Synergic Effect of TiO2 Filler on the Mechanical Properties of Polymer Nanocomposites
Abstract
1. Introduction
2. Polymeric Matrix
2.1. Matrix
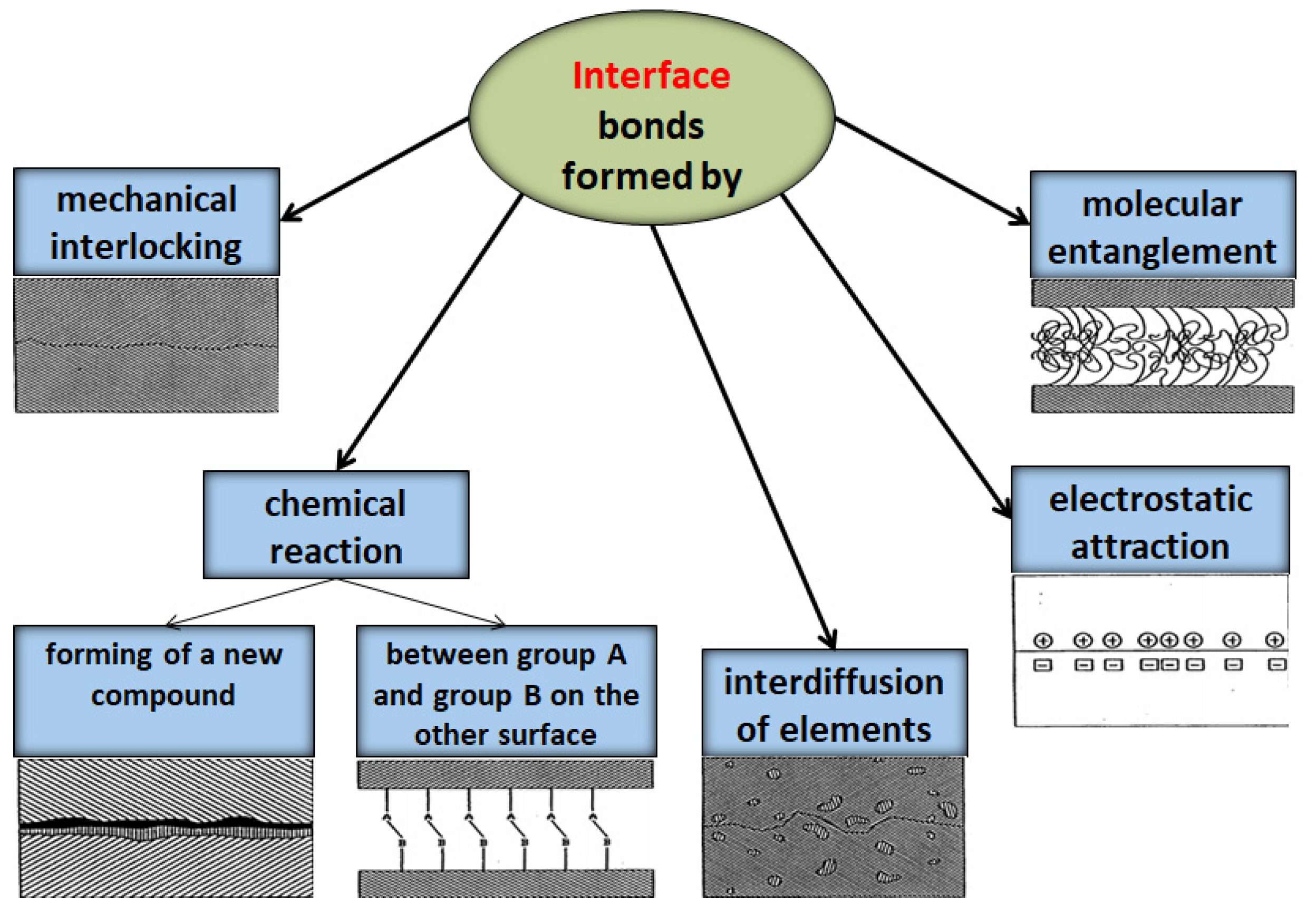
2.2. Matrix–Filler Interface
2.3. Fillers and Surface Modifications
- −
- The chemical interaction of the fillers with compounds that possess functional groups;
- −
- Chemical absorption on the surface of the particles of the filling material of some modifying agents;
- −
- Coating the filler particles with a suitable coupling agent.
3. Titanium Dioxide Nanoparticles
3.1. Size, Shape and Specific Surface Area of the Nanoparticles
3.2. Surface Modification of TiO2 Nanoparticles
- ✓
- Hydrolyzation of alkoxy groups obtaining silanol, which reacts with the mineral surface;
- ✓
- The condensation reaction between silanol molecules;
- ✓
- Bond formation between TiO2 nanoparticles and the organofunctional group.
3.3. Properties, Commercial Products and Applications
4. Polymeric Nanocomposites with TiO2 Filler
4.1. Preparation Methods
4.2. Mechanical Properties
4.2.1. The Nature of the Filler
4.2.2. The Nature of the Polymer Matrix
| Composites | Materials | Methods | Results-Mechanical Properties | Ref. |
|---|---|---|---|---|
| Thermoplastic matrix | ||||
| Polystyrene (PS)–TiO2 | Matrix: polystyrene. Filler: TiO2 (0.19 µm) Coupling agent: 3-amino ethoxy silane (0.1, 0.5 and 1 wt. %.). | Obtaining: mixing of matrix with TiO2-coupling agent Characterization: mechanical tests, SEM analysis. | Values of Young’s modulus, tensile strength, elongation at break, flexural strength increase with linearly filler concentration followed by a decrease beyond 15 wt. %. | [45] |
| Polyphenylene sulfide (PPS)–TiO2 | Matrix: polyphenylenesulphide (PPS) Filler: TiO2 (200 nm; 0, 5, 10, 15, 20, and 25 wt. %) | Obtaining: injection molding. Characterization: solid particle erosion test, three-point bending test, thermal analyzing methods. | The flexural modulus of composites increased with the increase in TiO2 concentration up to 10 wt. %, and then it decreases. TiO2 filler caused to reduce the erosion resistance of the PPS composites. | [128] |
| Polypropylene (PP)–TiO2 | Matrix: PP pellets; Filler: TiO2 (0, 10, 20 and 30 wt. %) | Obtaining: injection molding Characterization: mechanical properties: tensile stress, impact tests; TGA | The highest resilience value recorded for the sample with 20% TiO2 (37.09 ± 5.3 J/m). Tensile stress shows a decrease and the E modulus increase as the weight percent of TiO2 increases. | [124] |
| Polypropylene (PP)–TiO2 | Matrix: polypropylene (PP) Filler: TiO2 micropowder; TiO2 nanopowder titanate nanotubes (TiNT) | Obtaining: melt mixing; samples types PP*/TiX (PP*/mTiO2, PP*/nTiO2, PP*/TiNT) and samples with PP unmodified. Characterization: SEM analysis, TEM analysis, mechanical properties | The stiffness and microhardness of the PP–TiNT nanocomposites increase by 27% and, respectively, 33%. In the PP–nTiO2 nanocomposites, the increase in these mechanical characteristics is lower. | [126] |
| Polypropylene (PP)–TiO2 | Matrix: PP homopolymer Filler: TiO2 (0–3 wt. %) | Obtaining: melt compounding; Characterization: mechanical properties, thermogravimetric analysis, DSC, SEM analysis | The addition of TiO2 nanoparticles increases the mechanical properties of PP fibres. Tenacity is increased by 72.69% for the PP–TiO2 (3 wt. %) nanoparticle. Elongation at break of the PP fibres with TiO2 (1.5 wt. %) indicated an increase of 15.79%. | [135] |
| Polypropylene (PP)-rice husk–TiO2 | Matrix: polypropylene (PP) Filler: rice husk and TiO2 | Obtaining: injection molding Characterization: mechanical properties, SEM, TGA | Incorporating inorganic filler TiO2 into PP/RH significantly enhanced the green hybrid PP/RH/TiO2 composites mechanical properties and thermal stabilities. The maximum values of tensile strength and Young modulus were 41.2 MPa for PP/RH (10wt. %)/TiO2 (3wt. %), respectively, for PP/RH (40wt. %)/TiO2 (3wt. %) | [136] |
| polyurethane (TPU)–TiO2 | Matrix: polyurethane (TPU) matrix with multi-walled carbon nanotube (MWCNT); Filler: TiO2 (particle diameter—0.19 μm). | Obtaining: injection molding. Characterization: mechanical properties: tensile test, DMA, TGA, | The composites have good mechanical properties: tensile stress was 4.46 MPa, elongation at the break—49%, and Young’s Modulus— 9.17 MPa. | [134] |
| thermoplastic polyurethane (TPU)–TiO2 | Matrix: thermoplastic polyurethane Filler: TiO2 nano-particles Coupling agent: aminopropyl trimethoxy silane (APS) | Obtaining: mixing of matrix with filler; Characterization: elemental analysis, FTIR spectroscopy, TGA, mechanical properties. | For composite with TiO2 (3 wt.%), tensile strength and Young’s modulus were increased by 72% and 48.9, respectively. Higher values were obtained when modified TiO2 was used, at low percentages (1 wt.%). | [77] |
| polybutylene succinate (PBS)–TiO2 | Matrix: polybutylene succinate (PBS); Filler: TiO2 (20 nm; 0, 0.5, 1, 2, 5, and 10 wt. %) | Obtaining: vane extruder. Characterization: SEM, TEM, XRD, DSC, TGA, DMA; mechanical test, UV transmittance. | TiO2 has little effect on the impact strength of the composite material. The flexural modulus of composites improved by 36.3% with TiO2 (10 wt. %) addition. The tensile modulus of PBS–TiO2 (10 wt. %) was higher by 15.5% than that of pristine PBS. | [137] |
| polyetheretherketone (PEEK)–TiO2 | Matrix: PEEK. Filler: TiO2 powder (1, 3, 5 wt.%) | Obtaining: mixing and extrusion forming; Characterization: density and Melt Flow Index (MFI) measurement, DSC, UV thermal, mechanical test | E modulus increase with TiO2 content. The PEEK-1% TiO2 sample has a tensile strength higher than that of pristine PEEK. TiO2 (5% vol.) particles act effectively as UV blocker retarding the photo-degradation of PEEK. | [138] |
| poly(ethylene terephthalate) (PET)–TiO2 Poly(lactic acid) (PLA)–TiO2 | Matrix: poly(ethylene terephthalate) (PET) and poly(lactic acid) (PLA); Filler: TiO2 (20 nm); | Obtaining: extrusion forming; Characterization: analysis—DSC, XRD, SEM, DMTA, UV–Visible test, mechanical test. | The mechanical properties of PET–TiO2 and PLA–TiO2 composites have maximum values at a loading level of 3% TiO2. | [139] |
