Biophysico-Chemical Properties of Alginate Oligomers Obtained by Acid and Oxidation Depolymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Alginate Depolymerization
2.2.1. Preparation of Alginate Oligosaccharides by Acid Hydrolysis
2.2.2. Preparation of Alginate Oligosaccharides by Oxidation with Hydrogen Peroxide (H2O2)
2.3. Hydrolysis Yield
2.4. Reducing Sugar Assay and the Degree of Polymerization Calculation (DP)
2.5. Fourier Transform Infrared Spectrometry
2.6. Rheological Characterization of Alginate Oligosaccharides
2.7. Antioxidant Properties of Alginate and Alginate Oligosaccharides
2.7.1. Ferric Reducing Antioxidant Power (FRAP)
2.7.2. Free Radical Scavenging Activity (DPPH)
2.8. Antimicrobial Assay
2.9. Gel Preparation from Alginate Hydrolysates
2.10. Texture Profile Analysis (TPA) of Hydrolysed Alginate Gels
2.11. Statistical Data Analysis
3. Results and Discussion
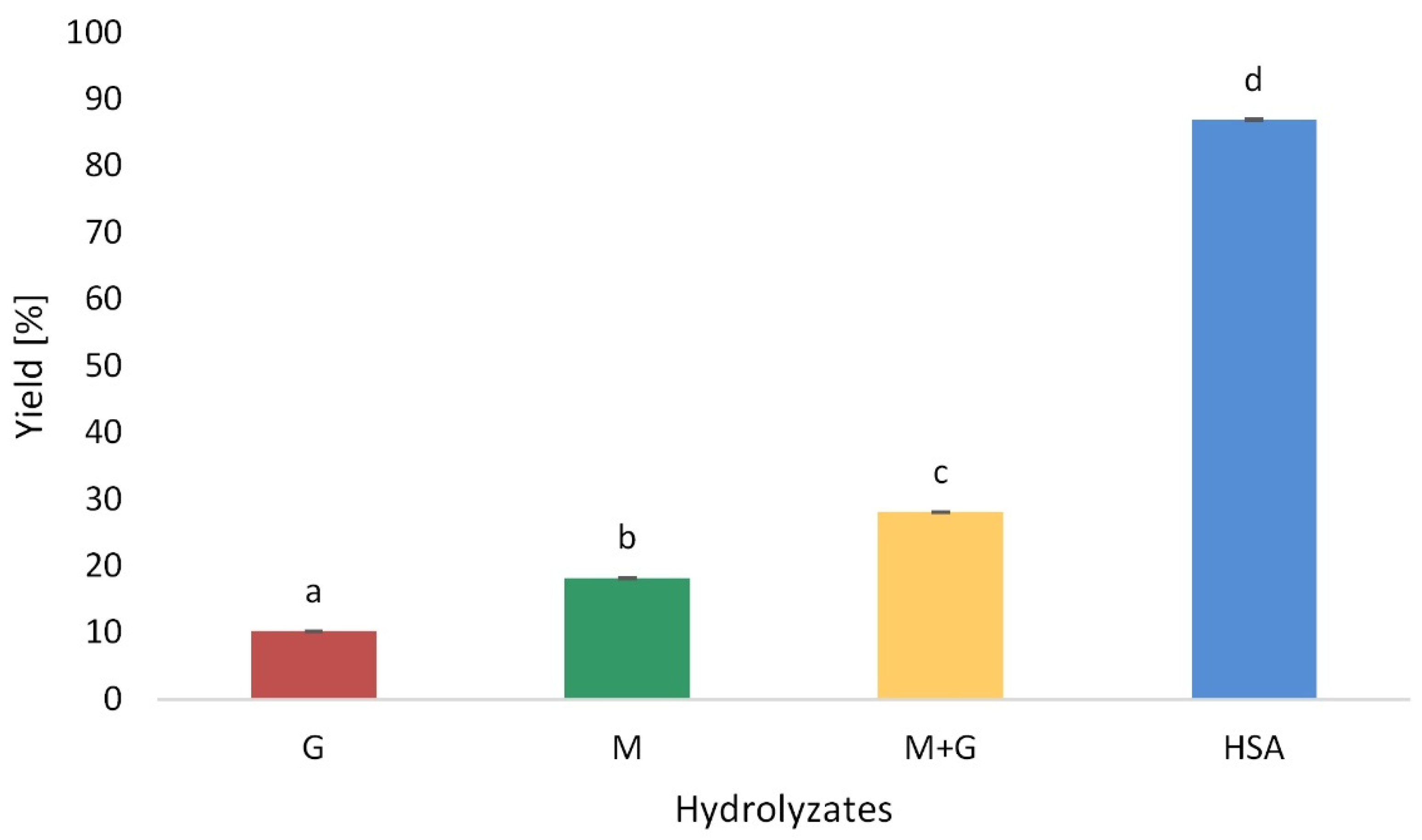
3.1. Hydrolysis Yield
3.2. Reducing Sugar Assay and the Degree of Polymerization Calculation (DP)
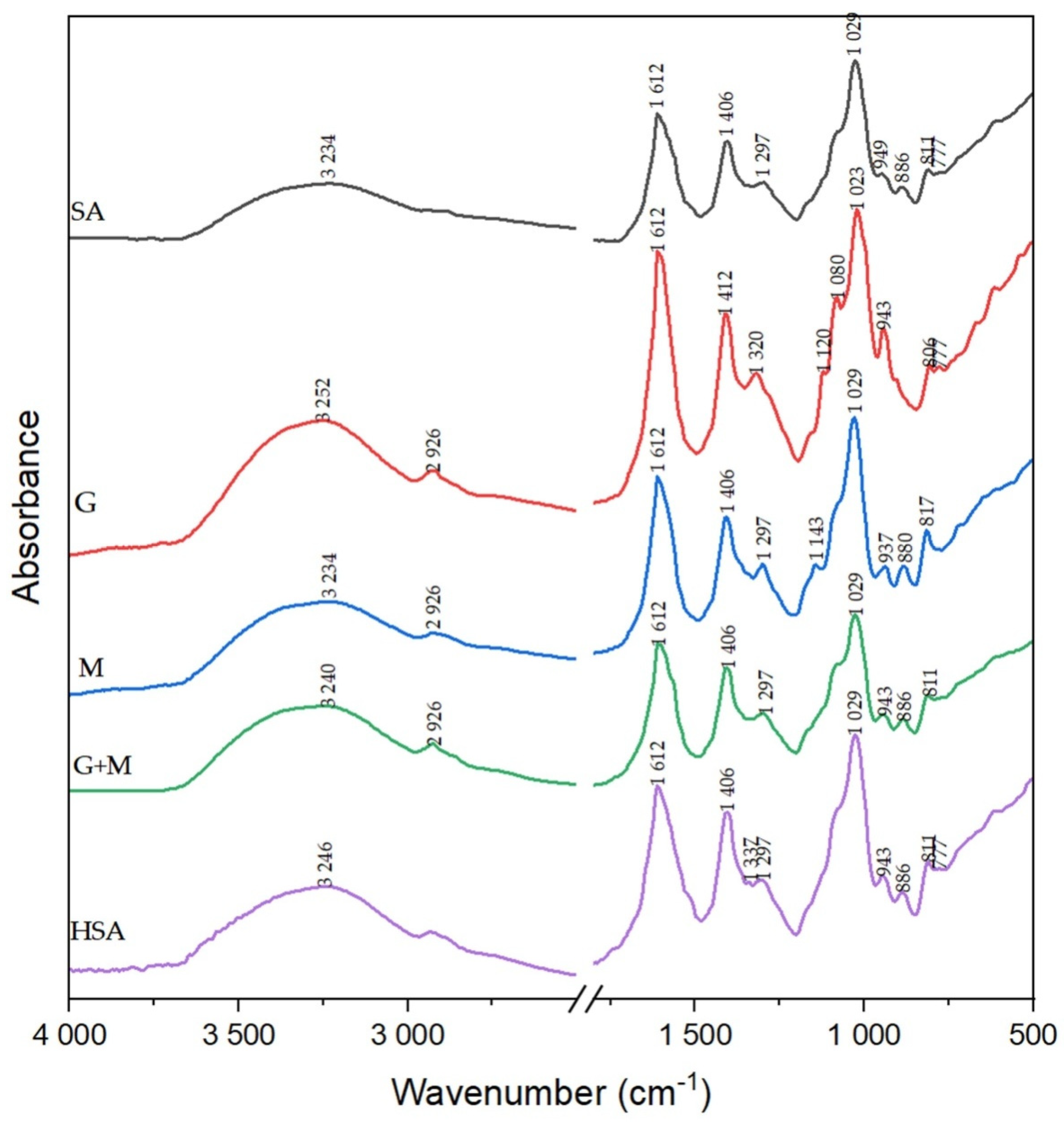
3.3. FTIR
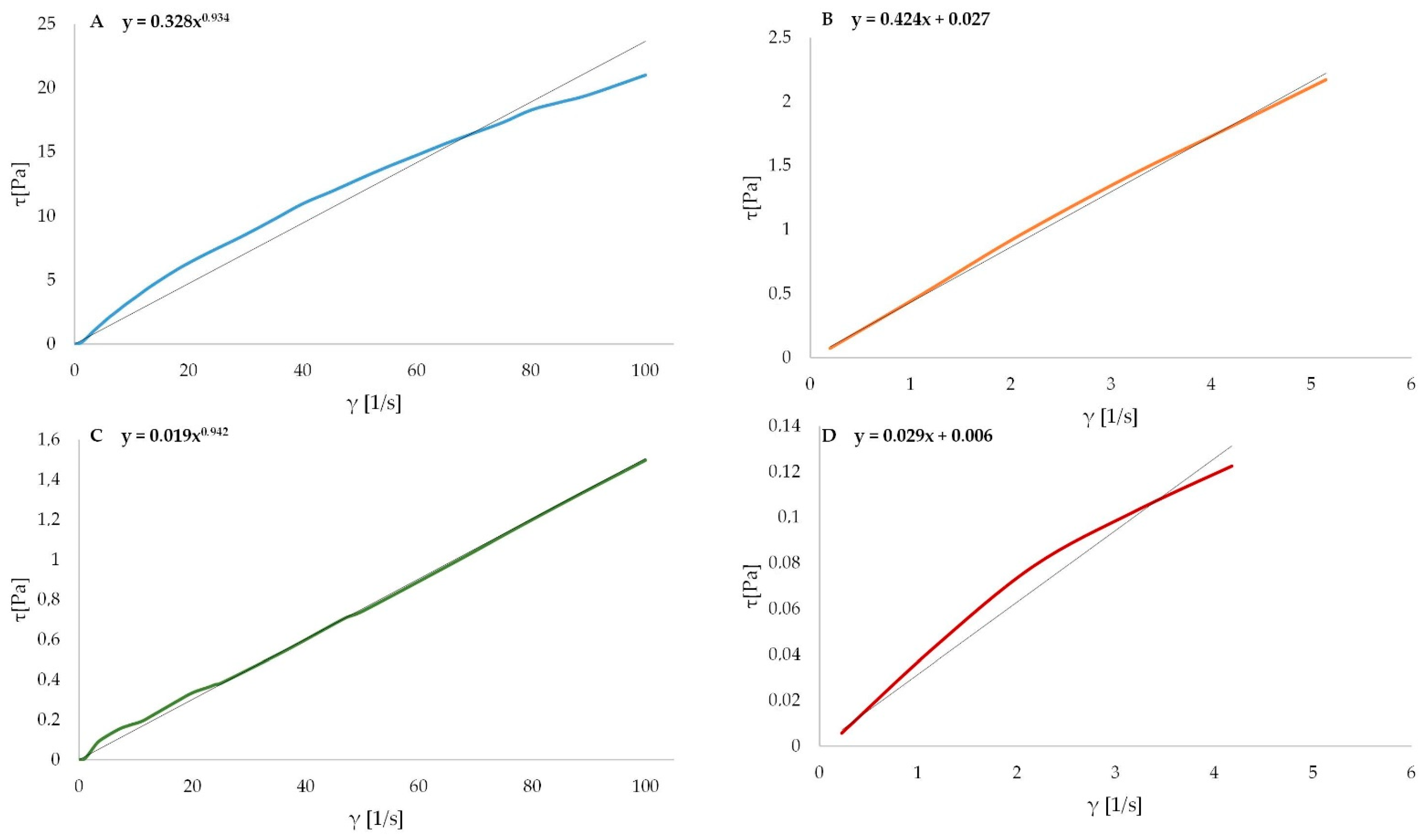
3.4. Rheological Characterization of Alginate Oligosaccharides
3.5. Antioxidant Properties
3.5.1. Ferric Reducing Antioxidant Power (FRAP)
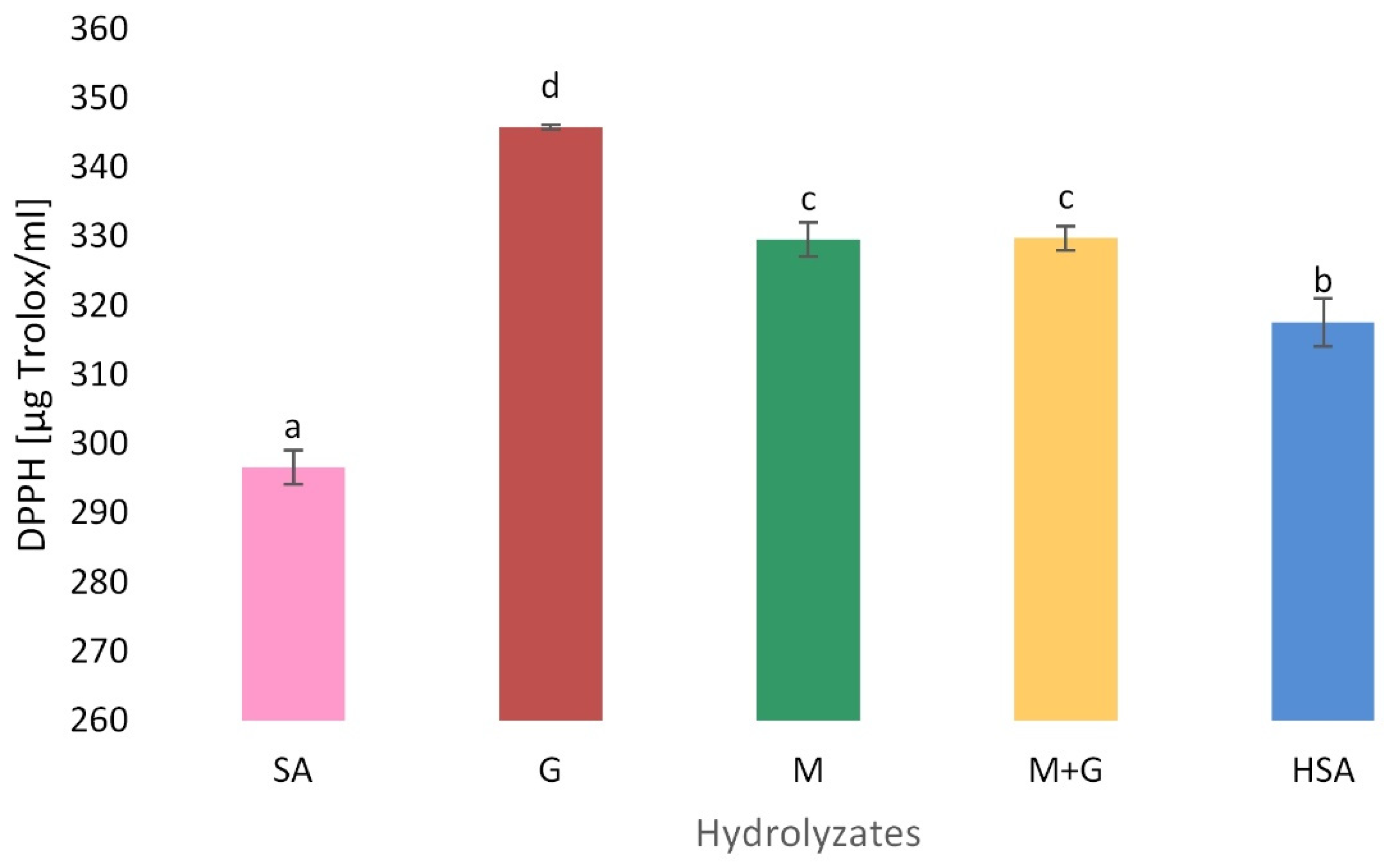
3.5.2. Free Radical Scavenging Activity (DPPH)
3.6. Antimicrobial Activity
3.7. TPA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Willför, S.; Xu, C. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact. Carbohydr. Diet. Fibre. 2015, 5, 31–61. [Google Scholar] [CrossRef]
- De Moura, F.A.; Macagnan, F.T.; Da Silva, L.P. Oligosaccharide production by hydrolysis of polysaccharides: A review. Int. J. Food Sci. Technol. 2015, 50, 275–281. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Functional oligosaccharides: Production, properties and applications. World J. Microbiol. Biotechnol. 2011, 27, 1119–1128. [Google Scholar] [CrossRef]
