The Effect of WS2 Nanosheets on the Non-Isothermal Cold- and Melt-Crystallization Kinetics of Poly(l-lactic acid) Nanocomposites
Abstract
:1. Introduction
2. Experimental Section
2.1. Solution Polymerization of PLLA
2.2. Preparation of PLLA/2D-WS2 Nanocomposites
2.3. Characterization Techniques of PLLA/2D-WS2 Nanocomposites
3. Results
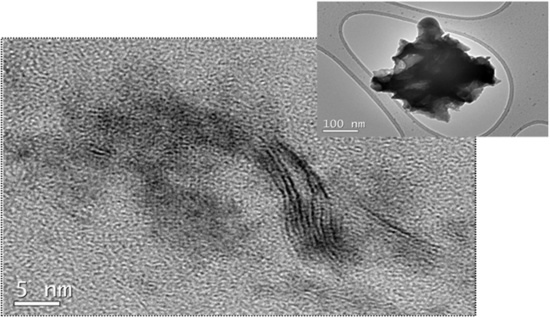
3.1. Morphology
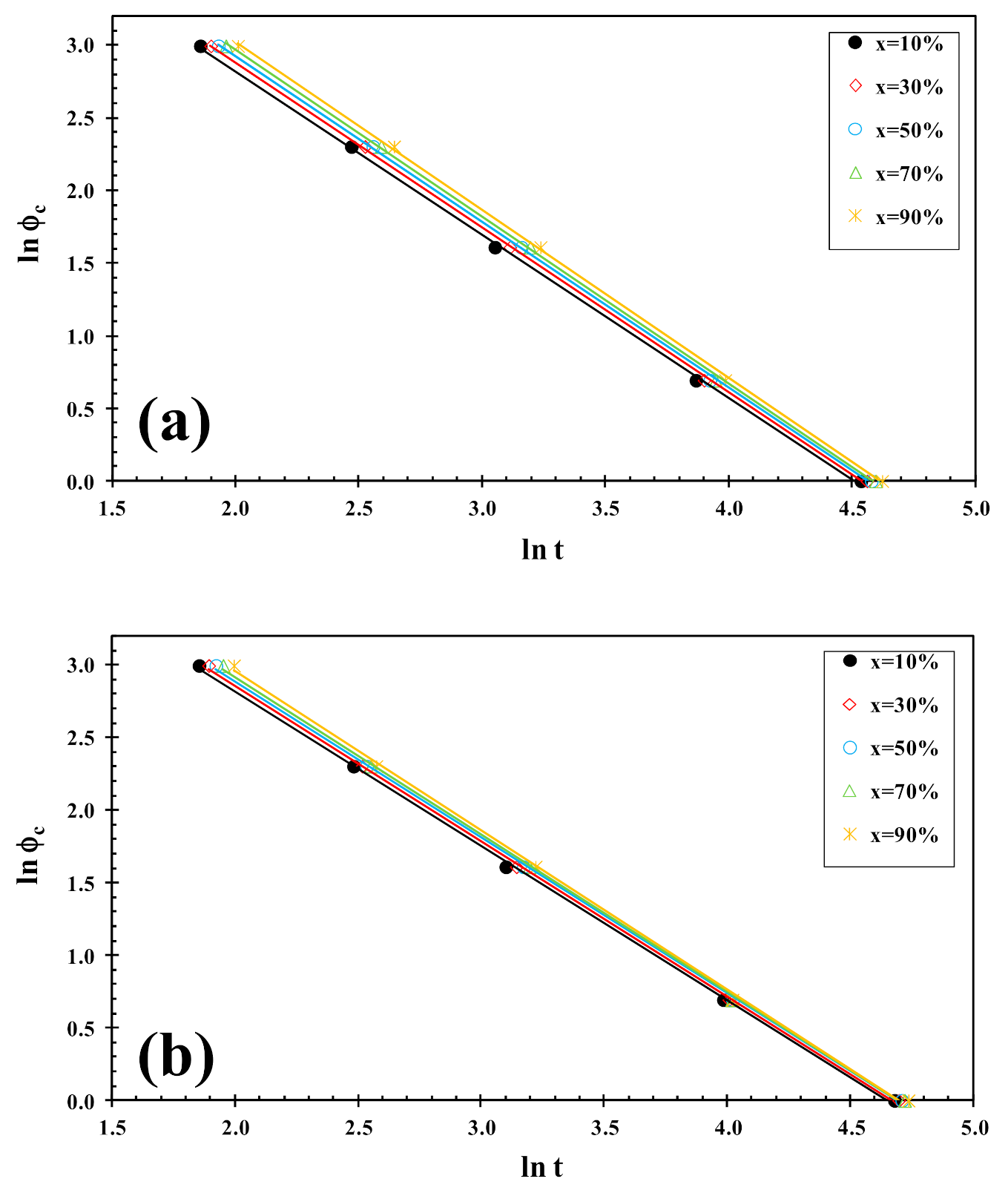
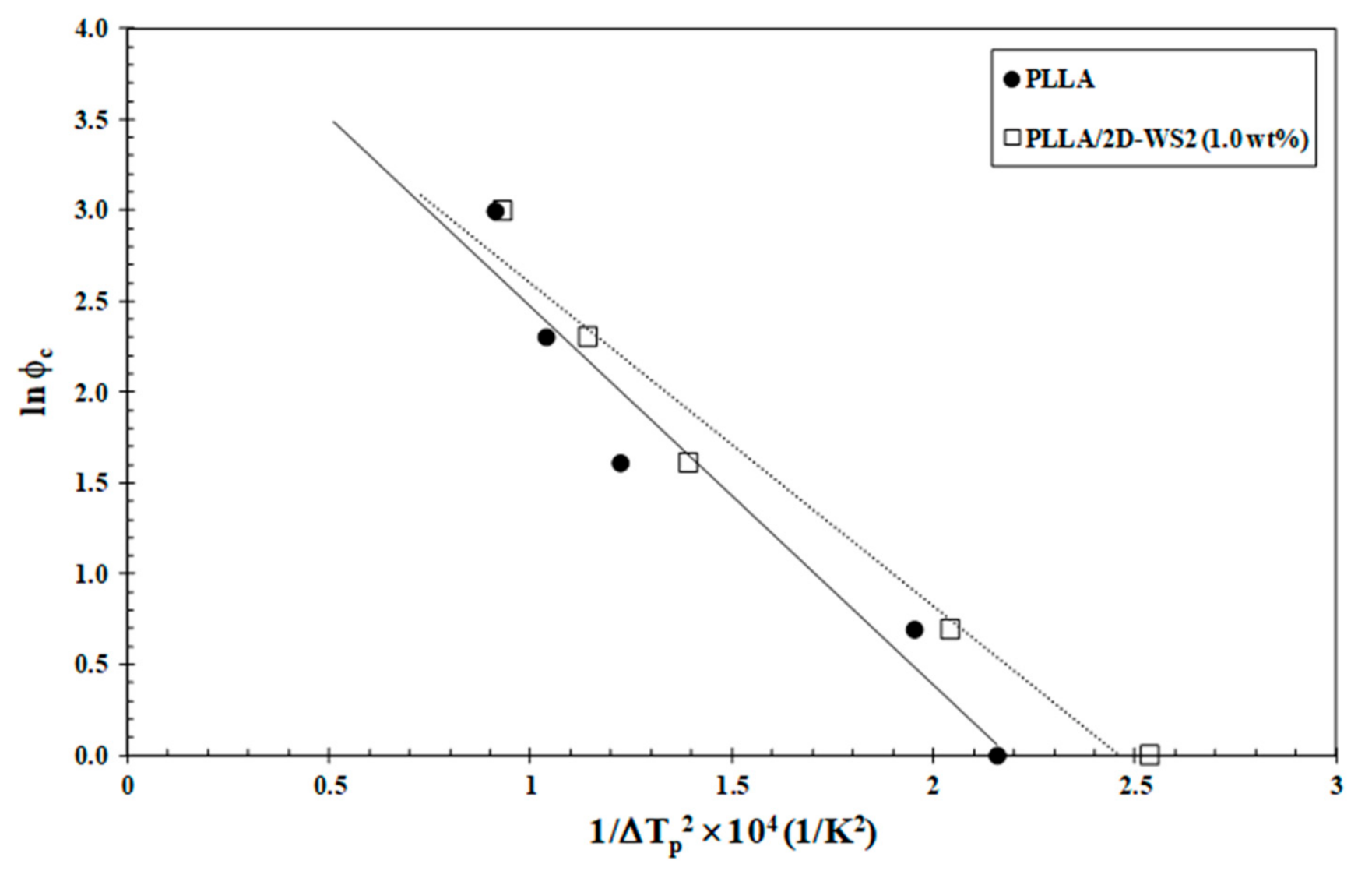
3.2. Non-Isothermal Cold-Crystallization
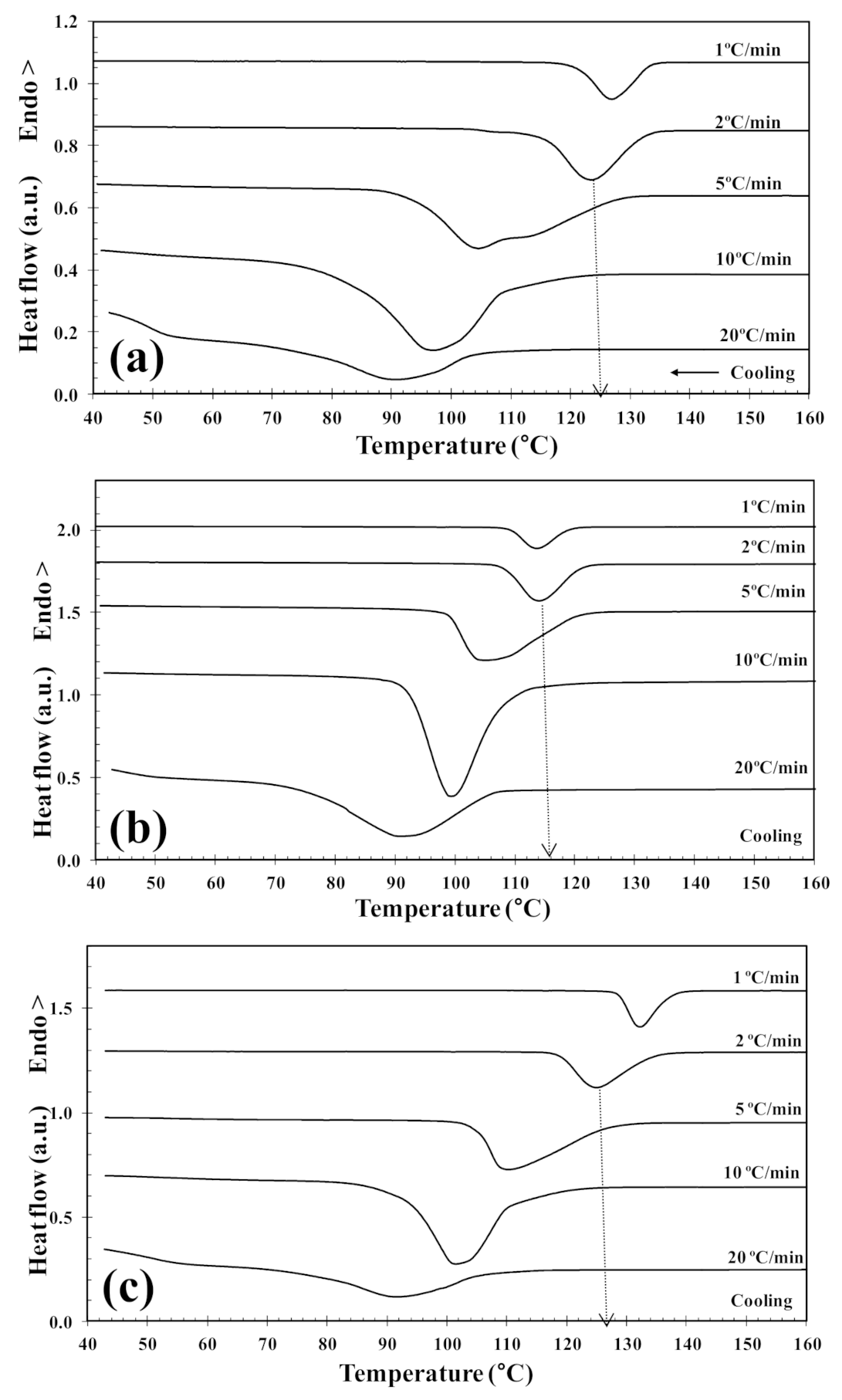
3.3. Non-Isothermal Melt-Crystallization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sinclair, R.G. The case for the polylactic acid as a commodity packaging plastic. Pure Appl. Chem. 1996, A33, 585–597. [Google Scholar] [CrossRef]
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic acid technology. Adv. Mater. 2000, 12, 1841–1846. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janrkor, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Saeidou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Wu, C.H.; Eder, G.; Janeschitz-Kriegl, H. Polymer crystallization dynamics, as reflected by differential scanning calorimetry. Part 2: Numerical simulations. Colloid Polym. Sci. 1993, 271, 1116–1128. [Google Scholar] [CrossRef]
- Burzic, I.; Pretschuh, C.; Kaineder, D.; Eder, G.; Smilek, J.; Masilko, J.; Kateryna, W. Impact modification of PLA using biobased biodegradable PHA biopolymers. Eur. Polym. J. 2019, 114, 32–38. [Google Scholar] [CrossRef]
- Ning, N.; Fu, S.; Zhang, W.; Chen, F.; Wang, K.; Deng, H.; Zhang, Q.; Fu, Q. Realizing the enhancement of interfacial interaction in semicrystalline polymer/filler composites via interfacial crystallization. Prog. Polym. Sci. 2012, 37, 1425–1455. [Google Scholar] [CrossRef]
