of polyamide 6 prepared in a fast scanning chip calorimeter. Polymer 2012, 53, 3994–4001. [Google Scholar] [CrossRef]
- Gohn, A.; Rhoades, A.M.; Wonderling, N.; Tighe, T.; Androsch, R. The effect of supercooling of the melt on the semicrystalline morphology of PA 66. Thermochim. Acta 2017, 655, 313–318. [Google Scholar] [CrossRef]
- Mollova, A.; Androsch, R.; Mileva, D.; Schick, C.; Benhamida, A. Effect of Supercooling on Crystallization of Polyamide 11. Macromolecules 2013, 46, 828–835. [Google Scholar] [CrossRef]
- Zhang, R.; Zhuravlev, E.; Schmelzer, J.W.P.; Androsch, R.; Schick, C. Steady-State Crystal Nucleation Rate of Polyamide 66 by Combining Atomic Force Microscopy and Fast-Scanning Chip Calorimetry. Macromolecules 2020, 53, 5560–5571. [Google Scholar] [CrossRef]
- Schick, C.; Mathot, V. Fast Scanning Calorimetry; Springer Nature Switzerland AG: Cham, Switzerland, 2016; pp. 1–795. [Google Scholar]
- Okuzaki, H.; Kuwabara, T.; Kunugi, T. Theoretical study of sorption-induced bending of polypyrrole films. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 2237–2246. [Google Scholar] [CrossRef]
- Pearce, R.; Vancso, G.J. Imaging of Melting and Crystallization of Poly(ethylene oxide) in Real-Time by Hot-Stage Atomic Force Microscopy. Macromolecules 1997, 30, 5843–5848. [Google Scholar] [CrossRef] [Green Version]
- Hobbs, J.; McMaster, T.; Miles, M.; Barham, P. Direct observations of the growth of spherulites of poly(hydroxybutyrate-co-valerate) using atomic force microscopy. Polymer 1998, 39, 2437–2446. [Google Scholar] [CrossRef]
- Godovsky, Y.K.; Magonov, S.N. Atomic Force Microscopy Visualization of Morphology and Nanostructure of an Ultrathin Layer of Polyethylene during Melting and Crystallization. Langmuir 2000, 16, 3549–3552. [Google Scholar] [CrossRef]
- Hobbs, J.K.; Miles, M.J. Direct Observation of Polyethylene Shish-Kebab Crystallization Using in-Situ Atomic Force Microscopy. Macromolecules 2001, 34, 353–355. [Google Scholar] [CrossRef]
- Hobbs, J.K.; Humphris, A.D.L.; Miles, M.J. In-Situ Atomic Force Microscopy of Polyethylene Crystallization. 1. Crystallization from an Oriented Backbone. Macromolecules 2001, 34, 5508–5519. [Google Scholar] [CrossRef]
- Kikkawa, Y.; Abe, H.; Fujita, M.; Iwata, T.; Inoue, Y.; Doi, Y. Crystal Growth in Poly(L-lactide) Thin Film Revealed by in situ Atomic Force Microscopy. Macromol. Chem. Phys. 2003, 204, 1822–1831. [Google Scholar] [CrossRef]
- Schönherr, H.; Waymouth, R.M.; Frank, C.W. Nucleation and Crystallization of Low-Crystallinity Polypropylene Followed in Situ by Hot Stage Atomic Force Microscopy. Macromolecules 2003, 36, 2412–2418. [Google Scholar] [CrossRef]
- Chan, C.-M.; Li, L. Direct Observation of the Growth of Lamellae and Spherulites by AFM. Polym. Phys. 2005, 188, 1–41. [Google Scholar] [CrossRef]
- Hobbs, J.K.; Farrance, O.E.; Kailas, L. How atomic force microscopy has contributed to our understanding of polymer crystallization. Polymer 2009, 50, 4281–4292. [Google Scholar] [CrossRef] [Green Version]
- Ono, Y.; Kumaki, J. In Situ Real-Time Observation of Polymer Folded-Chain Crystallization by Atomic Force Microscopy at the Molecular Level. Macromolecules 2018, 51, 7629–7636. [Google Scholar] [CrossRef]
- Schönherr, H.; Bailey, A.L.E.; Frank, C.W. Analyzing the Surface Temperature Depression in Hot Stage Atomic Force Microscopy with Unheated Cantilevers: Application to the Crystallization of Poly(ethylene oxide). Langmuir 2002, 18, 490–498. [Google Scholar] [CrossRef]
- Ivanov, D.A.; Amalou, Z.; Magonov, S.N. Real-Time Evolution of the Lamellar Organization of Poly(ethylene terephthalate) during Crystallization from the Melt: High-Temperature Atomic Force Microscopy Study. Macromolecules 2001, 34, 8944–8952. [Google Scholar] [CrossRef]
- Beekmans, L.; Vancso, G. Real-time crystallization study of poly(ϵ-caprolactone) by hot-stage atomic force microscopy. Polymer 2000, 41, 8975–8981. [Google Scholar] [CrossRef]