- Abd El-Mohdy, H.L. Radiation-induced degradation of sodium alginate and its plant growth promotion effect. Arab. J. Chem. 2019, 10, 431–438. [Google Scholar] [CrossRef] [Green Version]
- Piacentini, E. Encapsulation Efficiency. In Encyclopedia of Membranes, 1st ed.; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 706–707. [Google Scholar]
- Labre, F.; Mathieu, S.; Chaud, P.; Morvan, P.Y.; Vallée, R.; Helbert, W.; Fort, S. DMTMM-mediated amidation of alginate oligosaccharides aimed at modulating their interaction with proteins. Carbohydr. Polym. 2018, 184, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, S.; Li, X.; Yan, Q.; Reaney, M.J.T.; Jiang, Z. Alginate Oligosaccharides: Production, Biological Activities, and Potential Applications. Compr. Rev. Food Sci. 2019, 18, 1859–1881. [Google Scholar] [CrossRef] [Green Version]
- Muley, A.B.; Ladole, M.R.; Suprasanna, P.; Dalvi, S.G. Intensification in biological properties of chitosan after γ-irradiation. Int. J. Biol. Macromol. 2019, 131, 435–444. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, C.; Liu, X.; Mackie, A.; Zhang, L.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Impact of microfluidization and thermal treatment on the structure, stability and in vitro digestion of curcumin loaded zein-propylene glycol alginate complex nanoparticles. Food Res. Int. 2020, 138 (B), 109817. [Google Scholar] [CrossRef]
- Khajouei, A.R.; Keramat, J.; Hamdami, N.; Ursu, A.V.; Delattre, C.; Laroche, C.; Gardarin, C.; Lecerf, D.; Desbrières, J.; Djelveh, G. Extraction and characterization of an alginate from the Iranian brown seaweed Nizimuddinia zanardini. Int. J. Biol. Macromol. 2018, 118 (A), 1073–1081. [Google Scholar] [CrossRef]