| poly(L-lactide-co-ε-caprolactone) (PLCL)–TiO2 nanocomposites | Matrix: PLCL; Filler: TiO2 (20 nm) Coupling agent: silane coupling agent NH2(CH2)3Si(OC2H5)3 | Obtaining: solution casting method. Characterization: analysis—FTIR, DSC, TEM, tensile test, shape memory; | For composite with TiO2 (5%) the ultimate tensile strength and the elongation at break increase to 35.4 MPa and 444.6%, which are 113% and 11% higher than that of pure PLCL. | [140] |
| Poly(L-Lactide) (PLLA)–TiO2 | Matrix: poly(L-Lactide) (PLLA) Filler: TiO2 (<25 nm particle size) and Halloysite nanoclay (HNT) (Al2Si2O5(OH)4.2H2O); | Obtaining: compression molding. Characterization: mechanical test | Young modulus had a significant increase (p ≤ 0.05) with the addition of TiO2 up to 2.5 g TiO2/100 g PLLA. Regarding the tensile strength, better results were also achieved when adding 2.5 g TiO2/100g PLLA. | [141] |
| Poly(lactic acid) (PLA)–TiO2 | Matrix: PLA (4032D, 1.2–1.6% D-isomer lactide) Filler: TiO2 (20 nm); | Obtaining: injection molding; Characterization: SEM, TEM, dynamic rheological measurements, DSC, TGA, tensile testing, UV transmittance | Samples show a higher elongation at break, except for 15 wt. % TiO2. Elongations of nanocomposites with 1–2% TiO2 are about 19.1% and 24% higher than the pristine PLA. | [142] |
| Poly(lactic acid) (PLA)–TiO2 | Matrix: poly(lactic acid) (PLA) Filler: TiO2 (1, 3, 5, 10 wt.%) Coupling agent: c-methacryloxy propyltrimethoxy-silane) | Obtaining: in situ polymerization, Characterization: DSC, TGA, XDR, SEM, thermal and mechanical properties | The tensile strength, elongation at break, and Young’s modulus of PLA–TiO2 (3 wt.%) composites are improved to a certain degree compared with those of pristine PLA. | [143] |
| Thermosetting matrix | ||||
| epoxy–TiO2 nanocomposites | Matrix: mixture (resin + hardener); Filler: TiO2 (0.5, 1, 2, 3, 4, 5, 8 and 10% vol.); | Obtaining: mixing of resin + hardener and filler; Characterization: tensile test, dynamic mechanical analysis; | The incorporation of TiO2 nanoparticles into the epoxy resin improved flexural stiffness, flexural strength, and fracture toughness of the polymer. | [144] |
| epoxy–TiO2 nanocomposites | Matrix: epoxy resin Filler: TiO2 (5–40 nm, 0.5–2 wt.%); | Obtaining: mixing of matrix with filler; Characterization: thermal properties, mechanical properties, morphology, viscoelastic properties. | TiO2 composites with dimensions between 5–10 nm showed better properties than those with larger dimensions (20–50 nm). | [145] |
| epoxy–TiO2 nanocomposites | Matrix: mixture (resin+hardener); Filler: TiO2 (1, 3, 5, 10 wt.%) Coupling agent: methyl isobutyl-ketone; dodecylbenzene-sulfonic acid | Obtaining: mixing of matrix, filler and coupling agent; Characterization: FTIR, SEM, XRD, TGA, mechanical tests | The mechanical properties of materials are found to improve with TiO2, but degrade if the nano-TiO2 exceeds 3%. | [146] |
| epoxy–TiO2 nanocomposites | Matrix: epoxy resin (DER 331TM) Filler: TiO2 (220 nm, 50 nm and 17 nm crystal diameter); Coupling agent: isophorone diamine (IPDA) + salicylic acid. | Obtaining: mixing of matrix, filler and coupling agent; Characterization: mechanical test, XPS, SEM | The highest tensile stress values were found at 3 wt. % TiO2 (17 nm and 50 nm) and 5 wt. % TiO2 (220 nm). The maximum flexural properties were found at a lower TiO2 fraction of 1 wt.% only. | [147] |
| epoxy–TiO2 micro and nanocomposites | Matrix: epoxy resin:curing agent = 2:1 (wt. %) Filler: TiO2 (0.2 μm; 1, 5, 10, 15 wt. %); TiO2 (21 nm; 0.5, 1, 3 wt. %). | Obtaining: mixing with an electrical stirrer, Characterization: tensile test, tensile creep-recovery test, tensile stress relaxation tests, SEM. | TiO2 nanocomposites have better strength properties than TiO2 microcomposites due to the size of the particle. | [148] |
| vinyl ester resins–TiO2 nanocomposites | Matrix: vinyl ester:styrene monomers (55:45 wt. %) Filler: TiO2 (21 nm; 50 m2/g; 1, 2.5, and 5 wt. %). Coupling agent: polymeric coupling BYK-C 8000 | Obtaining: shear mixing and ultrasonication; Characterization: tensile test, flexural test, impact test, SEM | For nanocomposite with 0–2.5 wt. % TiO2, the tensile strength exhibits increasing tendency, while loading more than 2.5 wt. % leads to its decline. | [149] |
| epoxy resin–polyurethane (EP-PU)–TiO2 | Matrix: EP-PU epoxy resin; Filler: TiO2 (0.42 g/cm3; 25 nm) Coupling agent: isopropyl tri(dioctylpyrophosphate) titanate (TCA201) | Obtaining: mixing EP–PU and TCA201–TiO2 Characterization: FT-IR spectroscopy, SEM analysis, TGA analysis, mechanical properties, dielectric constant | The shear strength reached the maximum value (27.14 MPa) for EP–PU/TiO2 (3 wt. %) and its thermal decomposition temperature increase by 17.48 º C more than that of EP–PU matrix. The dielectric constant and dielectric loss showed 4.27 and 0.02, respectively. | [85] |
| Elastomeric matrix | ||||
| TiO2–natural rubber composites | Matrix: natural rubber (NR) Filler: TiO2 (KEMOX RC 800 PG) and the surface-modified nanosilica | Obtaining: hydraulic press under a pressure Characterization: stress relaxation measurements, SEM, AFM, effect of strain level, effect of ageing | The rate of stress relaxation was higher for silica-filled NR than TiO2-filled NR. This is due to the high degree of agglomeration in silica compared to TiO2. The relaxation rate increased with increasing TiO2 loading. | [150] |
| TiO2–natural rubber composite | Matrix: natural rubber stabilised with ammonia; Filler: TiO2 dispersion (2, 4 and 6 pphr) | Obtaining: TiO2 dispersions added in matrix; Characterisation: tesnsile test | The results showed improvement in both elongations at break and tensile strength data at low filler concentration (2 phr). | [151] |
| TiO2–natural rubber composites | Matrix: natural rubber latex centrifuged with ammonia; Filler: TiO2 (3 mm;.13 g/mL); TiO2 (15–40 nm; 4.26 g/mL); aditives: zinc oxide, stearic acid, N-cyclohexyl-benzothiazyl-sulphenamide, N2′-propyl-N-phenylenediamine, and S | Obtaining: TiO2 dispersion was immersed in natural rubber latex. Characterization: tensile test, SEM, TEM, XRD | The tensile strength of nano-sized TiO2-filled natural rubber composites (23.04 MPa) is superior to micro-sized TiO2-filled natural rubber composites (19.62 MPa) (for 6 phr of micro- and nano-s) | [152] |