- Jing, M.; Jiang, H.; Guo, Y.; Wu, Z.; Fu, Q. Transcrystallization of poly(l-lactic acid) on the surface of reduced graphene oxide fibers. RSC Adv. 2016, 6, 100090–100097. [Google Scholar] [CrossRef]
- Pluta, M.; Galeski, A.; Alexandre, M.; Paul, M.A.; Dubois, P. Polylactide/montmorillonite nanocomposites and microcomposites prepared by melt blending: Structure and some physical properties. J. Appl. Polym. Sci. 2002, 86, 1497–1506. [Google Scholar] [CrossRef]
- Safandowsk, M.; Rozanski, A.; Galeski, A. Plasticization of polylactide after solidification: An effectiveness and utilization for correct interpretation of thermal properties. Polymers 2020, 12, 561. [Google Scholar] [CrossRef]
- Jabbarzadeh, A. The origins of enhanced and retarded crystallization in nanocomposite polymers. Nanomaterials 2019, 9, 1472. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Ganesan, V.; Riggleman, R.A. Perspective: Outstanding theoretical questions in polymer-nanoparticle hybrids. J. Chem. Phys. 2017, 147, 020901. [Google Scholar] [CrossRef] [PubMed]
- Jabbarzadeh, A.; Halfina, B. Unravelling the effects of size, volume fraction and shape of nanoparticle additives on crystallization of nanocomposite polymers. Nanoscale Adv. 2019, 1, 4704–4721. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Maitra, U.; Waghmare, U.V. Extraordinary attributes of 2-dimensional MoS2 nanosheets. Chem. Phys. Lett. 2014, 609, 172–183. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Feng, X.; Yu, B.; Song, L.; Yeoh, G.H.; Hu, Y. Enhanced mechanical and barrier properties of polyurethane nanocomposite films with randomly distributed molybdenum disulfide nanosheets. Comp. Sci. Technol. 2016, 127, 142–148. [Google Scholar] [CrossRef]
