Synthesis, Characterization, and Evaluation of Nanoparticles Loading Adriamycin Based on 2-Hydroxypropyltrimethyl Ammonium Chloride Chitosan Grafting Folic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanoparticles
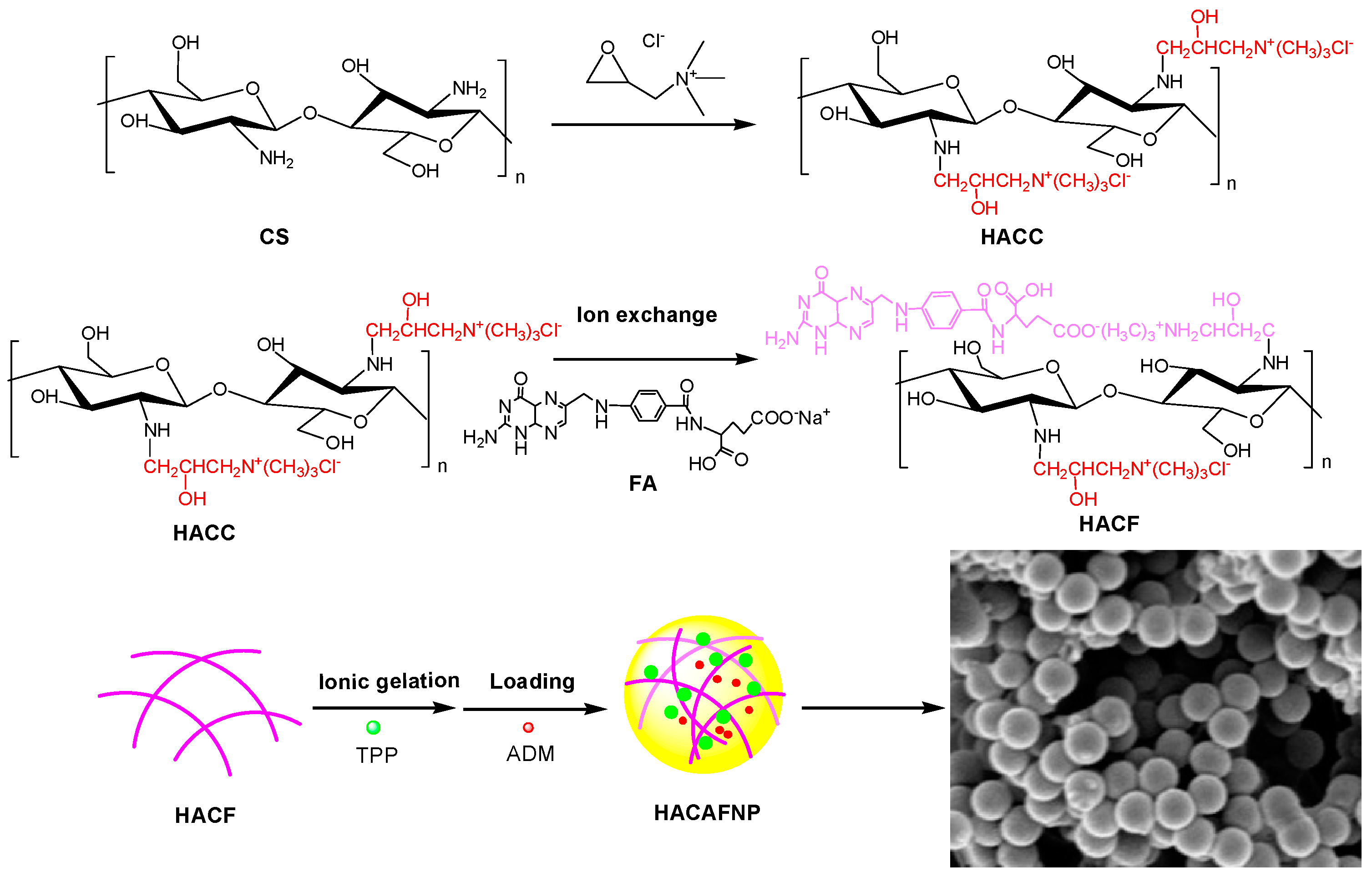
2.2.1. Preparation of Chitosan Derivatives
2.2.2. Preparation of Nanoparticles via HACC and HACF
Preparation of Nanoparticles via HACC
Preparation of Nanoparticles via HACF
2.3. Characterization of Chitosan Derivatives and Nanoparticles
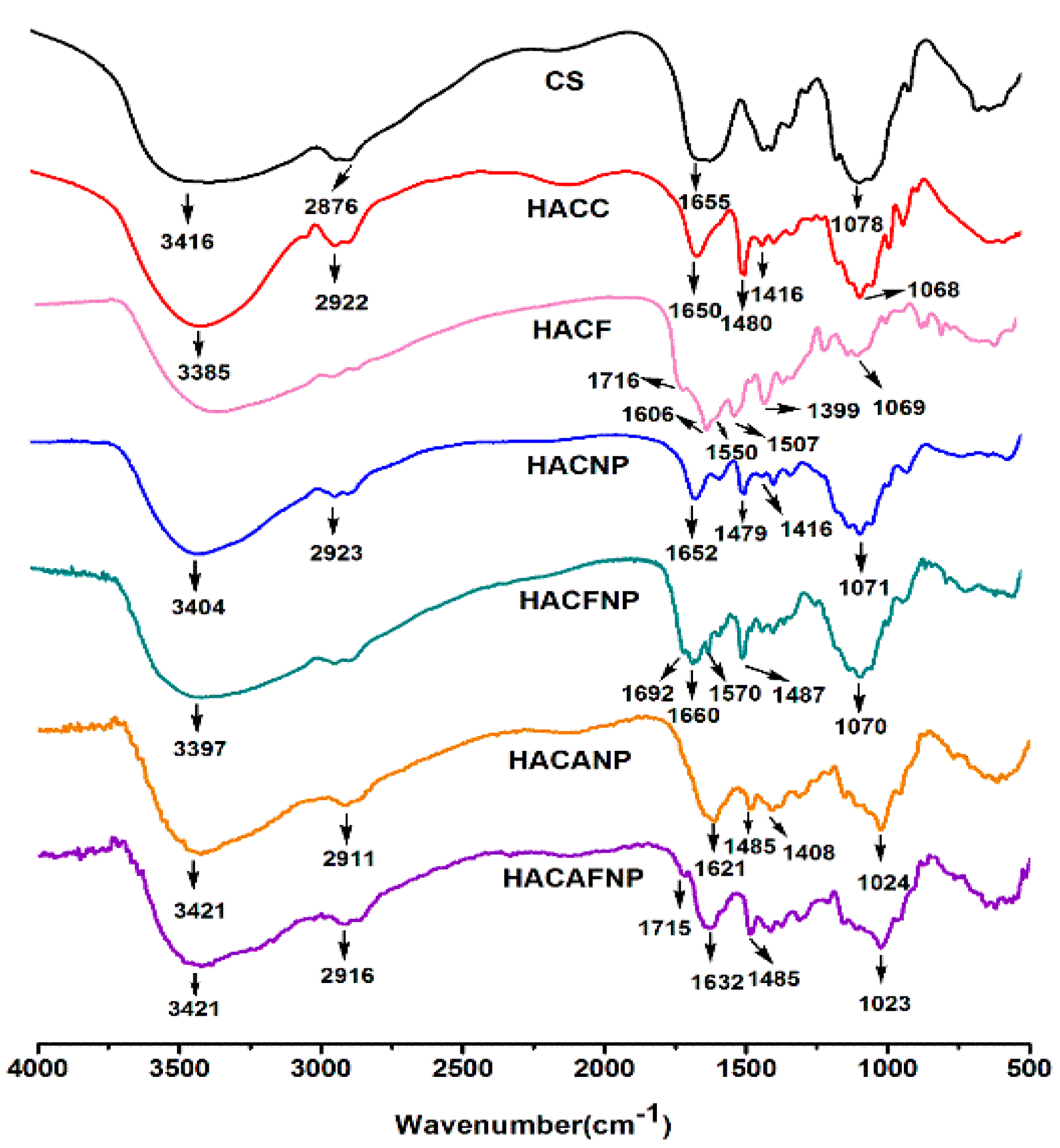
2.3.1. Fourier-Transform Infrared Spectroscopy
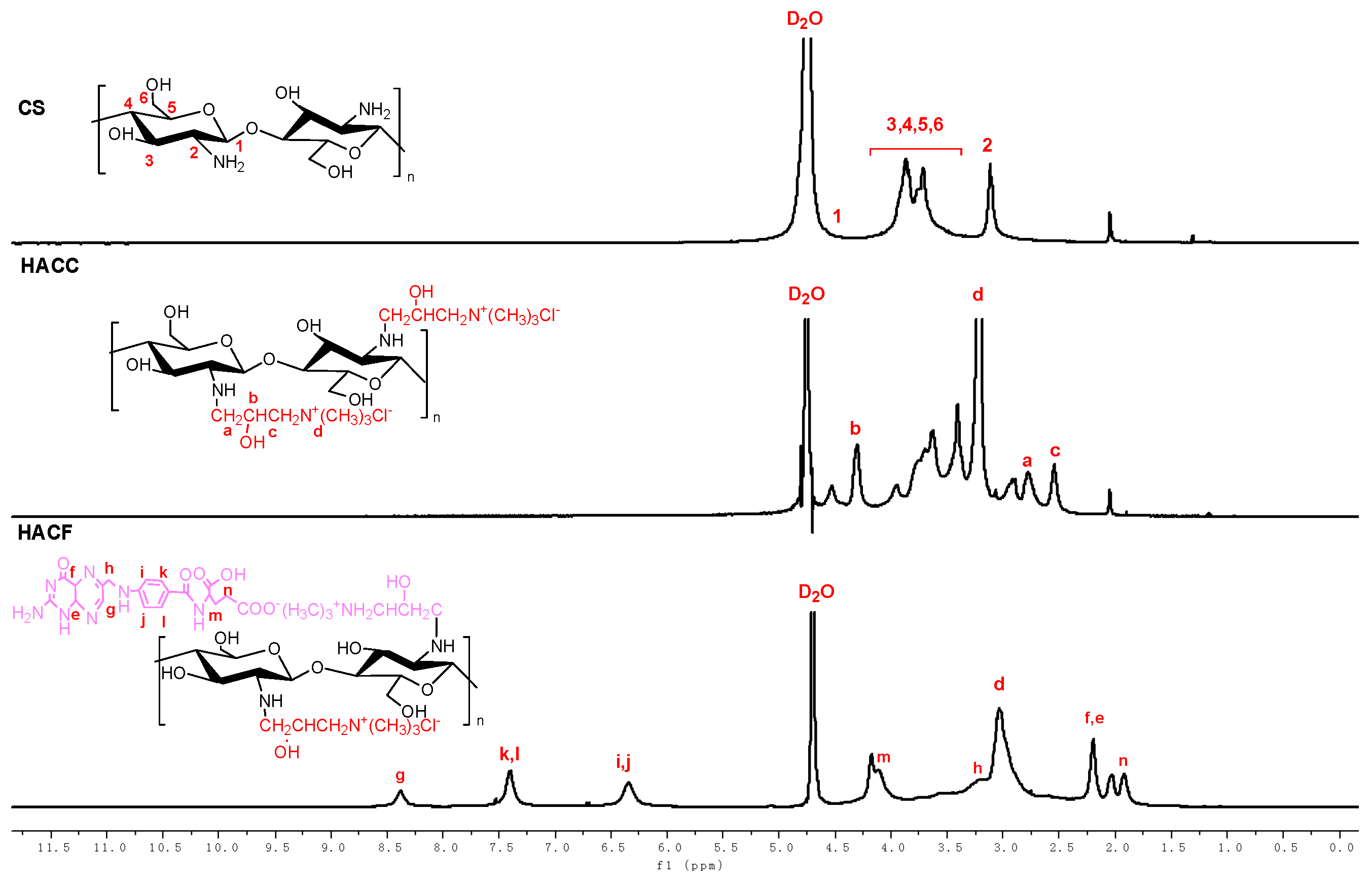
2.3.2. H Nuclear Magnetic Resonance Spectroscopy of Chitosan Derivatives
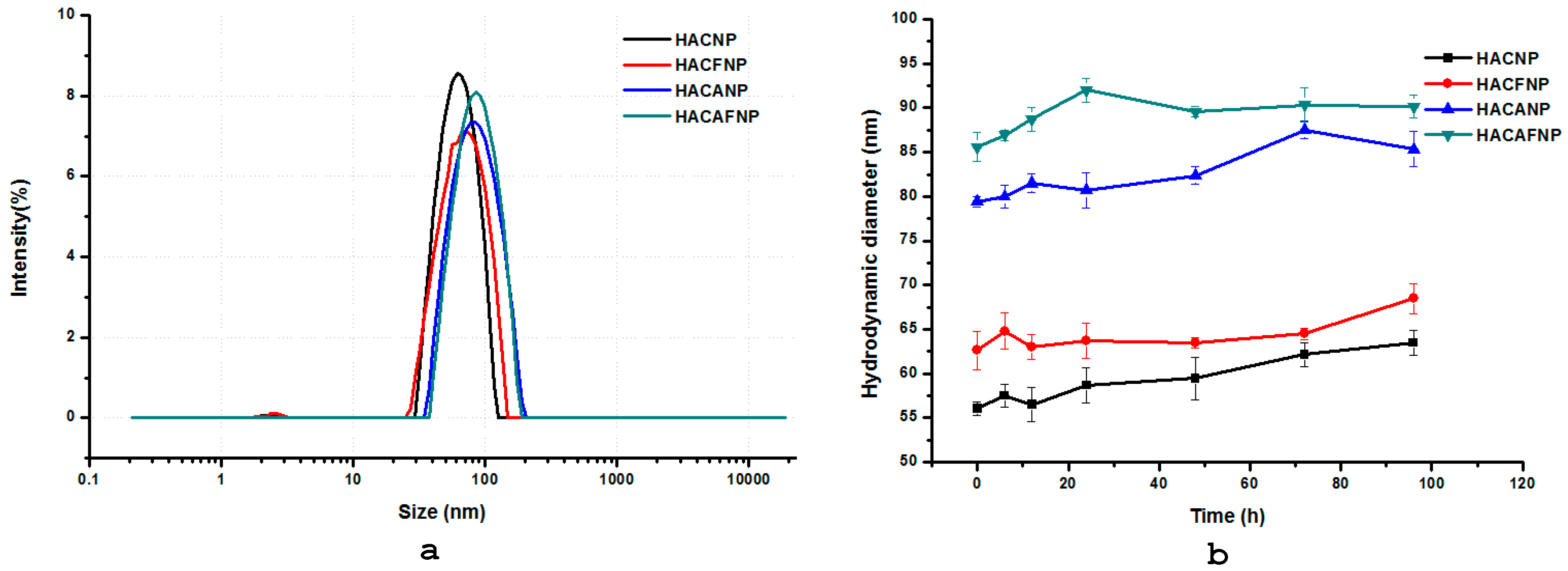
2.3.3. Hydrodynamic Diameter (nm), Zeta Potential (mV), and Nanoparticle Stability
2.3.4. Morphology
2.4. Entrapped Efficiency and Drug Loading of Nanoparticles
2.5. Swelling Degree
2.6. Evaluation of Sustained Release Performance
2.7. Antioxidant Assays
2.7.1. Superoxide-Radical Scavenging Activity Assay
2.7.2. DPPH Radical Scavenging Ability Assay
2.8. Cytotoxicity Assay
2.9. Statistical Analysis
3. Results and Discussions
3.1. Characterization of Nanoparticles
3.1.1. FTIR Spectra
3.1.2. H NMR Spectra and DS of Chitosan Derivatives
3.1.3. Hydrodynamic Diameter (nm), Zeta Potential (mV), and Nanoparticle Stability
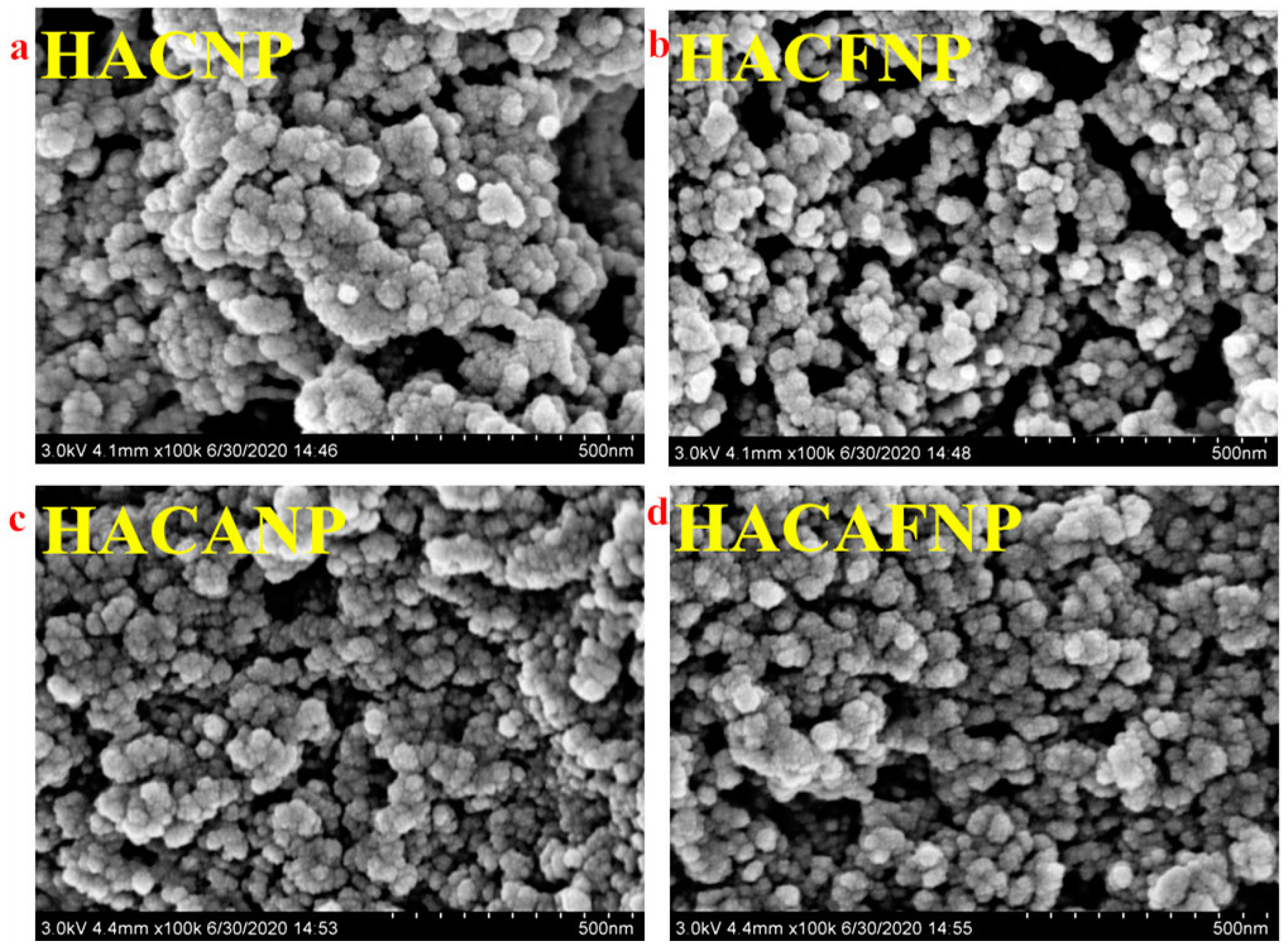
3.1.4. Morphology Analysis
3.2. Entrapped Efficiency and Drug Loading Analysis
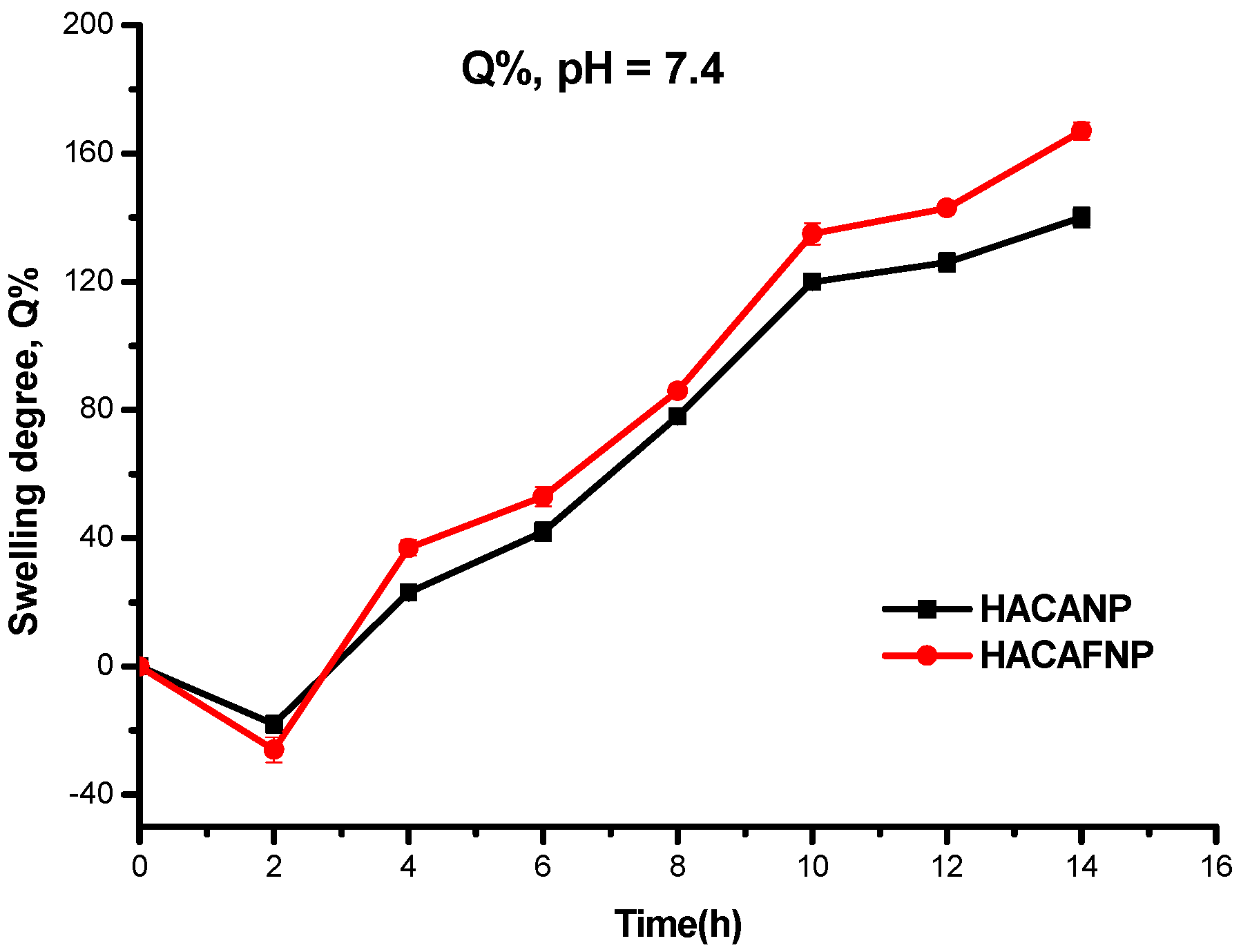
3.3. Swelling Degree Analysis
3.4. In Vitro Release of Chitosan Nanoparticles