| TiO2–natural rubber composites | Matrix: natural rubber; Filler: TiO2-15, 25, 45, 85 wt. % aditives: stearic acid, sulfur powder and zinc oxide; | Obtaining: compression molding; Characterization: mechanical properties; dynamic mechanical properties; thermal stability | TiO2 as filler allows obtaining materials with improved mechanical properties and thermal stability compared to the pristine natural rubber vulcanizates. | [153] |
| TiO2–chlorobutyl rubber composites | Matrix: chlorobutyl rubber (CBK 150) with 1.2% Cl; Filler: TiO2 (10–30 phr.) Additives: stearic acid, zinc oxide, sulfur, and zinc | Obtaining: mixing in a two-roll mill Characterization: mechanical properties, morphology (SEM, AFM), thermophysical measurements, diffusion experiments | The tensile strength of the composites increases by 250% when the filler loading goes to 40 phr (tensile modulus the same). | [133] |
| Acrylonitrile–Butadiene–Styrene–TiO2 nanocomposites | Matrix: acrylonitrile butadiene styrene (ABS) Fillers: TiO2 (25–50 nm; 0.5, 2.5, 5 and 10 wt. %) and ATO (size < 50 nm) | Obtaining: mechanical homogeniser. Characterization: SEM, AFM and Raman analysis, thermal properties, tensile test, flexural tests, micro-hardness tests. | The tensile strength of ABS/TiO2 and ABS/ATO nanocomposites increased by 7% at the 2.5 wt. % TiO2 filler, respectively, by 9.2% at 0.50 wt. % ATO filler. The modulus of elasticity increases up to 5 TiO2 wt. % and then decreases. | [31] |
- −
- Nature of filler and polymer matrix;
- −
- Amount of filler;
- −
- The distribution of filler, this should not form agglomerates in the samples;
- −
- Concentration of coupling agent for modifying of filler surface;
- −
- The method of obtaining, which is an essential factor.
4.3. Advantages, Limits and Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mittal, V. Optimization of Polymer Nanocomposite Properties; Wiley: Weinheim, Germany, 2010. [Google Scholar] [CrossRef]
- Schadler, L.S.; Brinson, L.C.; Sawyer, W.G. Polymer nanocomposites: A small part of the story. JOM 2007, 59, 53–60. [Google Scholar] [CrossRef]
- Young, R.; Kinloch, I.A.; Gong, L.; Novoselov, K. The mechanics of graphene nanocomposites: A review. Compos. Sci. Technol. 2012, 72, 1459–1476. [Google Scholar] [CrossRef]
- Oliviera, M.; Machado, A. Preparation of Polymer-Based Nanocomposites by Different Routes. In Nanocomposites: Synthesis, Characterization and Application; Wang, X., Ed.; NOVA Publishers: Hauppauge, NY, USA, 2013. [Google Scholar]
- Kumar, R. Polymer-Matrix Composites Types, Applications & Performance; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2014. [Google Scholar]
- Seferis, J. Role of the Polymeric Matrix in the Processing and Structural Properties of Composite Materials; Springer: New York, NY, USA, 2013. [Google Scholar]
- Venditti, I.; D’Amato, R.; Russo, M.V.; Falconieri, M. Synthesis of conjugated polymeric nanobeads for photonic bandgap materials. Sens. Actuators B Chem. 2007, 126, 35–40. [Google Scholar] [CrossRef]
- Soares, I.L.; Chimanowsky, J.P.; Luetkmeyer, L.; Da Silva, E.O.; Souza, D.D.H.S.; Tavares, M.I.B. Evaluation of the Influence of Modified TiO2 Particles on Polypropylene Composites. J. Nanosci. Nanotechnol. 2015, 15, 5723–5732. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.; Neto, R.C.; Santos, I.C.S.; Da Silva, E.O.; Tavares, M.I.B. Inorganic-organic hybrids based on poly (ε-Caprolactone) and silica oxide and characterization by relaxometry applying low-field NMR. Mater. Res. 2012, 15, 825–832. [Google Scholar] [CrossRef]
- Rubab, Z.; Afzal, A.; Siddiqi, H.M.; Saeed, S. Preparation, Characterization, and Enhanced Thermal and Mechanical Properties of Epoxy-Titania Composites. Sci. World J. 2014, 2014, 515739. [Google Scholar] [CrossRef] [PubMed]
- Kierys, A.; Zaleski, R.; Buda, W.; Pikus, S.; Dziadosz, M.; Goworek, J. Nanostructured polymer–titanium composites and titanium oxide through polymer swelling in titania precursor. Colloid Polym. Sci. 2012, 291, 1463–1470. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suzuki, N.; Kiba, S.; Kamachi, Y.; Miyamoto, N.; Yamauchi, Y. Unusual reinforcement of silicone rubber compounds containing mesoporous silica particles as inorganic fillers. Phys. Chem. Chem. Phys. 2012, 14, 3400–3407. [Google Scholar] [CrossRef]
- Da Silva, P.S.R.C.; Tavares, M.I.B. Solvent Effect on the Morphology of Lamellar Nanocomposites Based on HIPS. Mater. Res. 2015, 18, 191–195. [Google Scholar] [CrossRef]
- Cunha, A.D.P.C.B.; Tavares, M.I.B.; Silva, E.O.; Zaioncz, S. The Effect of Montmorillonite Clay on the Crystallinity of Poly(vinyl alcohol) Nanocomposites Obtained by Solution Intercalation and In Situ Polymerization. J. Nanosci. Nanotechnol. 2015, 15, 2814–2820. [Google Scholar] [CrossRef]
- Motaung, T.; Luyt, A.; Saladino, M.; Caponetti, E. Study of morphology, mechanical properties, and thermal degradation of polycarbonate-titania nanocomposites as function of titania crystalline phase and content. Polym. Compos. 2013, 34, 164–172. [Google Scholar] [CrossRef]
- Liaw, W.-C.; Cheng, Y.-L.; Liao, Y.-S.; Chen, C.-S.; Lai, S.-M. Complementary functionality of SiO2 and TiO2 in polyimide/silica–titania ternary hybrid nanocomposites. Polym. J. 2011, 43, 249–257. [Google Scholar] [CrossRef]
- Shishkovsky, I.V.; Scherbakov, V.I. Additive manufacturing of polymer composites with nano-titania inclusions. Laser Phys. Lett. 2021, 18, 066001. [Google Scholar] [CrossRef]
- Sanes, J.; Sánchez, C.; Pamies, R.; Avilés, M.-D.; Bermúdez, M.-D. Extrusion of Polymer Nanocomposites with Graphene and Graphene Derivative Nanofillers: An Overview of Recent Developments. Materials 2020, 13, 549. [Google Scholar] [CrossRef]
- Müller, C.M.; Laurindo, J.B.; Yamashita, F. Composites of thermoplastic starch and nanoclays produced by extrusion and thermopressing. Carbohydr. Polym. 2012, 89, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Kickelbick, G. Hybrid Materials: Synthesis, Characterization, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Almeida, A.D.S.; Tavares, M.I.B.; Da Silva, E.O.; Neto, R.P.C.; Moreira, L.A. Development of hybrid nanocomposites based on PLLA and low-field NMR characterization. Polym. Test. 2012, 31, 267–275. [Google Scholar] [CrossRef]