- Feng, X.; Wang, B.; Wang, X.; Wen, P.; Cai, W.; Hu, Y.; Liew, K.M. Molybdenum disulfide nanosheets as barrier enhancing nanofillers in thermal decomposition of polypropylene composites. Chem. Eng. J. 2016, 295, 278–287. [Google Scholar] [CrossRef]
- Zhou, K.; Gao, R.; Gui, Z.; Hu, Y. The effective reinforcements of functionalized MoS2 nanosheets in polymer hybrid composites by sol-gel technique. Compos. Part A 2017, 94, 1–9. [Google Scholar] [CrossRef]
- Naffakh, M.; Díez-Pascual, A.M.; Marco, C.; Ellis, G.; Gómez-Fatou, M.A. Opportunities and challenges in the use of inorganic fullerene-like nanoparticles to produce advanced polymer nanocomposites. Prog. Polym. Sci. 2013, 38, 1163–1231. [Google Scholar] [CrossRef]
- Naffakh, M.; Díez-Pascual, A.M. Thermoplastic polymer nanocomposites based on inorganic fullerene-like nanoparticles and inorganic nanotubes. Inorganics 2014, 2, 291–312. [Google Scholar] [CrossRef]
- Lalwani, G.; Henslee, A.M.; Farshid, B.; Parmar, P.; Lin, L.; Qin, Y.-X.; Kasper, F.K.; Mikos, A.G.; Sitharaman, B. Tungsten disulfide nanotubes reinforced biodegradable polymers for bone tissue engineering. Acta Biomater. 2013, 9, 8365–8373. [Google Scholar] [CrossRef] [PubMed]
- Naffakh, M.; Marco, C.; Ellis, G.; Cohen, S.R.; Laikhtman, A.; Rapoport, L.; Zak, A. Novel poly(3-hydroxybutyrate) nanocomposites containing WS2 inorganic nanotubes with improved thermal, mechanical and tribological properties. Mater. Chem. Phys. 2014, 147, 273–284. [Google Scholar] [CrossRef]