3.5. Antioxidant Activity Analysis
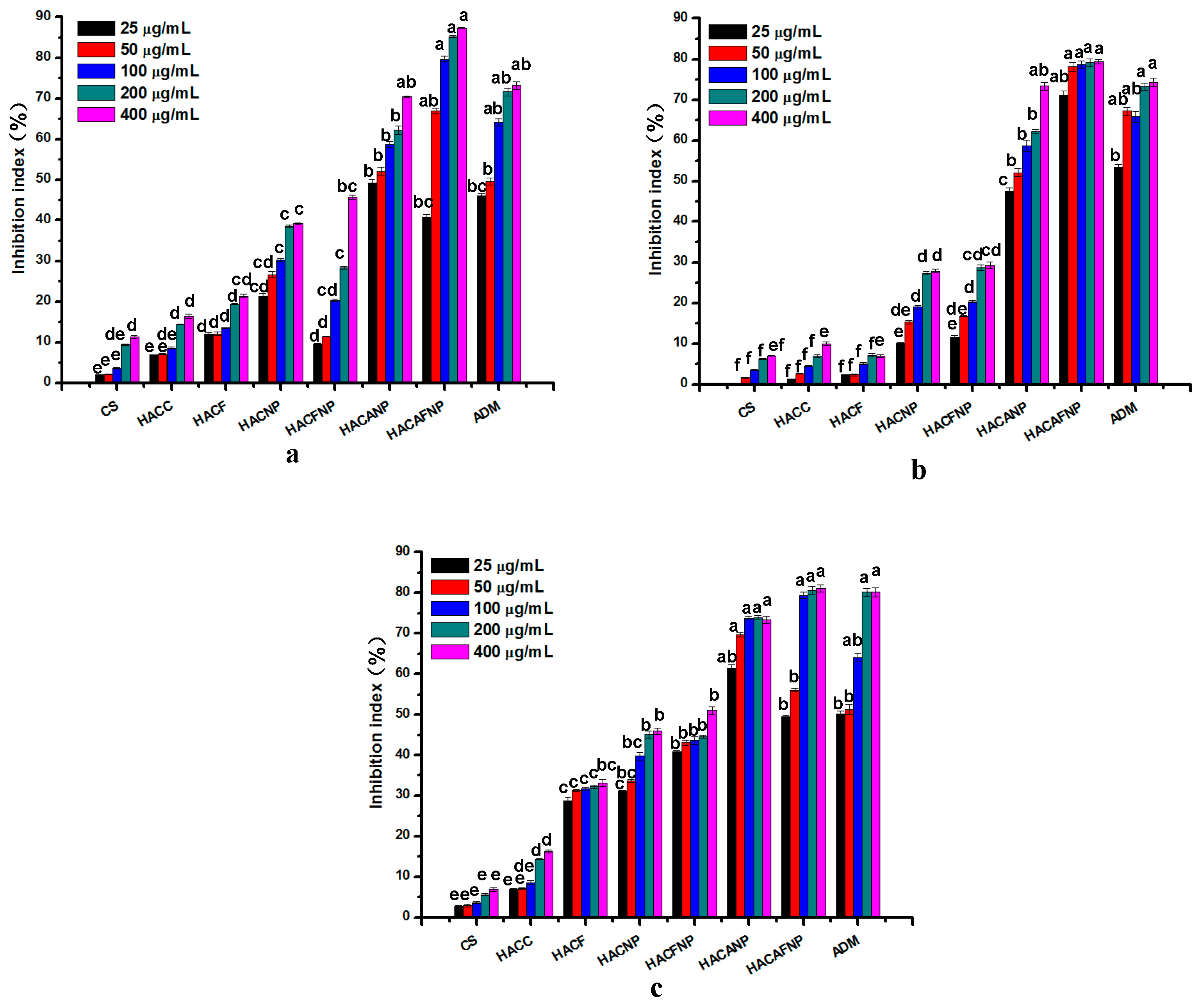
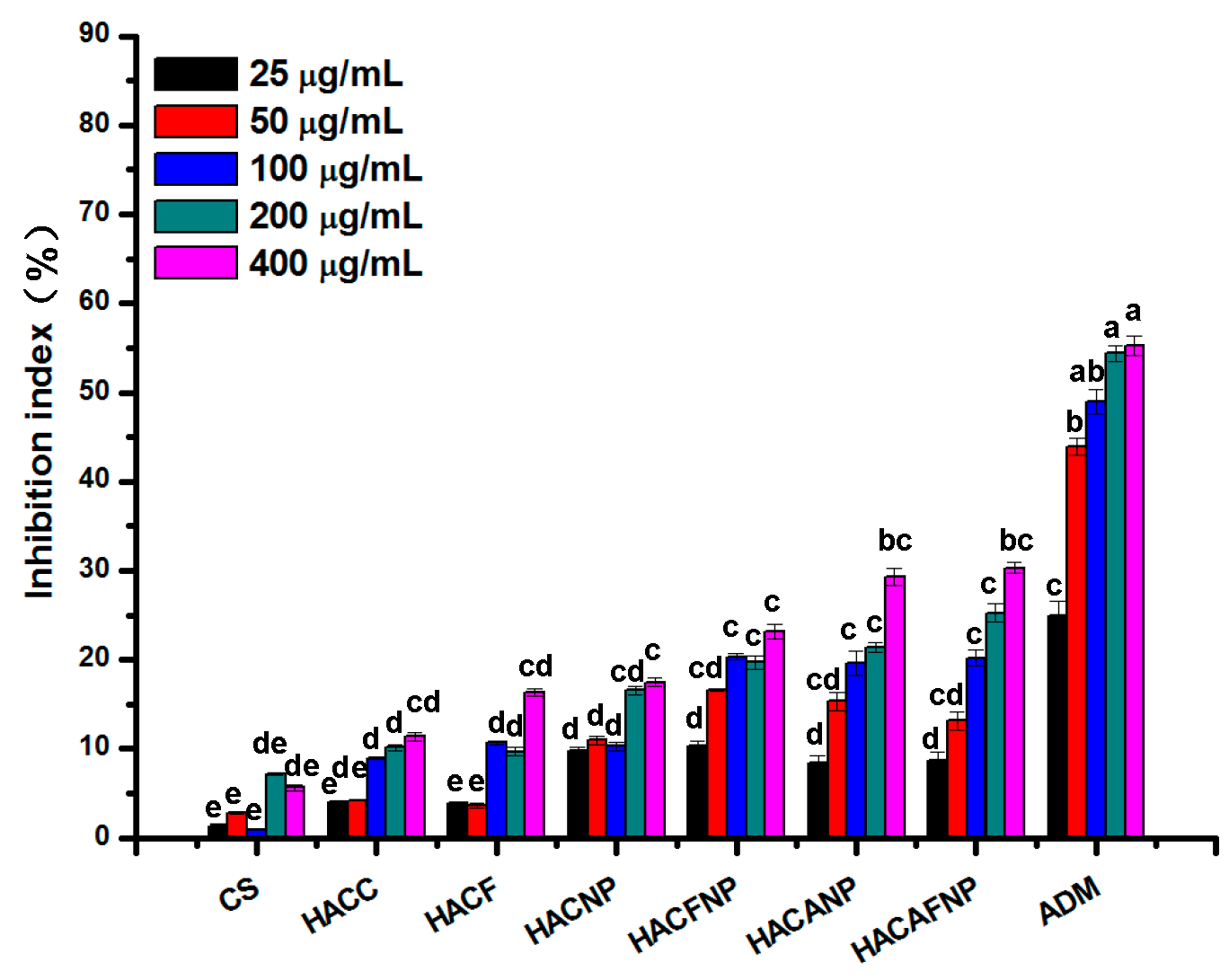
3.6. Cytotoxicity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mensah, K.B.; Mensah, A.B.B. Cancer control in Ghana: A narrative review in global context. Heliyon 2020, 6, e04564. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Li, M.; Jing, B.; An, M.; Yu, C.; Pinnock, C.B.; Zhu, Y.; Lam, M.T.; Liu, H. Long-Circulating Amphiphilic Doxorubicin for Tumor Mitochondria-Specific Targeting. ACS Appl. Mater. Interfaces 2018, 10, 43482–43492. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Pi, L.; Wen, H.; Yu, H.; Yang, X. Evaluation of novel magnetic targeting microspheres loading adriamycin based on carboxymethyl chitosan. J. Drug Deliv. Sci. Technol. 2020, 55, 101388. [Google Scholar] [CrossRef]
- He, S.; Li, A.; Zhang, W.; Zhang, L.; Liu, Y.; Li, K.; Qin, X. An integrated transcriptomics and network pharmacology approach to exploring the mechanism of adriamycin-induced kidney injury. Chem. Interact. 2020, 325, 109096. [Google Scholar] [CrossRef]
- Bhoopathy, S.; Inbakandan, D.; Rajendran, T.; Chandrasekaran, K.; Kasilingam, R.; Gopal, D. Curcumin loaded chitosan nanoparticles fortify shrimp feed pellets with enhanced antioxidant activity. Mater. Sci. Eng. C 2021, 120, 111737. [Google Scholar] [CrossRef]
- Xu, T.; Huang, C.; Qi, X.-T.; Yang, X.-C.; Zhang, N.; Cao, J.; Wang, C.; Zhu, H.; Yang, B.; He, Q.-J.; et al. 2-Bromopalmitate sensitizes osteosarcoma cells to adriamycin-induced apoptosis via the modulation of CHOP. Eur. J. Pharmacol. 2019, 844, 204–215. [Google Scholar] [CrossRef]