- Cheraghian, G. Effect of nano titanium dioxide on heavy oil recovery during polymer flooding. Pet. Sci. Technol. 2016, 34, 633–641. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Maniadi, A.; Koudoumas, E.; Liebscher, M.; Tzounis, L. Mechanical Properties of 3D-Printed Acrylonitrile–Butadiene–Styrene TiO2 and ATO Nanocomposites. Polymers 2020, 12, 1589. [Google Scholar] [CrossRef] [PubMed]
- Vidakis, N.; Maniadi, A.; Petousis, M.; Vamvakaki, M.; Kenanakis, G.; Koudoumas, E. Mechanical and Electrical Properties Investigation of 3D-Printed Acrylonitrile–Butadiene–Styrene Graphene and Carbon Nanocomposites. J. Mater. Eng. Perform. 2020, 29, 1909–1918. [Google Scholar] [CrossRef]
- Nasu, A.; Otsubo, Y. Rheology and UV-protecting properties of complex suspensions of titanium dioxides and zinc oxides. J. Colloid Interface Sci. 2007, 310, 617–623. [Google Scholar] [CrossRef]
- Uddin, M.; Mondal, D.P.; Morris, C.; Lopez, T.; Diebold, U.; Gonzalez, R.D. An in vitro controlled release study of valproic acid encapsulated in a titania ceramic matrix. Appl. Surf. Sci. 2011, 257, 7920–7927. [Google Scholar] [CrossRef]
- Fujihara, K.; Kumar, A.; Jose, R.; Ramakrishna, S.; Uchida, S. Spray deposition of electrospun TiO2 nanorods for dye-sensitized solar cell. Nanotechnology 2007, 18, 365709. [Google Scholar] [CrossRef]
- Andronic, L.; Enesca, A. Black TiO2 Synthesis by Chemical Reduction Methods for Photocatalysis Applications. Front. Chem. 2020, 8, 5489. [Google Scholar] [CrossRef]
- Saccà, A.; Carbone, A.; Gatto, I.; Pedicini, R.; Freni, A.; Patti, A.; Passalacqua, E. Composites Nafion-titania membranes for Polymer Electrolyte Fuel Cell (PEFC) applications at low relative humidity levels: Chemical physical properties and electrochemical performance. Polym. Test. 2016, 56, 10–18. [Google Scholar] [CrossRef]
- Fiorati, A.; Bellingeri, A.; Punta, C.; Corsi, I.; Venditti, I. Silver Nanoparticles for Water Pollution Monitoring and Treatments: Ecosafety Challenge and Cellulose-Based Hybrids Solution. Polymers 2020, 12, 1635. [Google Scholar] [CrossRef]
- Pantalei, S.; Zampetti, E.; Macagnano, A.; Bearzotti, A.; Venditti, I.; Russo, M. Enhanced Sensory Properties of a Multichannel Quartz Crystal Microbalance Coated with Polymeric Nanobeads. Sensors 2007, 7, 2920–2928. [Google Scholar] [CrossRef]
- Fratoddi, I.; Cartoni, A.; Venditti, I.; Catone, D.; O’Keeffe, P.; Paladini, A.; Toschi, F.; Turchini, S.; Sciubba, F.; Testa, G.; et al. Gold nanoparticles functionalized by rhodamine B isothiocyanate: A new tool to control plasmonic effects. J. Colloid Interface Sci. 2018, 513, 10–19. [Google Scholar] [CrossRef]
- Sharpe, L.H. The Interfacial Interactions în Polymeric Composites; Akovali, G., Ed.; Kluwer: Dordrecht, The Netherlands, 1993. [Google Scholar]
- Jesson, D.; Watts, J.F. The Interface and Interphase in Polymer Matrix Composites: Effect on Mechanical Properties and Methods for Identification. Polym. Rev. 2012, 52, 321–354. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Li, N.; Liao, J.; Zhao, S.; Wang, J.; Wang, S. Relationship between polymer–filler interfaces in separation layers and gas transport properties of mixed matrix composite membranes. J. Membr. Sci. 2015, 495, 252–268. [Google Scholar] [CrossRef]
- Drzal, L.T.; Rich, M.J.; Lloyd, P.F. Adhesion of Graphite Fibers to Epoxy Matrices: I. The Role of Fiber Surface Treatment. J. Adhes. 1983, 16, 1–30. [Google Scholar] [CrossRef]
- Berlin, A.A.; Volfson, S.t.A.; Enikilopian, N.S.; Negmatov, S.S. Principles of Polymer Composites; Akademic-Verlag: Berlin, Germany, 1985. [Google Scholar]
- Cassidy, P.E.; Yager, B.J. Coupling Agents As Adhesion Promoters în Reviews în Polymer Technology; Skeist, I., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1972; Volume 1. [Google Scholar]
- Kim, J.-K.; Mai, Y.-W. Engineered Interfaces in Fiber Reinforced Composites; Elsevier BV: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Fourche, G. An overview of the basic aspects of polymer adhesion. Part I: Fundamentals. Polym. Eng. Sci. 1995, 35, 957–967. [Google Scholar] [CrossRef]
- Morris, H.R.; Turner, J.F.; Munro, B.; Ryntz, R.A.; Treado, P.J. Chemical Imaging of Thermoplastic Olefin (TPO) Surface Architecture. Langmuir 1999, 15, 2961–2972. [Google Scholar] [CrossRef]
- Del Rio, F.W.; De Boer, M.; Knapp, J.A.; Reedy, E.D.; Clews, P.J.; Dunn, M. The role of van der Waals forces in adhesion of micromachined surfaces. Nat. Mater. 2005, 4, 629–634. [Google Scholar] [CrossRef]
- Lipatov, Y. Polymer Reinforcement; ChemTec Publishing: Scarborough, ON, Canada, 1995. [Google Scholar]
- Qin, R.-Y.; Schreiber, H. Adhesion at partially restructured polymer surfaces. Colloids Surf. A Physicochem. Eng. Asp. 1999, 156, 85–93. [Google Scholar] [CrossRef]
- Selvin, T.P.; Kuruvilla, J.; Sabu, T. Mechanical properties of titanium dioxide-filled polystyrene microcomposites. Mater. Lett. 2004, 58, 281–289. [Google Scholar] [CrossRef]
- De Armitt, C.; Rothon, R. Fillers and surface treatment. Plast. Addit. Compd. 2002, 4, 12–14. [Google Scholar] [CrossRef]
- Fronza, B.M.; Lewis, S.; Shah, P.K.; Barros, M.D.; Giannini, M.; Stansbury, J.W. Modification of filler surface treatment of composite resins using alternative silanes and functional nanogels. Dent. Mater. 2019, 35, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Mozetič, M. Surface Modification to Improve Properties of Materials. Materials 2019, 12, 441. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, S.; Shaikh, T.; Pandey, P. A Review on Polymer TiO2 Nanocomposites. Int. J. Eng. Res. Appl. 2013, 3, 1386–1391. [Google Scholar]
- Dalod, A.R.M.; Henriksen, L.; Grande, T.; Einarsrud, M.-A. Functionalized TiO2 nanoparticles by single-step hydrothermal synthesis: The role of the silane coupling agents. Beilstein J. Nanotechnol. 2017, 8, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, S.S. Synthesis of Titanium Dioxide (TiO2) Nanomaterials. J. Nanosci. Nanotechnol. 2006, 6, 906–925. [Google Scholar] [CrossRef]