- Naffakh, M.; Díez-Pascual, A.M. Nanocomposite biomaterials based on poly(ether-ether-ketone) (PEEK) and WS2 inorganic nanotubes. J. Mater. Chem. B 2014, 2, 4509–4520. [Google Scholar] [CrossRef] [PubMed]
- Naffakh, M.; Marco, C.; Ellis, G. Development of novel melt-processable biopolymer nanocomposites based on poly(l-lactic acid) and WS2 inorganic nanotubes. Cryst. Eng. Comm. 2014, 16, 5062–5072. [Google Scholar] [CrossRef]
- Naffakh, M.; Díez-Pascual, A.M. WS2 inorganic nanotubes reinforced poly(l-lactic acid)/hydroxyapatite hybrid composite biomaterials. RSC Adv. 2015, 5, 65514–65525. [Google Scholar] [CrossRef]
- Naffakh, M.; Fernández, M.; Shuttleworth, P.S.; García, A.M.; Moreno, D.A. Nanocomposite materials with poly(l-lactic Acid) and transition-metal dichalcogenide nanosheets 2D-TMDCs WS2. Polymers 2020, 12, 2699. [Google Scholar] [CrossRef]
- Martínez, J.; Martínez de Sarasa Buchaca, M.; de la Cruz-Martínez, F.; Alonso-Moreno, C.; Sánchez-Barba, L.F.; Fernandez-Baeza, J.; Rodríguez, A.M.; Rodríguez-Diéguez, A.; Castro-Osma, J.A.; Otero, A.; et al. Versatile organoaluminium catalysts based on heteroscorpionate ligands for the preparation of polyesters. Dalton Trans. 2018, 47, 7471–7479. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Kolloid Z. Z. Polym. 1973, 251, 980–990. [Google Scholar] [CrossRef]
- Chen, P.; Liang, X.; Xu, Y.; Zhou, Y.; Nie, W. Enhanced thermal and mechanical properties of PLA/MoS2 nanocomposites synthesized via the in-situ ring-opening polymerization. Appl. Surf. Sci. 2018, 440, 1143–1149. [Google Scholar] [CrossRef]