- Hu, W.; Xu, Z.; Zhu, S.; Sun, W.; Wang, X.; Tan, C.; Zhang, Y.; Zhang, G.; Xu, Y.; Tang, J. Small extracellular vesicle-mediated Hsp70 intercellular delivery enhances breast cancer adriamycin resistance. Free Radic. Biol. Med. 2021, 164, 85–95. [Google Scholar] [CrossRef]
- Pan, J.; Miao, D.; Chen, L. Germacrone reverses adriamycin resistance in human chronic myelogenous leukemia K562/ADM cells by suppressing MDR1 gene/P-glycoprotein expression. Chem. Biol. Interact. 2018, 288, 32–37. [Google Scholar] [CrossRef]
- Marangon, C.A.; Martins, V.; Ling, M.H.; Melo, C.C.; Plepis, A.; Meyer, R.L.; Nitschke, M. Combination of Rhamnolipid and Chitosan in Nanoparticles Boosts Their Antimicrobial Efficacy. ACS Appl. Mater. Interfaces 2020, 12, 5488–5499. [Google Scholar] [CrossRef] [PubMed]
- Oksal, E.; Pangestika, I.; Muhammad, T.S.T.; Mohamad, H.; Amir, H.; Kassim, M.N.I.; Andriani, Y. In vitro and in vivo studies of nanoparticles of chitosan-Pandanus tectorius fruit extract as new alternative treatment for hypercholesterolemia via Scavenger Receptor Class B type 1 pathway. Saudi Pharm. J. 2020, 28, 1263–1275. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Tan, W.; Zhang, J.; Wei, L.; Chen, Y.; Li, Q.; Dong, F.; Guo, Z. Synthesis, Characterization, and Antifungal Property of Hydroxypropyltrimethyl Ammonium Chitosan Halogenated Acetates. Mar. Drugs 2018, 16, 315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Sun, X.; Chen, Y.; Mi, Y.; Tan, W.; Miao, Q.; Li, Q.; Dong, F.; Guo, Z. Preparation of 2,6-diurea-chitosan oligosaccharide derivatives for efficient antifungal and antioxidant activities. Carbohydr. Polym. 2020, 234, 115903. [Google Scholar] [CrossRef]