- Huamani-Palomino, R.G.; Jacinto, C.R.; Alarcón, H.; Mejía, I.M.; López, R.C.; de Oliveira Silva, D.; Cavalheiro, E.T.G.; Venâncio, T.; Dávalos, J.Z.; Valderrama, A.C. Chemical modification of alginate with cysteine and its application for the removal of Pb(II) from aqueous solutions. Int. J. Biol. Macromol. 2019, 129, 1056–1068. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, S. Water-soluble β-1,3-glucan prepared by degradation of curdlan with hydrogen peroxide. Food Chem. 2019, 283, 302–304. [Google Scholar] [CrossRef]
- Falkeborg, M.; Cheong, L.Z.; Gianfico, C.; Sztukiel, K.M.; Kristensen, K.; Glasius, M.; Xu, X.; Guo, Z. Alginate oligosaccharides: Enzymatic preparation and antioxidant property evaluation. Food Chem. 2014, 164, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, A.; Xie, H.; Yu, W.; Xie, W.; Ma, X. Preparation of low molecular weight alginate by hydrogen peroxide depolymerization for tissue engineering. Carbohydr. Polym. 2010, 79, 660–664. [Google Scholar] [CrossRef]
- Sun, M.; Sun, C.; Xie, H.; Yan, S.; Yin, H. A simple method to calculate the degree of polymerization of alginate oligosaccharides and low molecular weight alginates. Carbohydr Res. 2019, 486, 107856. [Google Scholar] [CrossRef] [PubMed]
- Zimoch-Korzycka, A.; Kulig, D.; Jarmoluk, A.; Marycz, K.; Matuszczak, W. Study of Enzymatically Treated Alginate/Chitosan Hydrosols in Sponges Formation Process. Polymers 2016, 8, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP assay. Analytic. Biochem. 1996, 293, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Alqahtani, F.Y.; Aleanizy, F.S.; El Tahir, E.; Alquadeib, B.T.; Alsarra, I.A.; Alanazi, J.S.; Abdelhady, H.G. Preparation, characterization, and antibacterial activity of diclofenac-loaded chitosan nanoparticles. Saudi Pharm. J. 2019, 27, 82–87. [Google Scholar] [CrossRef]
- Bourne, M.C. Food Texture and Viscosity: Concept and Measurement, 2nd ed.; Academic Press: San Diego, CA, USA, 2002. [Google Scholar]