- Tao, P.; Li, Y.; Rungta, A.; Viswanath, A.; Gao, J.; Benicewicz, B.; Siegel, R.W.; Schadler, L.S. TiO2 nanocomposites with high refractive index and transparency. J. Mater. Chem. 2011, 21, 18623–18629. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Nano-TiO2 Reinforced PEEK/PEI Blends as Biomaterials for Load-Bearing Implant Applications. ACS Appl. Mater. Interfaces 2015, 7, 5561–5573. [Google Scholar] [CrossRef] [PubMed]
- Byranvand, M.M.; Kharat, A.N.; Fatholahi, L.; Beiranvand, Z.M. A review on synthesis of nano-TiO2 via different methods. JNS 2013, 3, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.; Lai, Q.; Fan, M. Review of the progress in preparing nano TiO2: An important environmental engineering material. J. Environ. Sci. 2014, 26, 2139–2177. [Google Scholar] [CrossRef] [PubMed]
- Hanemann, T.; Szabó, D.V. Polymer-Nanoparticle Composites: From Synthesis to Modern Applications. Materials 2010, 3, 3468–3517. [Google Scholar] [CrossRef]
- Laine, R.M.; Choi, J.; Lee, I. Organic–Inorganic Nanocomposites with Completely Defined Interfacial Interactions. Adv. Mater. 2001, 13, 800–803. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Ma, X.; Hu, M.; Wang, X.; Fan, P. Synthesis and characterization of epoxy resin modified with nano-SiO2 and γ-glycidoxypropyltrimethoxy silane. Surf. Coat. Technol. 2007, 201, 5269–5272. [Google Scholar] [CrossRef]
- Xu, X.; Li, B.; Lu, H.; Zhang, Z.; Wang, H. The interface structure of nano-SiO2/PA66 composites and its influence on material’s mechanical and thermal properties. Appl. Surf. Sci. 2007, 254, 1456–1462. [Google Scholar] [CrossRef]
- Li, X.; Cao, Z.; Zhang, Z.; Dang, H. Surface-modification in situ of nano-SiO2 and its structure and tribological properties. Appl. Surf. Sci. 2006, 252, 7856–7861. [Google Scholar] [CrossRef]
- Iijima, M.; Sato, N.; Lenggoro, I.W.; Kamiya, H. Surface modification of BaTiO3 particles by silane coupling agents in different solvents and their effect on dielectric properties of BaTiO3/epoxy composites. Colloids Surf. A Physicochem. Eng. Asp. 2009, 352, 88–93. [Google Scholar] [CrossRef]
- Balas, F.; Kokubo, T.; Kawashita, M.; Nakamura, T. Surface modification of organic polymers with bioactive titanium oxide without the aid of a silane-coupling agent. J. Mater. Sci. Mater. Electron. 2007, 18, 1167–1174. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Wen, Y.; Yuan, Y.; Wu, W.; Liu, C. Tunable wettability of monodisperse core-shell nano-SiO2 modified with poly(methylhydrosiloxane) and allyl-poly(ethylene glycol). Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 16–24. [Google Scholar] [CrossRef]
- Plueddemann, E.P. Silane Coupling Agents, 2nd ed.; Plenum Press: New York, NY, USA, 1991. [Google Scholar]
- Yosomiya, R.; Morimoto, K.; Nakajima, A.; Ikada, Y.; Suzuki, T.; Dharan, C.K.H. Adhesion and Bonding in Composites. J. Eng. Ind. 1991, 113, 117. [Google Scholar] [CrossRef]
- Konakanchi, A.; Alla, R.M.; Guduri, V. Silane Coupling Agents—Benevolent Binders in Composites. Trends Biomater. Artif. Organs 2017, 31, 108–113. [Google Scholar]
- Shokoohi, S.; Arefazar, A.; Khosrokhavar, R. Silane Coupling Agents in Polymer-based Reinforced Composites: A Review. J. Reinf. Plast. Compos. 2008, 27, 473–485. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, F.; Wang, C.; Wang, L.; Wang, X.; Chu, X.; Li, J.; Fang, X.; Wei, Z.; Wang, X. Hydrophobic modification of ZnO nanostructures surface using silane coupling agent. Polym. Compos. 2014, 35, 1204–1211. [Google Scholar] [CrossRef]
- Mallakpour, S.; Madani, M. The effect of the coupling agents KH550 and KH570 on the nanostructure and interfacial interaction of zinc oxide/chiral poly(amide–imide) nanocomposites containing l-leucine amino acid moieties. J. Mater. Sci. 2014, 49, 5112–5118. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Su, Y.-H.; Lai, J.-Y. In situ crosslinking of chitosan and formation of chitosan–silica hybrid membranes with using γ-glycidoxypropyltrimethoxysilane as a crosslinking agent. Polymer 2004, 45, 6831–6837. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, S.; Wu, L. De-Agglomeration and Dispersion Behavior of TiO2 Nanoparticles in Organic Media Using 3-Methacryloxypropyltrimethoxysilane as a Surface Modifier. J. Dispers. Sci. Technol. 2012, 33, 497–505. [Google Scholar] [CrossRef]
- Dang, Z.-M.; Xia, Y.-J.; Zha, J.-W.; Yuan, J.; Bai, J. Preparation and dielectric properties of surface modified TiO2 /silicone rubber nanocomposites. Mater. Lett. 2011, 65, 3430–3432. [Google Scholar] [CrossRef]
- Xu, Q.F.; Liu, Y.; Lin, F.-J.; Mondal, B.; Lyons, A.M. Superhydrophobic TiO2 –Polymer Nanocomposite Surface with UV-Induced Reversible Wettability and Self-Cleaning Properties. ACS Appl. Mater. Interfaces 2013, 5, 8915–8924. [Google Scholar] [CrossRef] [PubMed]
- Rahim-Abadi, M.M.; Mahdavian, A.R.; Gharieh, A.; Salehi-Mobarakeh, H. Chemical modification of TiO2 nanoparticles as an effective way for encapsulation in polyacrylic shell via emulsion polymerization. Prog. Org. Coat. 2015, 88, 310–315. [Google Scholar] [CrossRef]
- Toh-Ae, P.; Junhasavasdikul, B.; Lopattananon, N.; Sahakaro, K. Surface Modification of TiO2 Nanoparticles by Grafting with Silane Coupling Agent. Adv. Mater. Res. 2013, 844, 276–279. [Google Scholar] [CrossRef]
- Ambrósio, J.D.; Balarim, C.V.M.; De Carvalho, G.B. Preparation, characterization, and mechanical/tribological properties of polyamide 11/Titanium dioxide nanocomposites. Polym. Compos. 2014, 37, 1415–1424. [Google Scholar] [CrossRef]
- Sabzi, M.; Mirabedini, S.M.; Zohuriaan-Mehr, M.J.; Atai, M. Surface modification of TiO2 nano-particles with silane coupling agent and investigation of its effect on the properties of polyurethane composite coating. Prog. Org. Coat. 2009, 65, 222–228. [Google Scholar] [CrossRef]
- Rusu, G.; Rusu, E. Nylon 6/TiO2Composites by in situ Anionic Ring-Opening Polymerization of ϵ-Caprolactam: Synthesis, Characterization, and Properties. Int. J. Polym. Anal. Charact. 2011, 16, 561–583. [Google Scholar] [CrossRef]
- Zapata, P.A.; Palza, H.; Delgado, K.; Rabagliati, F.M. Novel antimicrobial polyethylene composites prepared by metallocenic in situ polymerization with TiO2-based nanoparticles. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 4055–4062. [Google Scholar] [CrossRef]