- Deng, L.; Taxipalati, M.; Zhang, A.; Que, F.; Wei, H.; Feng, F.; Zhang, H. Electrospun Chitosan/Poly(ethylene oxide)/Lauric Arginate Nanofibrous Film with Enhanced Antimicrobial Activity. J. Agric. Food Chem. 2018, 66, 6219–6226. [Google Scholar] [CrossRef] [PubMed]
- Alinejad, Y.; Bitar, C.M.E.; Villegas, K.M.; Perignon, S.; Hoesli, C.A.; Lerouge, S. Chitosan Microbeads Produced by One-Step Scalable Stirred Emulsification: A Promising Process for Cell Therapy Applications. ACS Biomater. Sci. Eng. 2019, 6, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Zhang, J.; Chen, Y.; Sun, X.; Tan, W.; Li, Q.; Guo, Z. New synthetic chitosan derivatives bearing benzenoid/heterocyclic moieties with enhanced antioxidant and antifungal activities. Carbohydr. Polym. 2020, 249, 116847. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, J.; Zhao, X.; Li, Q.; Dong, F.; Guo, Z. Preparation and physicochemical properties of antioxidant chitosan ascorbate/methylcellulose composite films. Int. J. Biol. Macromol. 2020, 146, 53–61. [Google Scholar] [CrossRef]
- Zhao, J.; Li, J.; Jiang, Z.; Tong, R.; Duan, X.; Bai, L.; Shi, J. Chitosan, N,N,N-trimethyl chitosan (TMC) and 2-hydroxypropyltrimethyl ammonium chloride chitosan (HTCC): The potential immune adjuvants and nano carriers. Int. J. Biol. Macromol. 2020, 154, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Iurciuc-Tincu, C.