Recycling of Wastes Plastics and Tires from Automotive Industry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.1.1. Rubber
- Sample 1: Granulate from recycled tire (size from 1.0 to 3.0 mm)—sample 1
- Sample 2: Granulate from recycled tire (size from 3.1 to 6.0 mm)—sample 2
- The granulate from recycled tires was produced by AVE SK-Kechnec plant Slovakia.
2.1.2. Plastics
- Analyzed plastic waste materials were taken away from the vehicle scrap yard.
- Plexiglass from the dashboard—sample 3
- Perforated headlamp or reflector—sample 4
- Inner fender—sample 5
- Shielding part of the dashboard—sample 6
- Front bumper-type 1—sample 7
- Front bumper-type 2—sample 8
- Car interior pillar panel—sample 9
- Interior accessories—comfort equipment for seats-sample 10
- Heating blower of cars—sample 11
- Plastic wheel hub—sample 12
2.2. Methods
2.2.1. Thermogravimetric Analysis
2.2.2. Pyrolysis and GC-MS Analysis (Py-GC-MS)
2.2.3. Calorimetry
3. Results and Discussion
3.1. TG Analysis
3.2. Py-GC-MS Analysis
3.3. Calorimetry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novotný, M.; Hipča, H.; Marušák, V. Analysis of the process of old vehicles recycling. In The State and Vision of Waste Recovery from the Automotive Industry SR, 1st ed.; Šooš, Ľ., Ed.; Spektrum STU: Bratislava, Slovakia, 2020; p. 285. (In Slovak) [Google Scholar]
- Krilek, J.; Samešová, D.; Čabalová, I.; Potkany, M.; Dado, M.; Kučera, M. Recycling and recovery of tires, rubber and plastics into the new products. In The State and Vision of Waste Recovery from the Automotive Industry SR, 1st ed.; Šooš, Ľ., Ed.; Spektrum STU: Bratislava, Slovakia, 2020; p. 285. (In Slovak) [Google Scholar]
- Zhang, H.; Chen, M. Current recycling regulations and technologies for the typical plastic components of end-of-life passenger vehicles: A meaningful lesson for China. J. Mat. Cycles Waste Manag. 2014, 16, 187–200. [Google Scholar] [CrossRef]
- Mleziva, J.; Šňupárek, J. Polymers—Production, Structure, Properties and Utilization, 2nd ed.; Sobotáles: Praha, Czech Republic, 2000; p. 544. [Google Scholar]
- Aristri, M.A.; Lubis, M.A.R.; Yadav, S.M.; Antov, P.; Papadopoulos, A.N.; Pizzi, A.; Fatriasari, W.; Ismayati, M.; Iswanto, A.H. Recent Developments in Lignin- and Tannin-Based Non-Isocyanate Polyurethane Resins for Wood Adhesives—A Review. Appl. Sci. 2021, 11, 4242. [Google Scholar] [CrossRef]
- Maddah, H.A. Polypropylene as a Promising Plastic: A Review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar]
- Smith, B. The Plastics Used in Automotives. 2018. Available online: https://www.azom.com/article.aspx?ArticleID=17014 (accessed on 27 May 2021).