- Nguyen, V.G.; Thai, H.; Mai, D.H.; Tran, H.T.; Tran, D.L.; Vu, M.T. Effect of titanium dioxide on the properties of polyethylene/ TiO2 nanocomposites. Compos. Part B Eng. 2013, 45, 1192–1198. [Google Scholar] [CrossRef]
- Dzžunuzović, E.; Marinović-Cincović, M.; Vuković, J.; Jeremić, K.; Nedeljkovic, J. Thermal properties of PMMA/ TiO2 nanocomposites prepared byin-situbulk polymerization. Polym. Compos. 2009, 30, 737–742. [Google Scholar] [CrossRef]
- Yuvaraj, H.; Kim, W.S.; Kim, J.T.; Kang, I.P.; Gal, Y.-S.; Kim, S.W.; Lim, K.T. Synthesis of Poly(methyl methacrylate) Encapsulated TiO2 Nanocomposite Particles in Supercritical CO2. Mol. Cryst. Liq. Cryst. 2009, 514, 355–365. [Google Scholar] [CrossRef]
- Qi, Y.; Xiang, B.; Tan, W.; Zhang, J. Hydrophobic surface modification of TiO2 nanoparticles for production of acrylonitrile-styrene-acrylate terpolymer/ TiO2 composited cool materials. Appl. Surf. Sci. 2017, 419, 213–223. [Google Scholar] [CrossRef]
- Weng, C.-C.; Wei, K.-H. Selective Distribution of Surface-Modified TiO2 Nanoparticles in Polystyrene-b-poly (Methyl Methacrylate) Diblock Copolymer. Chem. Mater. 2003, 15, 2936–2941. [Google Scholar] [CrossRef]
- Yufei, C.; Zhichao, L.; Junyan, T.; Qingyu, Z.; Yang, H. Characteristics and Properties of TiO2 /EP-PU Composite. J. Nanomater. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Zhao, J.; Milanova, M.; Warmoeskerken, M.M.; Dutschk, V. Surface modification of TiO2 nanoparticles with silane coupling agents. Colloids Surf. A Physicochem. Eng. Asp. 2012, 413, 273–279. [Google Scholar] [CrossRef]
- Wanag, A.; Sienkiewicz, A.; Rokicka-Konieczna, P.; Kusiak-Nejman, E.; Morawski, A.W. Influence of modification of titanium dioxide by silane coupling agents on the photocatalytic activity and stability. J. Environ. Chem. Eng. 2020, 8, 103917. [Google Scholar] [CrossRef]
- Mallakpour, S.; Barati, A. Efficient preparation of hybrid nanocomposite coatings based on poly(vinyl alcohol) and silane coupling agent modified TiO2 nanoparticles. Prog. Org. Coat. 2011, 71, 391–398. [Google Scholar] [CrossRef]
- Shakeri, A.; Yip, D.; Badv, M.; Imani, S.M.; Sanjari, M.; Didar, T.F. Self-Cleaning Ceramic Tiles Produced via Stable Coating of TiO2 Nanoparticles. Materials 2018, 11, 1003. [Google Scholar] [CrossRef]
- Klaysri, R.; Tubchareon, T.; Praserthdam, P. One-step synthesis of amine-functionalized TiO2 surface for photocatalytic decolorization under visible light irradiation. J. Ind. Eng. Chem. 2017, 45, 229–236. [Google Scholar] [CrossRef]
- Chen, Q.; Yakovlev, N.L. Adsorption and interaction of organosilanes on TiO2 nanoparticles. Appl. Surf. Sci. 2010, 257, 1395–1400. [Google Scholar] [CrossRef]
- Dinari, M.; Haghighi, A. Surface modification of TiO2 nanoparticle by three dimensional silane coupling agent and preparation of polyamide/modified-TiO2 nanocomposites for removal of Cr (VI) from aqueous solutions. Prog. Org. Coat. 2017, 110, 24–34. [Google Scholar] [CrossRef]
- Caris, C.; Kuijpers, R.; Van Herk, A.M.; German, A.L. Kinetics of (CO)polymerizations at the surface of inorganic submicron particles in emulsion-like systems. Makromol. Chem. Macromol. Symp. 1990, 35-36, 535–548. [Google Scholar] [CrossRef]
- Erdem, B.; Sudol, E.D.; Dimonie, V.L.; El-Aasser, M.S. Encapsulation of inorganic particles via miniemulsion polymerization. II. Preparation and characterization of styrene miniemulsion droplets containing TiO2 particles. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 4431–4440. [Google Scholar] [CrossRef]
- Rong, M.Z.; Zhang, M.Q.; Wang, H.B.; Zeng, H.M. Surface modification of magnetic metal nanoparticles through irradiation graft polymerization. Appl. Surf. Sci. 2002, 200, 76–93. [Google Scholar] [CrossRef]
- Yang, M.; Dan, Y. Preparation and characterization of poly(methyl methacrylate)/titanium oxide composite particles. Colloid Polym. Sci. 2005, 284, 243–250. [Google Scholar] [CrossRef]
- Milanesi, F.; Cappelletti, G.; Annunziata, R.; Bianchi, C.L.; Meroni, D.; Ardizzone, S. Siloxane− TiO2 Hybrid Nanocomposites. The Structure of the Hydrophobic Layer. J. Phys. Chem. C 2010, 114, 8287–8293. [Google Scholar] [CrossRef]
- Xiang, B.; Jiang, G.; Zhang, J. Surface modification of TiO2 nanoparticles with silane coupling agent for nanocomposite with poly(butyl acrylate). Plast. Rubber Compos. 2015, 44, 148–154. [Google Scholar] [CrossRef]
- Wang, C.; Mao, H.; Wang, C.; Fu, S. Dispersibility and Hydrophobicity Analysis of Titanium Dioxide Nanoparticles Grafted with Silane Coupling Agent. Ind. Eng. Chem. Res. 2011, 50, 11930–11934. [Google Scholar] [CrossRef]
- Godnjavec, J.; Znoj, B.; Vince, J.; Steinbucher, M.; Žnidaršič, A.; Venturini, P. Stabilization of rutile TiO2 nanoparticles with Glymo in polyacrylic clear coating. Mater. Tehnol. 2012, 46, 19–24. [Google Scholar]
- Yang, C.; Yang, C. Preparation of TiO2 particles and surface silanization modification for electronic ink. J. Mater. Sci. Mater. Electron. 2014, 25, 3285–3289. [Google Scholar] [CrossRef]
- Tangchantra, N.; Kruenate, J.; Aumnate, C.; Sooksomsong, T. The Effect of Surface Modification of TiO2 on Mechanical Properties of Polyethylene Composite Film. Adv. Mater. Res. 2010, 93-94, 300–303. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Nada, A.; Barakat, M.; Hamed, H.; Mohamed, N.; Veziroglu, T. Studies on the photocatalytic hydrogen production using suspended modified TiO2 photocatalysts. Int. J. Hydrogen Energy 2005, 30, 687–691. [Google Scholar] [CrossRef]
- Andronic, L.; Enesca, A.; Cazan, C.; Visa, M. TiO2–active carbon composites for wastewater photocatalysis. J. Sol.-Gel. Sci. Technol. 2014, 71, 396–405. [Google Scholar] [CrossRef]
- Sharma, S.K.; Vishwas, M.; Rao, K.N.; Mohan, S.; Reddy, D.S.; Gowda, K. Structural and optical investigations of TiO2 films deposited on transparent substrates by sol–gel technique. J. Alloys Compd. 2009, 471, 244–247. [Google Scholar] [CrossRef]
- Bayarri, B.; Giménez, J.; Curcó, D.; Esplugas, S. Photocatalytic degradation of 2,4-dichlorophenol by TiO2 /UV: Kinetics, actinometries and models. Catal. Today 2005, 101, 227–236. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Shimizu, N.; Ninomiya, K.; Ogino, C.; Rahman, M.M. Potential uses of titanium dioxide in conjunction with ultrasound for improved disinfection. Biochem. Eng. J. 2010, 48, 416–423. [Google Scholar] [CrossRef]