- Hoffman, J.D.; Miller, R.L. Kinetics of crystallization from the melt and chain folding in polyethylene fractions revisited: Theory and experiment. Polymer 1997, 38, 3151–3212. [Google Scholar] [CrossRef]
- Ferry, J.D. Viscoelastic Property of Polymers, 2nd ed.; Wiley: New York, NY, USA, 1980. [Google Scholar]
- He, Y.; Fan, Z.Y.; Wei, J.; Li, S.M. Morphology and melt crystallization of poly(l-lactide) obtained by ring opening polymerization of L-lactide with zinc catalyst. Polym. Eng. Sci. 2006, 46, 1583–1589. [Google Scholar] [CrossRef]
- Chen, H.C.; Chen, J.; Shao, L.; Yang, J.H.; Huang, T.; Zhang, N.; Wang, Y. Comparative study of poly(l-lactide) nanocomposites with organic montmorillonite and carbon nanotubes. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 183–196. [Google Scholar] [CrossRef]
- Liu, T.; Mo, Z.; Wang, S.; Zhang, H. Nonisothermal melt and cold crystallization kinetics of poly(aryl ether ether ketone ketone). Polym. Eng. Sci. 1997, 37, 568–575. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase changes 1. General theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. II. Transformation-time relations for random distribution of nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. III. Granulation, phase change, and microstructure. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetics of non-isothermal crystallization. Polymer 1971, 128, 150–158. [Google Scholar] [CrossRef]
- Naffakh, M.; Marco, C.; Gómez, M.A.; Jiménez, I. Unique nucleation activity of inorganic fullerene-like WS2 nanoparticles in polyphenylene sulfide nanocomposites: Isokinetic and isoconversional study of dynamic crystallization kinetics. J. Phys. Chem. B 2009, 113, 7107–7115. [Google Scholar] [CrossRef] [PubMed]