- Mann, D. Automotive Plastics and Composites: Worldwide Markets and Trends to 2007; Reinforce Plastics Magazine; Elsevier: Amsterdam, The Netherlands, 2007; p. 420. [Google Scholar]
- Lithner, D.; Nordensvan, I.; Dave, G. Comparative acute toxicity of leachates from plastic products made of polypropylene, polyethylene, PVC, acrylonitrile–butadiene–styrene, and epoxy to Daphnia magna. Environ. Sci. Pollut. Res. 2012, 19, 1763–1772. [Google Scholar] [CrossRef]
- Kulshreshtha, A.K. A Review of Commercial Polyblends Based on PVC, ABS, and PC. Polym. Plast. Technol. Eng. 1993, 32, 551–578. [Google Scholar] [CrossRef]
- Bulei, C.; Todor, M.P.; Heput, T.; Kiss, I. Direction for material recovery of used tires and their use in the production of new products intended for the industry of civil construction and pavements. IOP Conf. Ser. Mater. Sci. Eng. 2018, 294, 012064. [Google Scholar] [CrossRef]
- Thiounn, T.; Smith, R.C. Advances and approaches for chemical recycling of plastic waste—Review. J. Polym. Sci. 2020, 58, 1347–1364. [Google Scholar] [CrossRef] [Green Version]
- Wołosiewicz-Głąb, M.; Pięta, P.; Sas, S.; Grabowski, L. Plastic waste depolymerization as a source of energetic heating oils. E3S Web Conf. 2016, 14, 02044. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW)—A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef]
- Yuliansyah, A.T.; Prasetya, A.; Ramadhan, M.A.; Laksono, R. Pyrolisis of plastic waste to produce pyrolytic oil as an alternative fuel. Int. J. Technol. 2015, 6, 1076. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Sivakumar, N.; Manimekalai, T.K.; Sathiskumar, C. A review on pyrolisis of waste plastics to value added products. Elixir Online J. 2012, 29, 8291–8298. [Google Scholar]
- Cabalova, I.; Geffertova, J.; Bubenikova, T.; Krilek, J. Pyrolytic recovery as a prospective use of plastic waste materials. MM Sci. J. 2021. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Anuar, S.; Shafferina, D.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Arena, U.; Mastellone, M.L.; Scheirs, J. Fluidized Bed Pyrolysis of Plastic Wastes; John Wiley & Sons, Ltd.: Toronto, ON, Canada, 2006. [Google Scholar]
- Tukker, A. Plastics Waste—Feedstock Recycling, Chemical Recycling and Incineration; Rapra Review Reports; Rapra Technology Ltd.: Shrewsbury, UK, 2002; p. 136. [Google Scholar]
- Achilias, D.S.; Andriotis, L.; Koutsidis, I.A.; Louka, D.A.; Nianias, N.P.; Siafaka, P.; Tsagkalias, I.; Tsintzou, G. Recent advances in the chemical recycling of polymers (PP, PS, LDPE, HDPE, PVC, PC, Nylon, PMMA). In Material Recycling—Trends and Perspectives; Achilias, D.S., Ed.; InTech: Rijeka, Croatia, 2012; Volume 3, p. 64. [Google Scholar]
- Wong, S.L.; Ngadi, N.; Abdullah, T.A.T.; Inuwa, I.M. Conversion of low density polyethylene (LDPE) over ZSM-5 zeolite to liquid fuel. Fuel 2017, 192, 71–82. [Google Scholar] [CrossRef]