- Yang, Z.; Li, J.P.; Guan, H.S. Preparation and characterization of oligomannuronates from alginate degraded by hydrogen peroxide. Carbohydr. Polym. 2010, 58, 115–121. [Google Scholar] [CrossRef]
- Fenoradosoa, T.A.; Ali, G.; Delattre, C.; Laroche, C.; Petit, E.; Wadouachi, A.; Michaud, P. Extraction and characterization of an alginate from the brown seaweed Sargassum turbinarioides Grunow. J. Appl. Phycol. 2010, 22, 131–137. [Google Scholar] [CrossRef]
- Qin, Y. Alginate fibres: An overview of the production processes and applications in wound management. Polym. Int. 2008, 57, 171–180. [Google Scholar] [CrossRef]
- Du, B.; Song, Y.; Hu, X.; Liao, X.; Ni, Y.; Li, Q. Oligosaccharides prepared by acid hydrolysis of polysaccharides from pumpkin (Cucurbita moschata) pulp and their prebiotic activities. Int. J. Food Sci. Technol. 2011, 46, 982–987. [Google Scholar] [CrossRef]
- Mao, S.; Zhang, T.; Sun, W.; Ren, X. The depolymerization of sodium alginate by oxidative degradation. Pharm. Develop. Technol. 2012, 17, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.M.L.; Denis, M.F.L.; Piehl, L.L.; de Celis, E.R.; Buldain, G.Y.; Orto, V.C.D. Studies on the activation of hydrogen peroxide for color removal in the presence of a new Cu(II)-polyampholyte heterogeneous catalyst. Appl. Catal. B.–Environ. 2008, 82, 273–283. [Google Scholar] [CrossRef]
- Dima, S.-O.; Panaitescu, D.-M.; Orban, C.; Ghiurea, M.; Doncea, S.-M.; Fierascu, R.C.; Nistor, C.L.; Alexandrescu, E.; Nicolae, C.-A.; Trică, B. Bacterial Nanocellulose from Side-Streams of Kombucha Beverages Production: Preparation and Physical-Chemical Properties. Polymers 2017, 9, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leal, D.; Matsuhiro, B.; Rossi, M.; Caruso, F. FT-IR spectra of alginic acid block fractions in three species of brown seaweeds. Carbohydr Res. 2008, 343, 308–326. [Google Scholar] [CrossRef]