- Dobreva, A.; Gutzow, I. Activity of substrates in the catalyzed nucleation of glass-forming melts. I. Theory. J. Non-Cryst. Solids 1993, 162, 1–12. [Google Scholar] [CrossRef]
- Dobreva, A.; Gutzow, I. Activity of substrates in the catalyzed nucleation of glass-forming melts. II. Experimental evidence. J. Non-Cryst. Solids 1993, 162, 13–25. [Google Scholar] [CrossRef]
- Pan, P.; Liang, Z.; Cao, A.; Inoue, Y. Layered Metal Phosphonate Reinforced Poly(l-lactide) Composites with a Highly Enhanced Crystallization Rate. ACS Appl. Mater. Interfaces. 2009, 1, 402–411. [Google Scholar] [CrossRef] [PubMed]


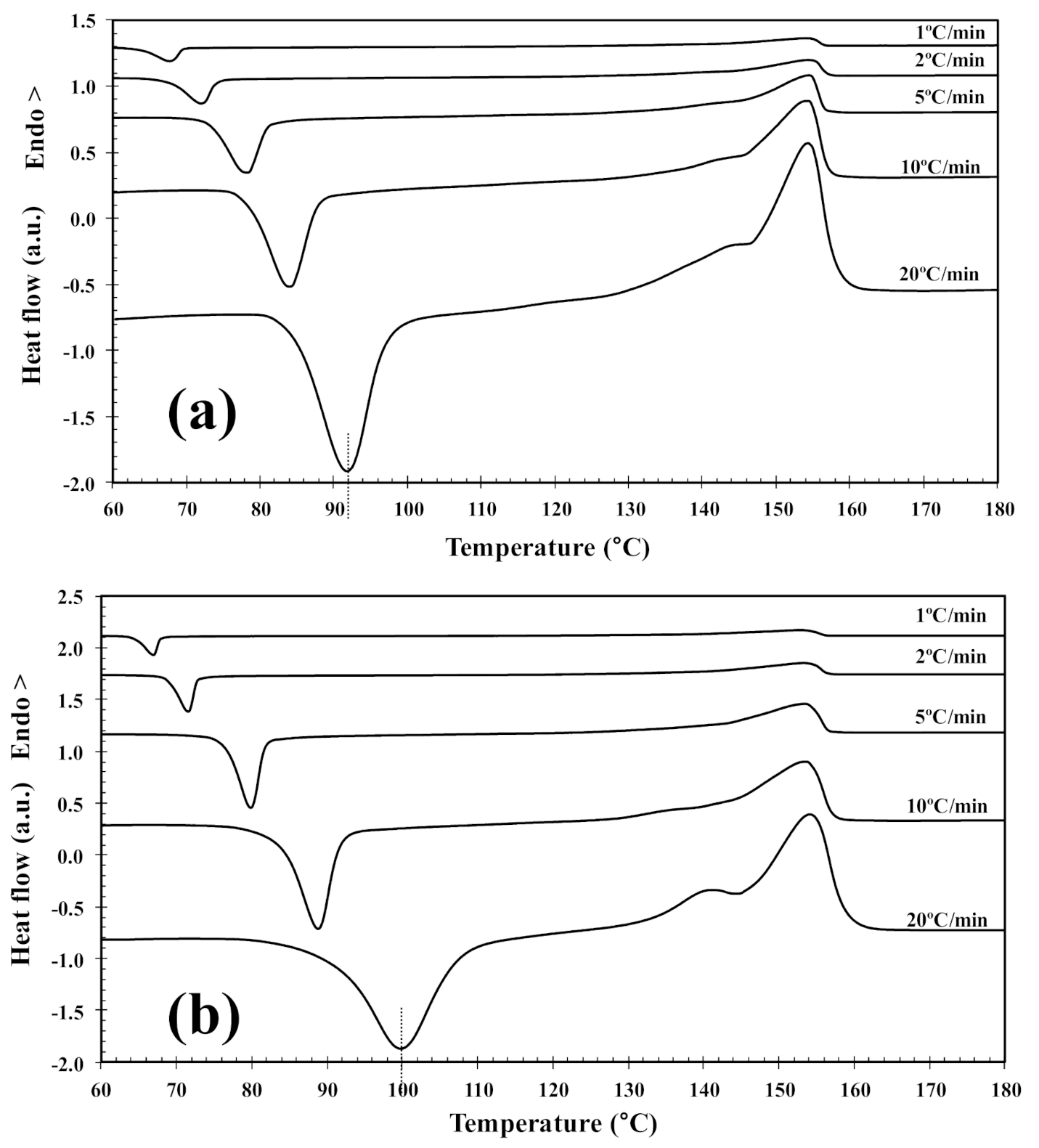
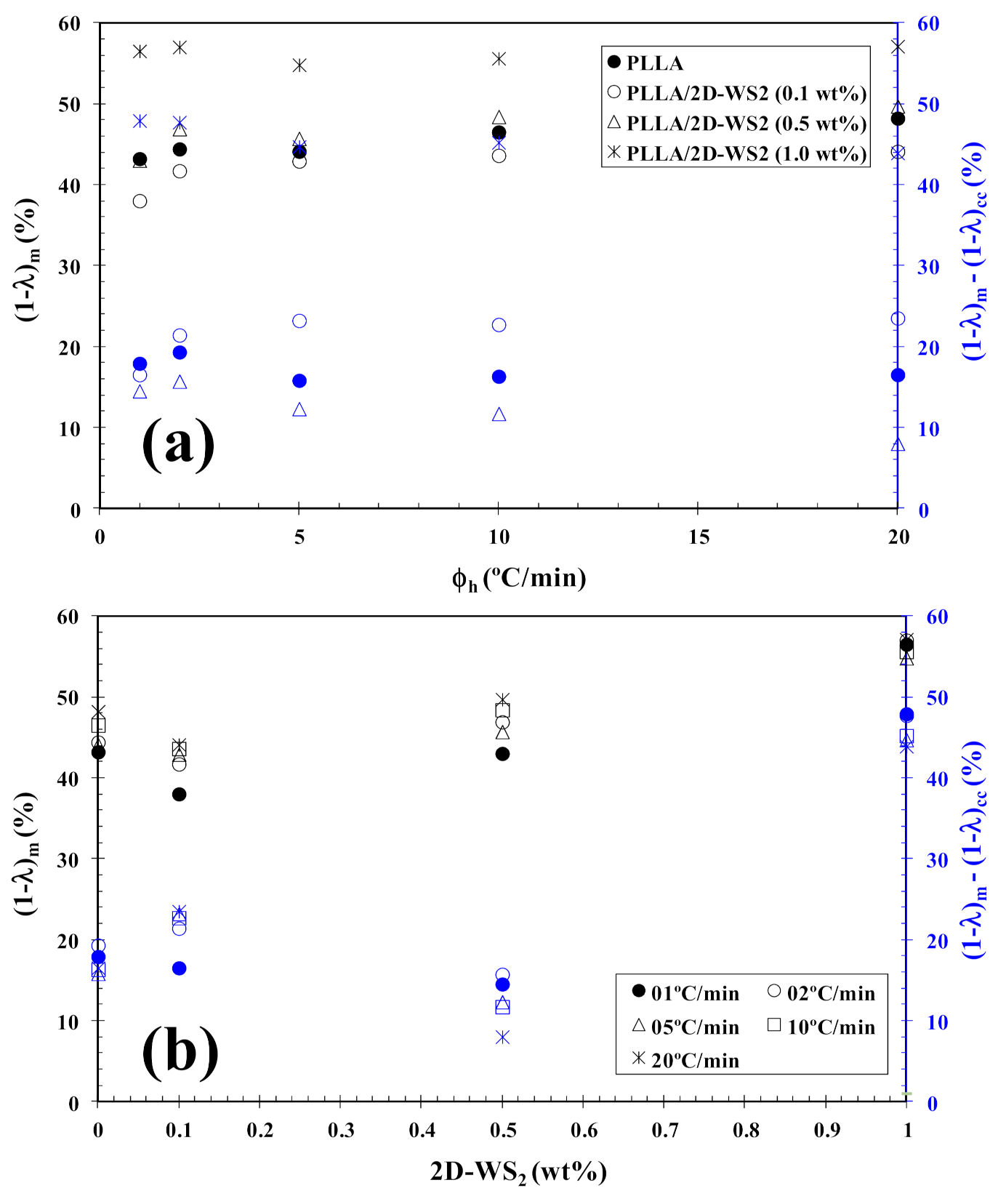




| 2D-WS2 (wt%) | ϕh (°C/min) | Tcc (°C) | (1−λ)cc (%) | Tm1 (°C) | Tm2 (°C) | (1−λ)m (%) | x a (%) | α a | f(T) a |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 67.8 | 25.3 | - | 154.0 | 43.2 | 10 | 1.30 | 4.18 | |
| 2 | 72.0 | 25.1 | 141.6 | 154.4 | 44.4 | 30 | 1.29 | 4.18 | |
| 0.0 | 5 | 78.3 | 28.3 | 143.7 | 154.5 | 44.1 | 50 | 1.30 | 4.27 |
| 10 | 84.0 | 30.2 | 143.8 | 154.1 | 46.5 | 70 | 1.29 | 4.30 | |
| 20 | 92.0 | 31.7 | 144.7 | 154.3 | 48.2 | 90 | 1.29 | 4.36 | |
| 1 | 68.9 | 21.5 | - | 149.9 | 38.0 | 10 | 1.31 | 4.07 | |
| 2 | 73.2 | 20.3 | - | 150.2 | 41.7 | 30 | 1.29 | 4.21 | |
| 0.1 | 5 | 80.5 | 19.7 | - | 150.8 | 42.9 | 50 | 1.29 | 4.30 |
| 10 | 87.0 | 20.9 | - | 151.5 | 43.6 | 70 | 1.30 | 4.30 | |
| 20 | 94.2 | 20.6 | - | 151.7 | 44.1 | 90 | 1.32 | 4.48 | |
| 1 | 67.0 | 28.5 | - | 152.9 | 43.0 | 10 | 1.34 | 4.34 | |
| 2 | 72.6 | 31.2 | - | 153.3 | 46.9 | 30 | 1.37 | 4.47 | |
| 0.5 | 5 | 79.9 | 33.4 | - | 153.5 | 45.7 | 50 | 1.38 | 4.54 |