- Achilias, D.S. Chemical Recycling of Poly(Methyl Methacrylate) by Pyrolysis. Potential use of the Liquid Fraction as a Raw Material for the Reproduction of the Polymer. Eur. Polym. J. 2007, 43, 2564–2575. [Google Scholar] [CrossRef]
- Kassargy, C.; Awad, S.; Burnens, G.; Kahine, K.; Tazerout, M. Experimental study of catalytic pyrolysis of polyethylene and polypropylene over USY zeolite and separation to gasoline and diesel-like fuels. J. Anal. Appl. Pyrolysis 2017, 127, 31–37. [Google Scholar] [CrossRef]
- ISO. Solid Mineral Fuels. Determination of Gross Calorific Value by the Bomb Calorimetric Method, and Calculation of Net Calorific Value; STN ISO 1928 (441352); ISO: Geneva, Switzerland, 2003. [Google Scholar]
- Fazli, A.; Rodrigue, D. Recycling Waste Tires into Ground Tire Rubber (GTR)/Rubber Compounds: A Review. J. Compos. Sci. 2020, 4, 103. [Google Scholar] [CrossRef]
- Saad, J.M.; Williams, P.T.; Zhang, Y.S.; Yao, D.; Yang, H.; Zhou, H. Comparison of waste plastics pyrolysis under nitrogen and carbon dioxide atmospheres: A thermogravimetric and kinetic study. J. Anal. Appl. Pyrol. 2021, 156, 105135. [Google Scholar] [CrossRef]
- Aboulkas, A.; Harfi, K.E.; Bouadili, A.E. Thermal degradation behaviors of polyethylene and polypropylene. Part I: Pyrolysis kinetics and mechanisms. Energy Convers. Manag. 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Ramarad, S.; Khalid, M.; Ratnam, C.T.; Abdullah, L.; Rashmi, W. Waste tire rubber in polymer blends: A review on the evolution, properties and future. Prog. Mater. Sci. 2015, 72, 100–140. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K.; Wang, X. Pyrolysis kinetic behaviour and Py-GC–MS analysis of waste dahlia flowers into renewable fuel and value-added chemicals. Fuel 2020, 260, 116338. [Google Scholar] [CrossRef]
- Kantarelis, E.; Donaj, P.; Yang, W.; Zabaniotou, A. Sustainable valorization of plastic wastes for energy with environmental safety via High-Temperature Pyrolysis (HTP) and High-Temperature Steam Gasification (HTSG). J. Hazard. Mater. 2009, 167, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Geffertová, J.; Geffert, A. Energy potential of the chosen wastes with biomass content. Acta Fac. Xylologiae 2011, 53, 93–99. (In Slovak) [Google Scholar]
- Wasilewski, R. Energy recovery from waste plastics. Chemic 2013, 67, 435–445. [Google Scholar]
- Panda, A.; Singh, R.K.; Mishra, D.K. Thermolysis of waste plastics to liquid fuel A suitable method for plastic waste management and production of value added products—A world prospective. Ren. Sustain. Energy Rev. 2010, 14, 233–248. [Google Scholar] [CrossRef]
- Zevenhoven, R.; Karlsson, M.; Hupa, M. Combustion and gasification properties of plastics particles. J. Air Waste Manag. Assoc. 1997, 47, 861–870. [Google Scholar] [CrossRef] [Green Version]
- Ionescu, G.; Bulmău, C. Estimation of energy potential for solid pyrolysis by-products using analytical methods. In Analytical Pyrolysis; Kusch, P., Ed.; Intech Open: London, UK, 2019. [Google Scholar]