- Batalha, L.S.; Pardini Gontijo, M.T.; Novaes de Carvalho Teixeira, A.V.; Gouvêa Boggione, D.M.; Soto Lopez, M.E.; Eller, M.R.; Santos Mendonça, R.C. Encapsulation in alginate-polymers improves stability and allows controlled release of the UFV-AREG1 bacteriophage. Int. Food Res. J. 2021, 139, 109947. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lin, Y.; Chen, X.; Zhao, B.; Zhang, J. Flow behavior, thixotropy and dynamical viscoelasticity of sodium alginate aqueous solutions. Food Hydrocoll. 2014, 38, 119–128. [Google Scholar] [CrossRef]
- Moraes, I.C.F.; Fasolin, L.H.; Cunha, R.L.; Menegalli, F.C. Dynamic and steady-shear rheological properties of xanthan and guar gums dispersed in yellow passion fruit pulp (Passiflora edulis f. flavicarpa). Braz. J. Chem. Eng. 2011, 03, 483–494. [Google Scholar] [CrossRef] [Green Version]
- Taherian, A.L.; Lacasse, P.; Bisakowski, B.; Pelletier, M.; Lanctôt, A.; Fustier, P. Rheological and thermogelling properties of commercials chitosan/β-glycerophosphate: Retention of hydrogel in water, milk and UF-milk. Food Hydrocoll. 2017, 63, 635–645. [Google Scholar] [CrossRef]
- Björn, A.; Segura de La Monja, P.; Karlsson, A.; Ejlertsson, J.; Svensson, B.H. Rheological Characterization; Kumar, S., Ed.; InTech: Houston, TX, USA, 2012; pp. 63–76. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Díaz, D.; Navaza, J.M. Rheology of aqueous solutions of food additives: Effect of concentration, temperature and blending. J. Food Eng. 2003, 56, 387–392. [Google Scholar] [CrossRef]
- Keleşoğlu, S.; Pettersen, B.H.; Sjöblom, J. Flow properties of water-in- North Sea heavy crude oil emulsions. J. Pet. Sci. Eng. 2012, 100, 14–23. [Google Scholar] [CrossRef]
- Yang, J.; Chen, S.; Fang, Y. Viscosity study of interactions between sodium alginate and CTAB in dilute solutions at different pH values. Carboh. Polym. 2009, 75, 333–337. [Google Scholar] [CrossRef]