-E.; Atanase, L.I.; Ochiuz, L.; Jérôme, C.; Sol, V.; Martin, P.; Popa, M. Curcumin-loaded polysaccharides-based complex particles obtained by polyelectrolyte complexation and ionic gelation. I-Particles obtaining and characterization. Int. J. Biol. Macromol. 2020, 147, 629–642. [Google Scholar] [CrossRef]
- Rata, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Bacaita, S.E.; Mihalache, C.; Daraba, O.-M.; Gherghel, D.; Popa, M. “In vitro” behaviour of aptamer-functionalized polymeric nanocapsules loaded with 5-fluorouracil for targeted therapy. Mater. Sci. Eng. C 2019, 103, 109828. [Google Scholar] [CrossRef]
- Matos, B.N.; Pereira, M.N.; Bravo, M.D.O.; Cunha-Filho, M.; Saldanha-Araújo, F.; Gratieri, T.; Gelfuso, G.M. Chitosan nanoparticles loading oxaliplatin as a mucoadhesive topical treatment of oral tumors: Iontophoresis further enhances drug delivery ex vivo. Int. J. Biol. Macromol. 2020, 154, 1265–1275. [Google Scholar] [CrossRef]
- Chen, Y.; Mi, Y.; Li, Q.; Dong, F.; Guo, Z. Synthesis of Schiff bases modified inulin derivatives for potential antifungal and antioxidant applications. Int. J. Biol. Macromol. 2020, 143, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Cartaya, A.; Lutz, H.; Maiocchi, S.; Nalesnik, M.; Bahnson, E. Delivery of Cinnamic Aldehyde Antioxidant Response Activating nanoParticles (ARAPas) for Vascular Applications. Antioxidants 2021, 10, 709. [Google Scholar] [CrossRef] [PubMed]
- Koseki, K.; Maekawa, Y.; Bito, T.; Yabuta, Y.; Watanabe, F. High-dose folic acid supplementation results in significant accumulation of unmetabolized homocysteine, leading to severe oxidative stress in Caenorhabditis elegans. Redox Biol. 2020, 37, 101724. [Google Scholar] [CrossRef]