- Olisa, Y.P.; Ajoko, T.J. Gross Calorific Value of Combustible Solid Waste in a Mass Burn Incineration Plant, Benin City, Nigeria. J. Air Waste Manag. Assoc. 2018, 22, 1377–1380. [Google Scholar] [CrossRef]
- Lunguleasa, A.; Spirchez, C.; Zeleniuc, O. Evaluation of the calorific values of wastes from some tropical wood species. Maderas Cienc. Tecnol. 2020, 22, 269–280. [Google Scholar] [CrossRef]
- Lieskovský, M.; Jankovský, M.; Trenčiansky, M.; Merganič, J.; Dvořák, J. Ash content vs. the economics of using wood chips for energy: Model based on data from central Europe. BioResources 2017, 12, 1579–1592. [Google Scholar] [CrossRef]
- Günther, B.; Gebauer, K.; Barkowski, R.; Rosenthal, M.; Bues, C.T. Calorific value of selected wood species and wood products. Eur. J. Wood Wood Prod. 2012, 70, 755–757. [Google Scholar] [CrossRef]
- Demirbas, A. Relationships between lignin contents and heating values of biomass. Energy Convers. Manag. 2001, 42, 183–188. [Google Scholar] [CrossRef]
- Kataki, R.; Konwer, D. Fuel wood characteristics of some in-digenous woody species of north-east India. Biomass Bioenergy 2001, 20, 17–23. [Google Scholar] [CrossRef]
- Sun, L.; Liu, X.M. Control Analysis of Production and Apparent Quality of Automobile Large Plastic Parts. Procedia Eng. 2011, 16, 438–443. [Google Scholar] [CrossRef] [Green Version]
- Moritomi, S.; Watanabe, T.; Kanazaki, S. Polypropylene compounds for automotive applications. Sumitomo Kagaku R&D Rep. 2010, 1, 1–16. [Google Scholar]
- Serrano, D.P.; Aguado, J.; Escola, J.M. Developing advanced catalysts for the conversion of polyolefinic waste plastics into fuels and chemicals. ACS Catal. 2012, 2, 1924–1941. [Google Scholar] [CrossRef]
- Kasar, P.; Sharma, D.K.; Ahmaruzzaman, M. Thermal and catalytic decomposition of waste plastics and its co-processing with petroleum residue through pyrolysis process. J. Clean. Prod. 2020, 265. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Nizami, A.S. Catalytic pyrolysis of plastic waste: A review. Process Saf. Environ. Prot. 2016, 102, 822–838. [Google Scholar] [CrossRef]
- Scheirs, J.; Kaminsky, W. Feedstock Recycling and Pyrolysis of Waste Plastics: Converting Waste Plastics into Diesel and Other Fuels; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Nakaji, Y.; Tamura, M.; Miyaoka, S.; Kumagai, S.; Tanji, M.; Nakagawa, Y.; Yoshioka, T.; Tomishige, K. Low-temperature catalytic upgrading of waste polyolefinic plastics into liquid fuels and waxes. Appl. Catal. B Environ. 2021, 285, 119805. [Google Scholar] [CrossRef]
- Mangesh, V.L.; Perumal, T.; Subramanian, S.; Padmanabhan, S. Clean energy from plastic: Production of hydroprocessed waste polypropylene pyrolysis oil utilizing a Ni–Mo/laponite catalyst. Energy Fuels 2020, 34, 8824–8836. [Google Scholar] [CrossRef]