| 10 | 88.8 | 36.7 | - | 153.5 | 48.4 | 70 | 1.39 | 4.60 | |
| 20 | 99.8 | 41.7 | 141.2 | 154.1 | 49.7 | 90 | 1.41 | 4.69 | |
| 1 | 71.9 | 8.6 | 164.4 | 167.8 | 56.5 | 10 | 1.29 | 4.14 | |
| 2 | 76.4 | 9.3 | 165.6 | 168.6 | 57.0 | 30 | 1.28 | 4.26 | |
| 1.0 | 5 | 83.0 | 10.1 | 166.1 | 169.0 | 54.8 | 50 | 1.26 | 4.31 |
| 10 | 89.3 | 10.4 | 166.4 | 169.1 | 55.6 | 70 | 1.26 | 4.35 | |
| 20 | 96.7 | 13.2 | 159.8 | 169.5 | 57.1 | 90 | 1.25 | 4.40 |
| 2D-WS2 (wt%) | ϕc (°C/min) | Tc (°C) | (1−λ)c (%) | Tcc (°C) | (1−λ)cc (%) | Tm1 (°C) | Tm2 (°C) | (1−λ)m (%) | x a (%) | α a | f(T) a |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 126.9 | 59.8 | - | - | - | 153.5 | 64.9 | 10 | −1.12 | 5.07 |
| 2 | 123.4 | 58.4 | - | - | - | 166.0 | 64.8 | 30 | −1.14 | 5.15 | |
| 5 | 104.5 | 50.2 | - | - | 167.4 | 171.9 | 58.0 | 50 | −1.14 | 5.21 | |
| 10 | 96.8 | 36.1 | 88.4 | 2.7 | - | 171.9 | 53.0 | 70 | −1.15 | 5.23 | |
| 20 | 90.2 | 9.0 | 94.2 | 24.2 | - | 172.1 | 50.2 | 90 | −1.16 | 5.34 | |
| 0.1 | 1 | 123.1 | 45.2 | - | - | - | 152.2 | 51.1 | 10 | −1.05 | 4.79 |
| 2 | 123.4 | 47.7 | - | - | - | 156.9 | 57.0 | 30 | −1.06 | 4.86 | |
| 5 | 120.8 | 48.6 | - | - | - | 160.5 | 58.6 | 50 | −1.07 | 4.91 | |
| 10 | 115.8 | 47.9 | - | - | - | 163.3 | 59.5 | 70 | −1.08 | 4.95 | |
| 20 | 99.5 | 37.7 | - | - | 165.0 | 170.1 | 56.0 | 90 | −1.08 | 5.01 | |
| 0.5 | 1 | 113.7 | 57.9 | - | - | 148.3 | 156.0 | 66.9 | 10 | −1.06 | 4.94 |
| 2 | 114.0 | 54.3 | - | - | 152.8 | 160.6 | 66.7 | 30 | −1.07 | 5.00 | |
| 5 | 105.0 | 51.7 | - | - | 158.4 | 165.3 | 66.2 | 50 | −1.08 | 5.04 | |
| 10 | 99.4 | 47.1 | - | - | 162.0 | 169.4 | 64.6 | 70 | −1.08 | 5.08 | |