- Xu, D.; Zuo, J.; Fang, Y.; Yan, Z.; Shi, J.; Gao, L.; Wang, Q.; Jiang, A. Effect of folic acid on the postharvest physiology of broccoli during storage. Food Chem. 2021, 339, 127981. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, Y.; Li, H.; Li, J.; Zhang, Y.; Liang, M.; Nie, J.; Wang, B.; Wang, X.; Huo, Y.; et al. Interaction of serum calcium and folic acid treatment on first stroke in hypertensive males. Clin. Nutr. 2021, 40, 2381–2388. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Kang, H.; Yang, W.; Sun, J.; Liu, C.; Cheng, G.; Rong, G.; Wang, X.; Wang, X.; Jin, Z.; et al. O -2′-Hydroxypropyltrimethyl ammonium chloride chitosan nanoparticles for the delivery of live Newcastle disease vaccine. Carbohydr. Polym. 2015, 130, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. Chitosan, hydroxypropyltrimethyl ammonium chloride chitosan and sulfated chitosan nanoparticles as adjuvants for inactivated Newcastle disease vaccine. Carbohydr. Polym. 2020, 229, 115423. [Google Scholar] [CrossRef]
- Zhou, J.; Zhai, Y.; Xu, J.; Zhou, T.; Cen, L. Microfluidic preparation of PLGA composite microspheres with mesoporous silica nanoparticles for finely manipulated drug release. Int. J. Pharm. 2021, 593, 120173. [Google Scholar] [CrossRef]
- Halder, S.; Ahmed, F.; Shuma, M.L.; Azad, M.; Kabir, E.R. Impact of drying on dissolution behavior of carvedilol-loaded sustained release solid dispersion: Development and characterization. Heliyon 2020, 6. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, W.; Wei, L.; Chen, Y.; Mi, Y.; Sun, X.; Li, Q.; Dong, F.; Guo, Z. Synthesis of urea-functionalized chitosan derivatives for potential antifungal and antioxidant applications. Carbohydr. Polym. 2019, 215, 108–118. [Google Scholar] [CrossRef]
- Yen, H.-J.; Young, Y.-A.; Tsai, T.-N.; Cheng, K.-M.; Chen, X.-A.; Chen, Y.-C.; Chen, C.-C.; Young, J.-J.; Hong, P.-d. Positively charged gold nanoparticles capped with folate quaternary chitosan: Synthesis, cytotoxicity, and uptake by cancer cells. Carbohydr. Polym. 2018, 183, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Sun, Y.; Chen, G.; Rong, G.; Kang, H.; Jin, Z.; Wang, X. Biological evaluation of N-2-hydroxypropyl trimethyl ammonium chloride chitosan as a carrier for the delivery of live Newcastle disease vaccine. Carbohydr. Polym. 2016, 149, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Scholzen, A.; Minigo, G.; David, C.; Apostolopoulos, V.; Mottram, P.L.; Plebanski, M. Pathogen recognition and development of particulate vaccines: Does size matter? Methods 2006, 40, 1–9. [Google Scholar] [CrossRef]
- Liu, C.; Fan, W.; Chen, X.; Liu, C.; Meng, X.; Park, H.J. Self-assembled nanoparticles based on linoleic-acid modified carboxymethyl-chitosan as carrier of adriamycin (ADR). Curr. Appl. Phys. 2007, 7, e125–e129. [Google Scholar] [CrossRef]
- Jing, Y.; Huang, J.; Yu, X. Maintenance of the antioxidant capacity of fresh-cut pineapple by procyanidin-grafted chitosan. Postharvest Biol. Technol. 2019, 154, 79–86. [Google Scholar] [CrossRef]