- Serrano, D.P.; Escola, J.M.; Briones, L.; Arroyo, M. Hydroprocessing of the LDPE thermal cracking oil into transportation fuels over Pd supported on hierarchical ZSM-5 catalyst. Fuel 2017, 206, 190–198. [Google Scholar] [CrossRef]
- Utami, M.; Wijaya, K.; Trisunaryanti, W. Pt-promoted sulfated zirconia as catalyst for hydrocracking of LDPE plastic waste into liquid fuels. Mater. Chem. Phys. 2018, 213, 548–555. [Google Scholar] [CrossRef]
- Escola, J.M.; Serrano, D.P.; Aguado, J.; Briones, L. Hydroreforming of the LDPE thermal cracking oil over hierarchical Ni/beta catalysts with different Ni particle size distributions. Ind. Eng. Chem. Res. 2015, 54, 6660–6668. [Google Scholar] [CrossRef]
| Plastic Material | Properties | Utilization | Source |
|---|---|---|---|
| Polypropylene-PP Polyethylene-PE | Low price; good adaptability; good performance and easy to recycle; | Thin-walled moldings; fuel tanks and hoses | [6] |
| Expanded polypropylene-EPP | Excellent mechanical properties-flexibility, compressive strength; ability of high energy absorption; accomplish the strictest criteria in the field of shock protection; thermal and sound insulation | In the car exterior and interior; as part of bumpers; seats; luggage compartments; headrests; carpet fillings | [3] |
| Polyvinyl chloride-PVC | High tensile strength; toughness; fire resistance; chemical resistance PVC without plasticizers (novodur) is resistant to water, acids, alkalis, organic compounds, oxygen, water vapour; high hardness; abrasion resistance; mechanical strength; good electrical insulating properties; high gloss and clarity; self-extinguishing; glues bonding and welding; undersides coating of cars and textile underlying | Softened as a surface layer of artificial leather; foil; moldings; profiles; hoses Unsoftened in the production of loaded moldings; profiles and sheets | [3,4,7] |
| Polyurethane-PUR | Increased comfort; corrosion resistance; insulation; sound absorption | Production of precision thin-walled moldings; parallel coupling sleeves and coupling dusters; for insulation and sealing tapes; textile lamination; packaging; insulation materials in construction industry; adhesives and fibers | [8] |
| Acrylonitrile butadiene styrene-ABS | Hard, shiny surface; attractive appearance; enables galvanic plating | For the complex and stressed moldings; grilles; radiator covers; ventilation; headlight frames; steering wheel covers; rear-view mirror housings; wheel hub covers; dashboards; large bonnet parts; safety panel cover layer; roof panel cover layer, vacuum drawn plates; surface and interior body parts | [3,9,10] |
| Copolymer of styrene and acrylonitrile-SAN | The most chemically resistant from the polystyrene materials and lasts for a long time even at a temperature of 85 °C | Covers with a good resistance to low temperatures; for glass fiber reinforced products; projector covers; cars interior | [2] |