| 20 | 90.6 | 19.8 | 82.5 | 14.7 | 154.8 | 170.4 | 57.7 | 90 | −1.10 | 5.15 | |
| 1 | 1 | 132.2 | 63.0 | - | - | - | 170.3 | 68.6 | 10 | −1.13 | 5.08 |
| 2 | 125.0 | 53.9 | - | - | - | 172.2 | 59.9 | 30 | −1.14 | 5.15 | |
| 5 | 110.2 | 44.2 | - | - | - | 172.5 | 50.9 | 50 | −1.15 | 5.20 | |
| 10 | 101.4 | 35.1 | - | - | - | 176.2 | 47.5 | 70 | −1.16 | 5.25 | |
| 20 | 91.3 | 10.0 | 99.7 | 17.4 | - | 175.6 | 42.6 | 90 | −1.17 | 5.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naffakh, M.; Rica, P.; Moya-Lopez, C.; Castro-Osma, J.A.; Alonso-Moreno, C.; Moreno, D.A. The Effect of WS2 Nanosheets on the Non-Isothermal Cold- and Melt-Crystallization Kinetics of Poly(l-lactic acid) Nanocomposites. Polymers 2021, 13, 2214. https://doi.org/10.3390/polym13132214
Naffakh M, Rica P, Moya-Lopez C, Castro-Osma JA, Alonso-Moreno C, Moreno DA. The Effect of WS2 Nanosheets on the Non-Isothermal Cold- and Melt-Crystallization Kinetics of Poly(l-lactic acid) Nanocomposites. Polymers. 2021; 13(13):2214. https://doi.org/10.3390/polym13132214
Chicago/Turabian StyleNaffakh, Mohammed, Pablo Rica, Carmen Moya-Lopez, José Antonio Castro-Osma, Carlos Alonso-Moreno, and Diego A. Moreno. 2021. "The Effect of WS2 Nanosheets on the Non-Isothermal Cold- and Melt-Crystallization Kinetics of Poly(l-lactic acid) Nanocomposites" Polymers 13, no. 13: 2214. https://doi.org/10.3390/polym13132214
APA StyleNaffakh, M., Rica, P., Moya-Lopez, C., Castro-Osma, J. A., Alonso-Moreno, C., & Moreno, D. A. (2021). The Effect of WS2 Nanosheets on the Non-Isothermal Cold- and Melt-Crystallization Kinetics of Poly(l-lactic acid) Nanocomposites. Polymers, 13(13), 2214. https://doi.org/10.3390/polym13132214