Nanofiber-Mâché Hollow Ball Mimicking the Three-Dimensional Structure of a Cyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
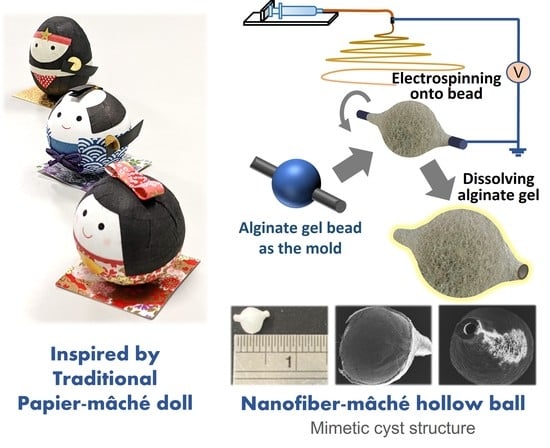
2.2. Fabrication of Nanofiber-Mâché Ball
2.3. Morphological Observation
2.4. Surface Characterization
2.5. Cell Culture with Nanofiber-Mâché Ball
2.6. Statistical Analysis
3. Results
3.1. Morphology of Nanofiber-Mâché Ball
3.2. Dimension and Geometry of Nanofiber
3.3. Characterization of Nanofiber Surface
3.4. Cell Adhesion
3.5. Cell Orientation
3.6. ECM Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faltaous, A.A.; Leigh, E.C.; Ray, P.; Wolbert, T.T. A Rare Transformation of Epidermoid Cyst into Squamous Cell Carcinoma: A Case Report with Literature Review. Am. J. Case Rep. 2019, 20, 1141–1143. [Google Scholar] [CrossRef] [PubMed]
- Osborn, A.G.; Preece, M.T. Intracranial Cysts: Radiologic-Pathologic Correlation and Imaging Approach. Radiology 2006, 239, 650–664. [Google Scholar] [CrossRef] [PubMed]
- Khoshnood, N.; Zamanian, A. A comprehensive review on scaffold-free bioinks for bioprinting. Bioprinting 2020, 19, e00088. [Google Scholar] [CrossRef]
- Cho, M.S.; Kim, S.J.; Ku, S.Y.; Park, J.H.; Lee, H.; Yoo, D.H.; Park, U.C.; Song, S.A.; Choi, Y.M.; Yu, H.G. Generation of retinal pigment epithelial cells from human embryonic stem cell-derived spherical neural masses. Stem Cell Res. 2012, 9, 101–109. [Google Scholar] [CrossRef]
- Théry, M. Micropatterning as a tool to decipher cell morphogenesis and functions. J. Cell Sci. 2010, 123, 4201–4213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, M.; Furusawa, K.; Mizutani, T.; Kawabata, K.; Haga, H. Three-dimensional morphogenesis of MDCK cells induced by cellular contractile forces on a viscous substrate. Sci. Rep. 2015, 5, 14208. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.S.; Ahn, C.B.; Son, K.H.; Lee, J.W. Motility Improvement of Biomimetic Trachea Scaffold via Hybrid 3D-Bioprinting Technology. Polymers 2021, 13, 971. [Google Scholar] [CrossRef]
- Chung, I.-M.; Enemchukwu, N.O.; Khaja, S.D.; Murthy, N.; Mantalaris, A.; García, A.J. Bioadhesive hydrogel microenvironments to modulate epithelial morphogenesis. Biomaterials 2008, 29, 2637–2645. [Google Scholar] [CrossRef] [Green Version]
- Wakuda, Y.; Nishimoto, S.; Suye, S.; Fujita, S. Native collagen hydrogel nanofibres with anisotropic structure using core-shell electrospinning. Sci. Rep. 2018, 8, 6248. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Wakuda, Y.; Matsumura, M.; Suye, S. Geometrically customizable alginate hydrogel nanofibers for cell culture platforms. J. Mater. Chem. B 2019, 7, 6556–6563. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Hibino, T.; Suye, S.; Fujita, S. Electrospun collagen core/poly-l-lactic acid shell nanofibers for prolonged release of hydrophilic drug. RSC Adv. 2021, 11, 5703–5711. [Google Scholar] [CrossRef]
- Yang, L.; Shi, J.; Zhou, X.; Cao, S. Hierarchically organization of biomineralized alginate beads for dual stimuli-responsive drug delivery. Int. J. Biol. Macromol. 2015, 73, 1–8. [Google Scholar] [CrossRef]
- Li, H.; Jiang, F.; Ye, S.; Wu, Y.; Zhu, K.; Wang, D. Bioactive apatite incorporated alginate microspheres with sustained drug-delivery for bone regeneration application. Mater. Sci. Eng. C 2016, 62, 779–786. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-W.; Zhu, S.; Wu, S.-R.; Wang, P.; Han, G.-Z. Response behavior of ion-sensitive hydrogel based on crown ether. Colloids Surf. A Physicochem. Eng. Asp. 2013, 417, 140–145. [Google Scholar] [CrossRef]
- Hu, T.; Lo, A.C.Y. Collagen–Alginate Composite Hydrogel: Application in Tissue Engineering and Biomedical Sciences. Polymers 2021, 13, 1852. [Google Scholar] [CrossRef] [PubMed]