- Tuan, N.M.; Nha, N.T.; Tuyen, N.H. Low-temperature synthesis of nano-TiO2 anatase on nafion membrane for using on DMFC. J. Phys. Conf. Ser. 2009, 187, 012040. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Armstrong, G.; Canales-Vazquez, J.; García, R.; Bruce, P.G. Lithium-Ion Intercalation into TiO2-B Nanowires. Adv. Mater. 2005, 17, 862–865. [Google Scholar] [CrossRef]
- Richards, B.S.; Cotter, J.E.; Honsberg, C.B. Enhancing the surface passivation of TiO2 coated silicon wafers. Appl. Phys. Lett. 2002, 80, 1123–1125. [Google Scholar] [CrossRef]
- Pinto, D.; Bernardo, L.; Amaro, A.; Lopes, S. Mechanical properties of epoxy nanocomposites using titanium dioxide as reinforcement—A review. Constr. Build. Mater. 2015, 95, 506–524. [Google Scholar] [CrossRef]
- Sahu, M.; Satapathy, A. Thermal Characteristics of Polypropylene Composites Filled with TiO2. In Proceedings of the ASEAI 2014, Bangkok, Thailand, 11–13 April 2013; Volume 49, pp. 1747–1752. [Google Scholar]
- Peng, X.; Ding, E.; Xue, F. In situ synthesis of TiO2/polyethylene terephthalate hybrid nanocomposites at low temperature. Appl. Surf. Sci. 2012, 258, 6564–6570. [Google Scholar] [CrossRef]
- Mourad, A.-H.I.; Mozumder, M.S.; Mairpady, A.; Pervez, H.; Kannuri, U.M. On the Injection Molding Processing Parameters of HDPE-TiO2 Nanocomposites. Materials 2017, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Zhil’Tsova, T.; Oliveira, M.; Ferreira, J. Relative influence of injection molding processing conditions on HDPE acetabular cups dimensional stability. J. Mater. Process. Technol. 2009, 209, 3894–3904. [Google Scholar] [CrossRef]
- Serrano, C.; Cerrada, M.; Fernandez-Garcia, M.; Ressia, J.; Valles, E.M. Rheological and structural details of biocidal iPP-TiO2 nanocomposites. Eur. Polym. J. 2012, 48, 586–596. [Google Scholar] [CrossRef]
- Umek, P.; Huskić, M.; Škapin, A.S.; Florjančič, U.; Zupančič, B.; Emri, I.; Arčon, D. Structural and mechanical properties of polystyrene nanocomposites with 1D titanate nanostructures prepared by an extrusion process. Polym. Compos. 2008, 30, 1318–1325. [Google Scholar] [CrossRef]
- Somani, P.R.; Marimuthu, R.; Mulik, U.; Sainkar, S.; Amalnerkar, D. High piezoresistivity and its origin in conducting polyaniline/TiO2 composites. Synth. Met. 1999, 106, 45–52. [Google Scholar] [CrossRef]
- Feng, W.; Sun, E.; Fujii, A.; Wu, H.; Niihara, K.; Yoshino, K. Synthesis and Characterization of Photoconducting Polyaniline-TiO2 Nanocomposite. Bull. Chem. Soc. Jpn. 2000, 73, 2627–2633. [Google Scholar] [CrossRef]
- Xia, H.; Wang, Q. Ultrasonic Irradiation: A Novel Approach To Prepare Conductive Polyaniline/Nanocrystalline Titanium Oxide Composites. Chem. Mater. 2002, 14, 2158–2165. [Google Scholar] [CrossRef]
- Alghamdi, M.N. Titanium Dioxide Reinforced Polypropylene Composites: Preparation and Characterization PP-TiO2 Composites. Int. J. Eng. Res. Technol. 2016, 5, 633–637. [Google Scholar]
- Vladuta, C.; Andronic, L.; Duta, A. Effect of TiO2, nanoparticles on the interface in the PET-rubber composites. J. Nanosci. Nanotechnol. 2010, 10, 2518–2526. [Google Scholar] [CrossRef] [PubMed]
- Mikešová, J.; Šlouf, M.; Gohs, U.; Popelková, D.; Vacková, T.; Vu, N.H.; Kratochvíl, J.; Zhigunov, A. Nanocomposites of polypropylene/titanate nanotubes: Morphology, nucleation effects of nanoparticles and properties. Polym. Bull. 2014, 71, 795–818. [Google Scholar] [CrossRef]
- Sreekumar, P.; Al-Harthi, M.A.; De, S. Reinforcement of starch/polyvinyl alcohol blend using nano-titanium dioxide. J. Compos. Mater. 2012, 46, 3181–3187. [Google Scholar] [CrossRef]
- Bora, M.Ö.; Çoban, O.; Avcu, E.; Fidan, S.; Sınmazçelik, T. The effect of TIO2 filler content on the mechanical, thermal, and tribological properties of TiO2 /PPS composites. Polym. Compos. 2013, 34, 1591–1599. [Google Scholar] [CrossRef]
- Saluja, P.S.; Tiwari, J.K.; Gupta, G. Preparation and Thermal Behaviour of Polyester Composite Filled with TiO2. Int. Res. J. Eng. Technol. 2017, 4, 3135–3141. [Google Scholar]
- Byrne, M.T.; McCarthy, J.E.; Bent, M.; Blake, R.; Gun’Ko, Y.K.; Horvath, E.; Konya, Z.; Kukovecz, A.; Kiricsi, I.; Coleman, J.N. Chemical functionalisation of titania nanotubes and their utilisation for the fabrication of reinforced polystyrene composites. J. Mater. Chem. 2007, 17, 2351–2358. [Google Scholar] [CrossRef]
- Dong, Y.; Gui, Z.; Hu, Y.; Wu, Y.; Jiang, S. The influence of titanate nanotube on the improved thermal properties and the smoke suppression in poly(methyl methacrylate). J. Hazard. Mater. 2012, 209-210, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Alexandru, M. On the morphology and potential application of polydimethylsiloxane-silica-titania composites. Express Polym. Lett. 2011, 5, 188–196. [Google Scholar] [CrossRef]
- Saritha, A.; Joseph, K.; Boudenne, A.; Thomas, S. Mechanical, thermophysical, and diffusion properties of TiO2-filled chlorobutyl rubber composites. Polym. Compos. 2011, 32, 1681–1687. [Google Scholar] [CrossRef]
- Manap, A.; Mahalingam, S.; Vaithylingam, R.; Abdullah, H. Mechanical, thermal and morphological properties of thermoplastic polyurethane composite reinforced by multi-walled carbon nanotube and titanium dioxide hybrid fillers. Polym. Bull. 2020, 1–18. [Google Scholar] [CrossRef]
- Esthappan, S.K.; Kuttappan, S.K.; Joseph, R. Thermal and mechanical properties of polypropylene/titanium dioxide nanocomposite fibers. Mater. Des. 2012, 37, 537–542. [Google Scholar] [CrossRef]
- Awang, M.; Mohd, W.R.W.; Sarifuddin, N. Study the effects of an addition of titanium dioxide (TiO2) on the mechanical and thermal properties of polypropylene-rice husk green composites. Mater. Res. Express 2019, 6, 075311. [Google Scholar] [CrossRef]
- Huang, J.; Lu, X.; Zhang, N.; Yang, L.; Yan, M.; Liu, H.; Zhang, G.; Qu, J. Study on the properties of nano-TiO2 /polybutylene succinate composites prepared by vane extruder. Polym. Compos. 2013, 35, 53–59. [Google Scholar] [CrossRef]
- Bragaglia, M.; Cherubini, V.; Nanni, F. PEEK-TiO2 composites with enhanced UV resistance. Compos. Sci. Technol. 2020, 199, 108365. [Google Scholar] [CrossRef]