- Abedalwafa, M.; Wang, F.; Wang, L.; Li, C. Biodegradable poly-epsilon-caprolactone (PCL) for tissue engineering applications: A review. Biomaterials 2009, 30, 780–788. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Yusoh, K. A review on the recent research of polycaprolactone (PCL). Adv. Mater. Res. 2015, 1134, 249–255. [Google Scholar] [CrossRef]
- Goldberg, D. A review of the biodegradability and utility of poly(caprolactone). J. Polym. Environ. 1995, 3, 61–67. [Google Scholar] [CrossRef]
- Dolynchuk, O.; Kolesov, I.; Jehnichen, D.; Reuter, U.; Radusch, H.-J.; Sommer, J.-U. Reversible Shape-Memory Effect in Cross-Linked Linear Poly(ε-caprolactone) under Stress and Stress-Free Conditions. Macromolecules 2017, 50, 3841–3854. [Google Scholar] [CrossRef]
- Ohta, T.; Kawasaki, K. Equilibrium morphology of block copolymer melts. Macromolecules 1986, 19, 2621–2632. [Google Scholar] [CrossRef]
- Phillips, P.J.; Rensch, G.J.; Taylor, K.D. Crystallization studies of poly(ɛ-caprolactone).I. Morphology and kinetics. J. Polym. Sci. Part B Polym. Phys. 1987, 25, 1725–1740. [Google Scholar] [CrossRef]
- Vion, J.M.; Jerome, R.; Teyssie, P.; Aubin, M.; Prudhomme, R.E. Synthesis, characterization, and miscibility of caprolactone random copolymers. Macromolecules 1986, 19, 1828–1838. [Google Scholar] [CrossRef]
- Goulet, L.; Prud’Homme, R.E. Crystallization kinetics and melting of caprolactone random copolymers. J. Polym. Sci. Part B Polym. Phys. 1990, 28, 2329–2352. [Google Scholar] [CrossRef]
- Koleske, J.V.; Lundberg, R.D. Lactone polymers. I. Glass transition temperature of poly-ɛ-caprolactone by means on compatible polymer mixtures. J. Polym. Sci. Part A-2 Polym. Phys. 1969, 7, 795–807. [Google Scholar] [CrossRef]
- He, Y.; Inoue, Y. Novel FTIR method for determining the crystallinity of poly(ε-caprolactone). Polym. Int. 2000, 49, 623–626. [Google Scholar] [CrossRef]
- Zhuravlev, E.; Schmelzer, J.W.; Wunderlich, B.; Schick, C. Kinetics of nucleation and crystallization in poly(ɛ-caprolactone) (PCL). Polymer 2011, 52, 1983–1997. [Google Scholar] [CrossRef]
- Chen, H.-L.; Li, L.-J.; Ou-Yang, W.-C.; Hwang, J.C.; Wong, W.-Y. Spherulitic Crystallization Behavior of Poly(epsilon-caprolactone) with a Wide Range of Molecular Weight. Macromolecules 1997, 30, 1718–1722. [Google Scholar] [CrossRef]
- Ikehara, T.; Jinnai, H.; Kaneko, T.; Nishioka, H.; Nishi, T. Local lamellar structures in banded spherulites analyzed by three-dimensional electron tomography. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 1122–1125. [Google Scholar] [CrossRef]
- Mareau, V.H.; Prud’Homme, R.E. In-Situ Hot Stage Atomic Force Microscopy Study of Poly(ε-caprolactone) Crystal Growth in Ultrathin Films. Macromolecules 2005, 38, 398–408. [Google Scholar] [CrossRef]
- Varga, J.; Ehrenstein, G.W. High-temperature hedritic crystallization of the β-modification of isotactic polypropylene. Colloid Polym. Sci. 1997, 275, 511–519. [Google Scholar] [CrossRef]
- Zhuravlev, E.; Wurm, A.; Pötschke, P.; Androsch, R.; Schmelzer, J.W.; Schick, C. Kinetics of nucleation and crystallization of poly(ε-caprolactone)—Multiwalled carbon nanotube composites. Eur. Polym. J. 2014, 52, 1–11. [Google Scholar] [CrossRef]
- Anfatec. Available online: http://www.anfatec.de/anfatec/mic.html (accessed on 11 May 2021).
- Zhuravlev, E.; Schick, C. Fast scanning power compensated differential scanning nano-calorimeter: 1. The device. Thermochim. Acta 2010, 505, 1–13. [Google Scholar] [CrossRef]
- Zhuravlev, E.; Schick, C. Fast scanning power compensated differential scanning nano-calorimeter: 2. Heat capacity analysis. Thermochim. Acta 2010, 505, 14–21. [Google Scholar] [CrossRef]
- Minakov, A.A.; van Herwaarden, A.W.; Wien, W.; Wurm, A.; Schick, C. Advanced nonadiabatic ultrafast nanocalorimetry and superheating phenomenon in linear polymers. Thermochim. Acta 2007, 461, 96–106. [Google Scholar] [CrossRef]
- Poly(epsilon-caprolactone) (PCL) Heat Capacity, Enthalpy, Entropy, Gibbs Energy: Datasheet from “The Advanced THermal Analysis System (ATHAS) Databank—Polymer Thermodynamics”. Available online: https://materials.springer.com/polymerthermodynamics/docs/athas_0049 (accessed on 15 May 2021).