- Xiao, Q.; Tong, Q.; Lim, L.-T. Pullulan-sodium alginate based edible films: Rheological properties of film forming solutions. Carboh. Polym. 2012, 87, 1689–1695. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, P.; Wang, B.; Zhan, Y.; Zhang, J. Study on rheological behavior of sodium alginate and its blend solution. China Synt. Fib. Ind. 2007, 30, 30–32. [Google Scholar]
- Nelson, A.Z.; Schweizer, K.S.; Rauzan, B.M.; Nuzzo, R.G.; Vermant, J.; Ewoldt, R.H. Designing and transforming yield-stress fluids. Curr. Opin. Solid State Mater. Sci. 2019, 23, 100758. [Google Scholar] [CrossRef]
- Zhao, X.; Li, B.; Xue, C.; Sun, L. Effect of molecular weight on the antioxidant property of low molecular weight alginate from Laminaria japonica. J. Appl. Phycol. 2012, 24, 295–300. [Google Scholar] [CrossRef]
- Şen, M.; Atik, H. The antioxidant properties of oligo sodium alginates prepared by radiation-induced degradation in aqueous and hydrogen peroxide solutions. Radiat. Phys. Chem. 2012, 81, 816–822. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Goma, A.M.; Hifney, A.F.; Abdel-Gawad, K.M. Optimization of alginate alkaline extraction technology from Sargassum latifolium and its potential antioxidant and emulsifying properties. Carboh. Polym. 2017, 157, 1903–1912. [Google Scholar] [CrossRef]
- Kelishomi, Z.H.; Goliaei, B.; Mahdavi, H.; Nikoofar, A.; Rahimi, M.; Moosavi-Movahedi, A.A.; Bigdeli, B. Antioxidant activity of low molecular weight alginate produced by thermal treatment. Food Chem. 2016, 196, 897–902. [Google Scholar] [CrossRef]
- Sari-Chmayssem, N.; Taha, S.; Mawlawi, H. Extracted and depolymerized alginates from brown algae Sargassum vulgare of Lebanese origin: Chemical, rheological, and antioxidant properties. J Appl Phycol. 2016, 28, 1915–1929. [Google Scholar] [CrossRef]