| Polymethylmethacrylate-PMMA | Clarity; colorlessness in large thicknesses (92% light transmission); resistance to weathering, water, diluted acids and hydroxides; heat resistance up to 80 °C; low surface hardness | Glazing caravans and vehicles | [3] |
| Polycarbonate-PC | Technically important plastic construction; high light transmission; high impact strength; good electrical insulating properties; high mechanical tensile strength; low water absorption; resistance to UV radiation; chemical and dimensional stability up to 140 °C | Highly resistant moldings | [7] |
| Polyamide-PA | High hardness; toughness; abrasion resistance; good electrical insulating properties | Part of stressed parts of the handles; window controls and sliding bushes in the form of construction plastics such as bearings; gears; coils; anticorrosive coatings of metals; electrical insulating layers; cords for tires; conveyor belts; carpet fibers; nets | [2] |
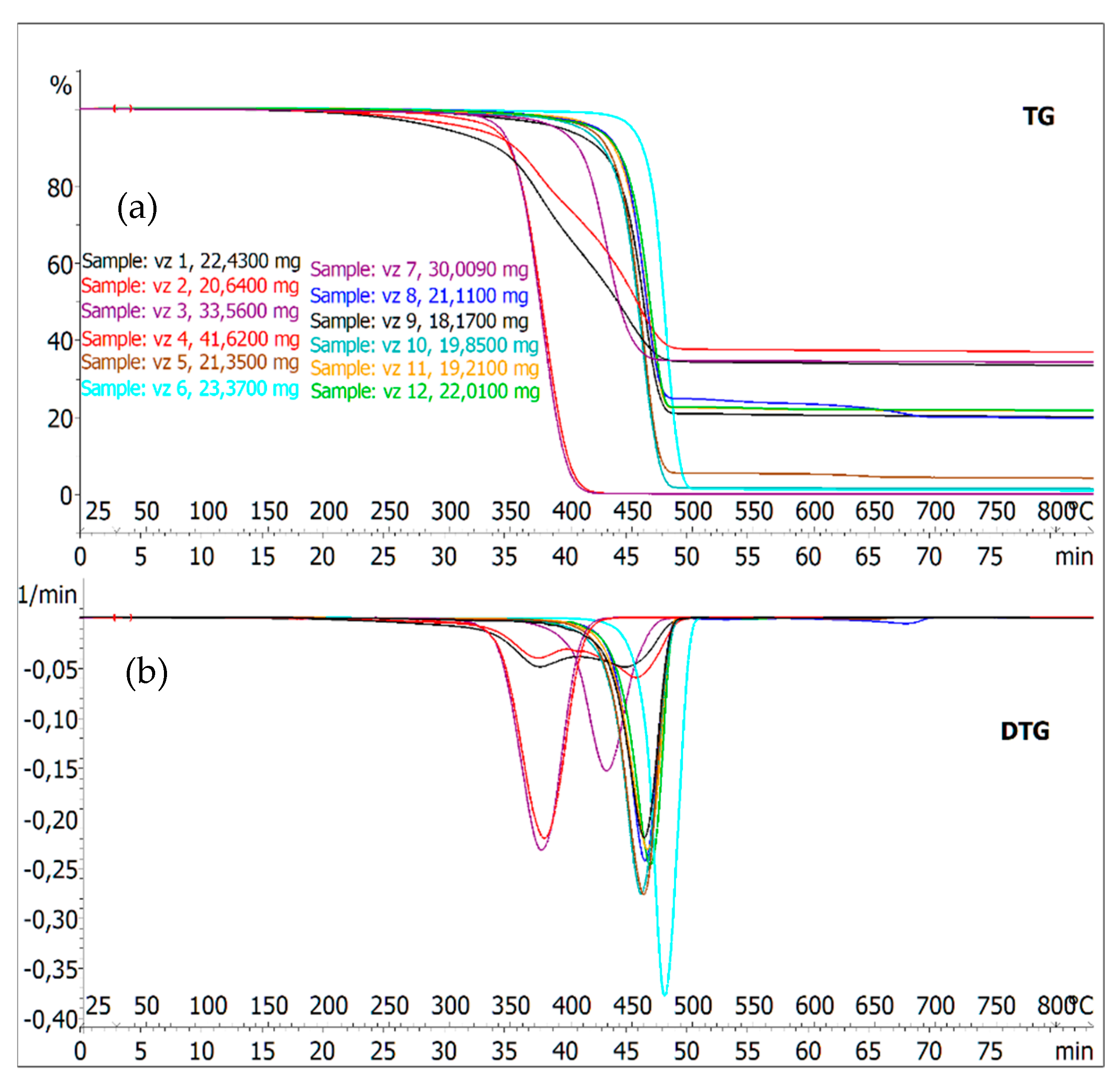
| Sample | (Tmax) [°C] | Weight Loss at Tmax [%] | Weight Loss at 800 °C [%] |
|---|---|---|---|
| 1 | 375.9/452.8 | 21.04/55.57 | 66.67 |
| 2 | 374.7/459.3 | 16.13/50.89 | 61.77 |
| 3 | 379.2 | 56.06 | 100 |
| 4 | 377.5 | 52.39 | 100 |
| 5 | 463.4 | 61.52 | 95.84 |
| 6 | 480.4 | 53.13 | 99.06 |
| 7 | 431.6 | 36.53 | 65.75 |
| 8 | 464.4 | 43.94 | 80.38 |
| 9 | 464.1 | 50.48 | 80.08 |
| 10 | 461.1 | 65.51 | 98.42 |
| 11 | 466.5 | 49.34 | 77.76 |
| 12 | 467.4 | 53.72 | 78.31 |
| Sample/Property | Calorific Value (MJ/kg) | Ash Content (%) |
|---|---|---|
| 1 | 36.441 ± 0.783 | 7.36 ± 2.40 |
| 2 | 37.051 ± 0.565 | 7.51 ± 3.19 |
| 3 | 26.521 ± 0.012 | 0.12 ± 0.06 |
| 4 | 26.478 ± 0.085 | 0.05 ± 0.01 |
| 5 | 44.558 ± 0.131 | 2.39 ± 0.10 |
| 6 | 34.784 ± 0.063 | 18.32 ± 0.16 |
| 7 | 26.261 ± 0.061 | 18.31 ± 0.23 |
| 8 | 45.245 ± 0.050 | 0.80 ± 0.01 |
| 9 | 35.795 ± 0.045 | 20.56 ± 0.04 |
| 10 | 35.921 ± 0.055 | 19.71 ± 0.01 |
| 11 | 45.130 ± 0.092 | 0.76 ± 0.25 |
| 12 | 40.493 ± 0.050 | 1.27 ± 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čabalová, I.; Ház, A.; Krilek, J.; Bubeníková, T.; Melicherčík, J.; Kuvik, T. Recycling of Wastes Plastics and Tires from Automotive Industry. Polymers 2021, 13, 2210. https://doi.org/10.3390/polym13132210
Čabalová I, Ház A, Krilek J, Bubeníková T, Melicherčík J, Kuvik T. Recycling of Wastes Plastics and Tires from Automotive Industry. Polymers. 2021; 13(13):2210. https://doi.org/10.3390/polym13132210
Chicago/Turabian StyleČabalová, Iveta, Aleš Ház, Jozef Krilek, Tatiana Bubeníková, Ján Melicherčík, and Tomáš Kuvik. 2021. "Recycling of Wastes Plastics and Tires from Automotive Industry" Polymers 13, no. 13: 2210. https://doi.org/10.3390/polym13132210
APA StyleČabalová, I., Ház, A., Krilek, J., Bubeníková, T., Melicherčík, J., & Kuvik, T. (2021). Recycling of Wastes Plastics and Tires from Automotive Industry. Polymers, 13(13), 2210. https://doi.org/10.3390/polym13132210