- Androsch, R. Melt-crystallization, glass transition and morphology of a (R)-3-hydroxybutyrate pentamer. Eur. Polym. J. 2007, 43, 93–108. [Google Scholar] [CrossRef]
- Du, Z.-X.; Yang, Y.; Xu, J.-T.; Fan, Z.-Q. Effect of molecular weight on spherulitic growth rate of poly(ε-caprolactone) and poly(ε-caprolactone)-b-poly(ethylene glycol). J. Appl. Polym. Sci. 2007, 104, 2986–2991. [Google Scholar] [CrossRef]
- Wu, T.; Yu, L. Surface Crystallization of Indomethacin Below T g. Pharm. Res. 2006, 23, 2350–2355. [Google Scholar] [CrossRef]
- McMillan, P. The crystallisation of glasses. J. Non Cryst. Solids 1982, 52, 67–76. [Google Scholar] [CrossRef]
- De Cupere, V.; Rouxhet, P. Surface crystallization of poly(ethylene terephtalate) studied by atomic force microscopy. Polymer 2002, 43, 5571–5576. [Google Scholar] [CrossRef]
- Jukes, P.C.; Das, A.; Durell, M.; Trolley, D.; Higgins, A.M.; Geoghegan, M.; Macdonald, J.E.; Jones, R.; Brown, A.S.; Thompson, P. Kinetics of Surface Crystallization in Thin Films of Poly(ethylene terephthalate). Macromolecules 2005, 38, 2315–2320. [Google Scholar] [CrossRef]
- Durell, M.; MacDonald, J.; Trolley, D.; Wehrum, A.; Jukes, P.C.; Jones, R.A.L.; Walker, C.J.; Brown, S. The role of surface-induced ordering in the crystallisation of PET films. EPL 2002, 58, 844. [Google Scholar] [CrossRef]
- Zia, Q.; Ingolič, E.; Androsch, R. Surface and bulk morphology of cold-crystallized poly(ethylene terephthalate). Colloid Polym. Sci. 2010, 288, 819–825. [Google Scholar] [CrossRef]
- Crist, B.; Schultz, J.M. Polymer spherulites: A critical review. Prog. Polym. Sci. 2016, 56, 1–63. [Google Scholar] [CrossRef]
- Keith, H.D.; Padden, F.J. Banding in Polyethylene and Other Spherulites. Macromolecules 1996, 29, 7776–7786. [Google Scholar] [CrossRef]
- Woo, E.M.; Nurkhamidah, S. Surface Nanopatterns of Two Types of Banded Spherulites in Poly(nonamethylene terephthalate) Thin Films. J. Phys. Chem. B 2012, 116, 5071–5079. [Google Scholar] [CrossRef] [PubMed]
- Woo, E.M.; Lugito, G.; Tsai, J.-H.; Müller, A.J. Hierarchically Diminishing Chirality Effects on Lamellar Assembly in Spherulites Comprising Chiral Polymers. Macromolecules 2016, 49, 2698–2708. [Google Scholar] [CrossRef]
- Nagarajan, S.; Woo, E.M. Three-dimensional periodic architecture in Poly(ε-caprolactone) crystallized in bulk aggregates. Polymer 2020, 210, 123059. [Google Scholar] [CrossRef]
- Hua, C.; Chen, Z.; Xu, Q.; He, L. Ring-banded spherulites in PCL and PCL/MWCNT solution-casting films and effect of compressed CO2 on them. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 784–792. [Google Scholar] [CrossRef]
- Wunderlich, B. Macromolecular Physics V2; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Du, M.; Zhuravlev, E.; Androsch, R.; Schick, C. Surface Crystal Nucleation and Growth in Poly (ε-caprolactone): Atomic Force Microscopy Combined with Fast Scanning Chip Calorimetry. Polymers 2021, 13, 2008. https://doi.org/10.3390/polym13122008
Zhang R, Du M, Zhuravlev E, Androsch R, Schick C. Surface Crystal Nucleation and Growth in Poly (ε-caprolactone): Atomic Force Microscopy Combined with Fast Scanning Chip Calorimetry. Polymers. 2021; 13(12):2008. https://doi.org/10.3390/polym13122008
Chicago/Turabian StyleZhang, Rui, Mengxue Du, Evgeny Zhuravlev, René Androsch, and Christoph Schick. 2021. "Surface Crystal Nucleation and Growth in Poly (ε-caprolactone): Atomic Force Microscopy Combined with Fast Scanning Chip Calorimetry" Polymers 13, no. 12: 2008. https://doi.org/10.3390/polym13122008
APA StyleZhang, R., Du, M., Zhuravlev, E., Androsch, R., & Schick, C. (2021). Surface Crystal Nucleation and Growth in Poly (ε-caprolactone): Atomic Force Microscopy Combined with Fast Scanning Chip Calorimetry. Polymers, 13(12), 2008. https://doi.org/10.3390/polym13122008