- Farhoodi, M.; Dadashi, S.; Mousavi, S.M.A.; Sotudeh-Gharebagh, R.; Emam-Djomeh, Z.; Oromiehie, A.; Hemmati, F. Influence of TiO2 Nanoparticle Filler on the Properties of PET and PLA Nanocomposites. Polym. Korea 2012, 36, 745–755. [Google Scholar] [CrossRef]
- Lu, X.-L.; Lü, X.-Q.; Wang, J.-Y.; Sun, Z.-J.; Tong, Y.-X. Preparation and shape memory properties of TiO2/PLCL biodegradable polymer nanocomposites. Trans. Nonferrous Met. Soc. China 2013, 23, 120–127. [Google Scholar] [CrossRef]
- Alberton, J.; Martelli, S.M.; Fakhouri, F.M.; Soldi, V. Mechanical and moisture barrier properties of titanium dioxide nanoparticles and halloysite nanotubes reinforced polylactic acid (PLA). IOP Conf. Ser. Mater. Sci. Eng. 2014, 64, 012010. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, J.; Yang, L.; Chen, R.; Zou, W.; Lin, X.; Qu, J. Preparation, characterization and properties of PLA/TiO2 nanocomposites based on a novel vane extruder. RSC Adv. 2015, 5, 4639–4647. [Google Scholar] [CrossRef]
- Zhuang, W.; Liu, J.; Zhang, J.H.; Hu, B.X.; Shen, J. Preparation, characterization, and properties of TiO2/PLA nanocomposites by in situ polymerization. Polym. Compos. 2009, 30, 1074–1080. [Google Scholar] [CrossRef]
- Wetzel, B.; Rosso, P.; Haupert, F.; Friedrich, K. Epoxy nanocomposites—Fracture and toughening mechanisms. Eng. Fract. Mech. 2006, 73, 2375–2398. [Google Scholar] [CrossRef]
- Chatterjee, A.; Islam, M.S. Fabrication and characterization of TiO2–epoxy nanocomposite. Mater. Sci. Eng. A 2008, 487, 574–585. [Google Scholar] [CrossRef]
- Huang, K.S.; Nien, Y.H.; Chen, J.S.; Shieh, T.R.; Chen, J.W. Synthesis and properties of epoxy/TiO2 composite materials. Polym. Compos. 2006, 27, 195–200. [Google Scholar] [CrossRef]
- Al-Turaif, H.A. Effect of nano TiO2 particle size on mechanical properties of cured epoxy resin. Prog. Org. Coat. 2010, 69, 241–246. [Google Scholar] [CrossRef]
- Papanicolaou, G.; Kontaxis, L.; Manara, A. Viscoelastic behaviour and modelling of nano and micro TiO2 powder-epoxy resin composites. Ciênc. Tecnol. Mater. 2016, 28, 138–146. [Google Scholar] [CrossRef]
- Salehian, H.; Jahromi, S.A.J. Effect of titanium dioxide nanoparticles on mechanical properties of vinyl ester-based nanocomposites. J. Compos. Mater. 2015, 49, 2365–2373. [Google Scholar] [CrossRef]
- Meera, A.P.; Said, S.; Grohens, Y.; Luyt, A.S.; Thomas, S. Tensile Stress Relaxation Studies of TiO2 and Nanosilica Filled Natural Rubber Composites. Ind. Eng. Chem. Res. 2009, 48, 3410–3416. [Google Scholar] [CrossRef]
- Ochigbo, S.S.; Luyt, A.S. Mechanical and Morphological Properties of Films Based on Ultrasound Treated Titanium Dioxide Dispersion/Natural Rubber Latex. Int. J. Compos. Mater. 2011, 1, 7–13. [Google Scholar] [CrossRef]
- Hayeemasae, N.; Rathnayake, W.; Ismail, H. Nano-sized TiO2-reinforced natural rubber composites prepared by latex compounding method. J. Vinyl Addit. Technol. 2017, 23, 200–209. [Google Scholar] [CrossRef]
- Datta, J.; Kosiorek, P.; Włoch, M. Effect of high loading of titanium dioxide particles on the morphology, mechanical and thermo-mechanical properties of the natural rubber-based composites. Iran. Polym. J. 2016, 25, 1021–1035. [Google Scholar] [CrossRef]




| Modification Agent of TiO2 Surface | Chemical Structure | Polymer–TiO2 Nanocomposite | Ref |
|---|---|---|---|
| 3-(trimethoxysilyl)propyl methacrylate, KH–570 |  | silicone rubber–TiO2 nanocomposite | [72] |
| fluoro silane | H3Si–F | HDPE–TiO2 nanocomposite | [73] |
| glycidyl methacrylate |  | methyl methacrylate–butyl acrylate/dimethylaminoethyl methacrylate–butyl acrylate–acrylic acid–TiO2 nanoparticles | [74] |
| bis-(3-triethoxysilylpropyl) tetrasulfide (TESPT) |  | rubber–TiO2 nanocomposite | [75] |
| 3-amino propyl trimethoxy silane |  | PA11–TiO2 nanocomposite;PU-TiO2 composites; | [76,77] |
| 3-amino propyl triethoxy silane |  | nylon 6/TiO2 composites;PS–TiO2 microcompositespolyurethane–TiO2 composites;polyamide–TiO2 nanocomposites | [45,77,78] |
| hexadecyl trimethoxy silane |  | PE–TiO2 nanocomposite | [79] |
| vinyl trimethoxy silane (VTMS) |  | LDPE–TiO2 nanocomposite | [80] |
| 6-palmitate ascorbic acid |  | PMMA–TiO2 nanocomposite | [81] |
| 3-methacryloxy propyl trimethoxy silane |  | PMMA–TiO2 nanocomposite;acrylonitrile–styrene-acrylate terpolymer–TiO2 composite;PS-b-PMMA–TiO2 nanocomposite | [82,83] |
| cetyl trimethylammonium chloride (TMAC) amphiphilics |  | PS-b-PMMA–TiO2 nanocomposite | [84] |
| isopropyl tri(dioctylpyrophosphate) titanate (TCA201) |  | EP-PU/TiO2 composite | [85] |
| 3-isocyanato propyl trimethoxy silane |  | polymer–TiO2 | [86] |
| Application | Properties |
|---|---|
| Photocatalysis | Particularly in anatase from under ultraviolet light |
| Self-cleaning and anti-fogging glass | Spiked with nitrogen ions or droplet with metal oxides under UV–visible light |
| Hydrolysis catalyst | Super hydrophilicity, deodorizing, sterilizing, anti-fouling; chemical resistance |
| Dye-sensitized solar cells | Strong oxidative potential for develop OH radicals |
| Pigments, opacifiers, cosmetic, UV absorber | Brightness, high reflective index, high reflective optical, perfect white, opacity, nontoxic to human life |
| Polymer Nanocomposites | Conventional Polymer Composites |
|---|---|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazan, C.; Enesca, A.; Andronic, L. Synergic Effect of TiO2 Filler on the Mechanical Properties of Polymer Nanocomposites. Polymers 2021, 13, 2017. https://doi.org/10.3390/polym13122017
Cazan C, Enesca A, Andronic L. Synergic Effect of TiO2 Filler on the Mechanical Properties of Polymer Nanocomposites. Polymers. 2021; 13(12):2017. https://doi.org/10.3390/polym13122017
Chicago/Turabian StyleCazan, Cristina, Alexandru Enesca, and Luminita Andronic. 2021. "Synergic Effect of TiO2 Filler on the Mechanical Properties of Polymer Nanocomposites" Polymers 13, no. 12: 2017. https://doi.org/10.3390/polym13122017
APA StyleCazan, C., Enesca, A., & Andronic, L. (2021). Synergic Effect of TiO2 Filler on the Mechanical Properties of Polymer Nanocomposites. Polymers, 13(12), 2017. https://doi.org/10.3390/polym13122017