- Abdel-Hakeem, M.A.; Abdel-Haseb, O.M.; Abdel-Ghany, S.E.; Cevik, E.; Sabit, H. Doxorubicin loaded on chitosan-protamine nanoparticles triggers apoptosis via downregulating Bcl-2 in breast cancer cells. J. Drug Deliv. Sci. Technol. 2020, 55, 101423. [Google Scholar] [CrossRef]


| Compounds | Yields (%) | DS (%) |
|---|---|---|
| CS | - | - |
| HACC | 74.25 | 70.17 |
| HACF | 69.43 | 48.16 |
| Samples | Hydrodynamic Diameter (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| HACNP | 56.7 ± 0.74 | 0.21 ± 0.16 | +24.67 ± 0.34 |
| HACFNP | 62.6 ± 2.15 | 0.22 ± 0.06 | +23.54 ± 1.06 |
| HACANP | 79.4 ± 0.60 | 0.24 ± 0.09 | +23.83 ± 1.24 |
| HACAFNP | 85.6 ± 2.04 | 0.23 ± 0.14 | +21.06 ± 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, Y.; Zhang, J.; Zhang, L.; Li, Q.; Cheng, Y.; Guo, Z. Synthesis, Characterization, and Evaluation of Nanoparticles Loading Adriamycin Based on 2-Hydroxypropyltrimethyl Ammonium Chloride Chitosan Grafting Folic Acid. Polymers 2021, 13, 2229. https://doi.org/10.3390/polym13142229
Mi Y, Zhang J, Zhang L, Li Q, Cheng Y, Guo Z. Synthesis, Characterization, and Evaluation of Nanoparticles Loading Adriamycin Based on 2-Hydroxypropyltrimethyl Ammonium Chloride Chitosan Grafting Folic Acid. Polymers. 2021; 13(14):2229. https://doi.org/10.3390/polym13142229
Chicago/Turabian StyleMi, Yingqi, Jingjing Zhang, Lulin Zhang, Qing Li, Yuanzheng Cheng, and Zhanyong Guo. 2021. "Synthesis, Characterization, and Evaluation of Nanoparticles Loading Adriamycin Based on 2-Hydroxypropyltrimethyl Ammonium Chloride Chitosan Grafting Folic Acid" Polymers 13, no. 14: 2229. https://doi.org/10.3390/polym13142229