- Sen, M. Effects of molecular weight and ratio of guluronic acid to mannuronic acid on the antioxidant properties of sodium alginate fractions prepared by radiation-induced degradation. Appl. Radiat. Isot. 2011, 69, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Jiang, X.; Gong, J. Antibacterial activity of lyase-depolymerized products of alginate. J. Appl. Phycol. 2005, 17, 57–60. [Google Scholar] [CrossRef]
- Pritchard, M.; Powell, L.; Khan, S. The antimicrobial effects of the alginate oligomer OligoG CF-5/20 are independent of direct bacterial cell membrane disruption. Sci. Rep. 2017, 7, 44731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhein-Knudsen, N.; Tutor Ale, M.; Ajalloueian, F.; Meyer, A.S. Characterization of alginates from Ghanaian brown seaweeds: Sargassum spp. and Padina spp. Food Hydrocolloids 2017, 71, 236–244. [Google Scholar] [CrossRef]
- Mutlu, C.; Tontul, S.A.; Erbaş, M. Production of a minimally processed jelly candy for children using honey instead of sugar. LWT 2018. [Google Scholar] [CrossRef]
- Yusof, N.; Jaswir, I.; Jamal, P.; Jami, M. Texture Profile Analysis (TPA) of the jelly dessert prepared from halal gelatin extracted using High Pressure Processing (HPP). Mal. J. Fund. Appl. Sci. 2019, 15, 604–608. [Google Scholar] [CrossRef]
- Larsen, B.E.; Bjørnstad, J.; Pettersen, E.O.; Tønnesen, H.H.; Melvik, J.E. Rheological characterization of an injectable alginate gel system. BMC Biotechnol. 2015, 15, 29. [Google Scholar] [CrossRef] [Green Version]
- Roopa, B.S.; Bhattacharya, S. Alginate gels: I. Characterization of textural attributes. J. Food Eng. 2008, 85, 123–131. [Google Scholar] [CrossRef]
- Park, R.M.; Nguyen, N.H.T.; Lee, S.M.; Kim, Y.H.; Min, J. Alginate oligosaccharides can maintain activities of lysosomes under low pH condition. Sci Rep. 2021, 11, 11504. [Google Scholar] [CrossRef]


| Parameters | Variants | ||||
|---|---|---|---|---|---|
| SA | G | M | M+G | HSA | |
| RSC (%) | 2.56 a ± 0.02 | 25.74 d ± 0.06 | 30.02 e ± 0.08 | 20.97 c ± 0.08 | 18.95 b ± 0.06 |
| DP (–) | 40.28 e ± 0.34 | 4.19 b ± 0.01 | 3.60 a ± 0.01 | 5.14 c ± 0.02 | 5.68 d ± 0.02 |
| Variants | Ostwald de Waele Model | Herschel–Bulkley’s Model | Apparent Viscosity η (mPa•s) | |
|---|---|---|---|---|
| Consistency Index k (Pa·s) | Flow Behavior Index n (-) | Yield Stress τ0 (Pa) | ||
| SA | 0.334 a ± 0.017 | 0.918 a ± 0.066 | 0.030 a ± 0.003 | 261.140 a ± 6.025 |
| G | 0.009 b ± 0.003 | 0.869 ab ± 0.123 | 0.005 b ± 0.002 | 3.580 b ± 1.032 |
| M | 0.003 b ± 0.002 | 0.742 b ± 0.050 | 0.002 b ± 0.001 | 2.330 b ± 0.250 |
| M+G | 0.004 b ± 0.002 | 0.822 ab ± 0.039 | 0.003 b ± 0.002 | 4.130 b ± 0.191 |
| HSA | 0.017 b ± 0.002 | 0.955 a ± 0.025 | 0.006 b ± 0.002 | 15.010 b ± 0.302 |
| Variants | Hd (N) | Sp (mm) | Coh (–) | Gu (N) | Ch (Nxmm) |
|---|---|---|---|---|---|
| SA | 71.25 a ± 3.99 | 0.46 a ± 0.01 | 0.40 a ± 0.02 | 28.50 a ± 2.46 | 13.11 a ± 1.23 |
| HSA | 13.68 b ± 1.81 | 0.42 b ± 0.02 | 0.16 b ± 001 | 2.19 b ± 0.39 | 0.92 b ± 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimoch-Korzycka, A.; Kulig, D.; Król-Kilińska, Ż.; Żarowska, B.; Bobak, Ł.; Jarmoluk, A. Biophysico-Chemical Properties of Alginate Oligomers Obtained by Acid and Oxidation Depolymerization. Polymers 2021, 13, 2258. https://doi.org/10.3390/polym13142258
Zimoch-Korzycka A, Kulig D, Król-Kilińska Ż, Żarowska B, Bobak Ł, Jarmoluk A. Biophysico-Chemical Properties of Alginate Oligomers Obtained by Acid and Oxidation Depolymerization. Polymers. 2021; 13(14):2258. https://doi.org/10.3390/polym13142258
Chicago/Turabian StyleZimoch-Korzycka, Anna, Dominika Kulig, Żaneta Król-Kilińska, Barbara Żarowska, Łukasz Bobak, and Andrzej Jarmoluk. 2021. "Biophysico-Chemical Properties of Alginate Oligomers Obtained by Acid and Oxidation Depolymerization" Polymers 13, no. 14: 2258. https://doi.org/10.3390/polym13142258