- Chueh, B.; Zheng, Y.; Torisawa, Y.; Hsiao, A.Y.; Ge, C.; Hsiong, S.; Huebsch, N.; Franceschi, R.; Mooney, D.J.; Takayama, S. Patterning alginate hydrogels using light-directed release of caged calcium in a microfluidic device. Biomed. Microdevices 2010, 12, 145–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebia, R.A.; Rozet, S.; Tamada, Y.; Tanaka, T. Biodegradable PHBH/PVA blend nanofibers: Fabrication, characterization, in vitro degradation, and in vitro biocompatibility. Polym. Degrad. Stab. 2018, 154, 124–136. [Google Scholar] [CrossRef]
- Qin, Q.; Takarada, W.; Kikutani, T. Fiber Structure Development of PHBH through Stress-Induced Crystallization in High-Speed Melt Spinning Process. J. Fiber Sci. Technol. 2017, 73, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Rebia, R.A.; Shizukuishi, K.; Tanaka, T. Characteristic changes in PHBH isothermal crystallization monofilaments by the effect of heat treatment and dip-coating in various solvents. Eur. Polym. J. 2020, 134, 109808. [Google Scholar] [CrossRef]
- Nomura, S.; Kawai, H.; Kimura, I.; Kagiyama, M. General description of orientation factors in terms of expansion of orientation distribution function in a series of spherical harmonics. J. Polym. Sci. Part A 2 Polym. Phys. 1970, 8, 383–400. [Google Scholar] [CrossRef]
- Batnyam, O.; Suye, S.I.; Fujita, S. Direct cryopreservation of adherent cells on an elastic nanofiber sheet featuring a low glass-transition temperature. RSC Adv. 2017, 7, 51264–51271. [Google Scholar] [CrossRef] [Green Version]
- Hoang, V.T.; Trinh, C.T.; Nguyen, C.H.; Chansomphou, V.; Chansomphou, V.; Tran, T.T.T. Overview of epidermoid cyst. Eur. J. Radiol. Open 2019, 6, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Reissis, D.; Pfaff, M.J.; Patel, A.; Steinbacher, D.M. Craniofacial dermoid cysts: Histological analysis and inter-site comparison. Yale J. Biol. Med. 2014, 87, 349–357. [Google Scholar] [PubMed]
- Ishii, N.; Fukazawa, E.; Aoki, T.; Kishi, K. Combined extracranial and intracranial approach for resection of dermoid cyst of the sphenoid bone with a cutaneous sinus tract across the frontal branch of the facial nerve. Arch. Craniofacial Surg. 2019, 20, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Azab, W.; Salaheddin, W.; Alsheikh, T.; Nasim, K.; Nasr, M. Colloid cysts posterior and anterior to the foramen of Monro: Anatomical features and implications for endoscopic excision. Surg. Neurol. Int. 2014, 5, 124. [Google Scholar] [CrossRef] [PubMed]
- Pedrizzetti, G.; La Canna, G.; Alfieri, O.; Tonti, G. The vortex—an early predictor of cardiovascular outcome? Nat. Rev. Cardiol. 2014, 11, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Badylak, S.F. Extracellular Matrix as an Inductive Scaffold for Functional Tissue Reconstruction. In Translating Regenerative Medicine to the Clinic; Elsevier: Amsterdam, The Netherlands, 2016; pp. 11–29. ISBN 9780128005521. [Google Scholar]
- Zeug, A.; Stawarski, M.; Bieganska, K.; Korotchenko, S.; Wlodarczyk, J.; Dityatev, A.; Ponimaskin, E. Current microscopic methods for the neural ECM analysis. In Progress in Brain Research; Elsevier B.V.: Amsterdam, The Netherlands, 2014; Volume 214, pp. 287–312. ISBN 9780444634863. [Google Scholar]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawton, M.T.; Quiñones-Hinojosa, A.; Chang, E.F.; Yu, T. Thrombotic Intracranial Aneurysms: Classification Scheme and Management Strategies in 68 Patients. Neurosurgery 2005, 56, 441–454. [Google Scholar] [CrossRef]
- Iihara, K.; Murao, K.; Sakai, N.; Soeda, A.; Ishibashi-Ueda, H.; Yutani, C.; Yamada, N.; Nagata, I. Continued growth of and increased symptoms from a thrombosed giant aneurysm of the vertebral artery after complete endovascular occlusion and trapping: The role of vasa vasorum. J. Neurosurg. 2003, 98, 407–413. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.-Y.; Hashimoto, N.; Kitai, R.; Suye, S.-i.; Fujita, S. Nanofiber-Mâché Hollow Ball Mimicking the Three-Dimensional Structure of a Cyst. Polymers 2021, 13, 2273. https://doi.org/10.3390/polym13142273
Huang W-Y, Hashimoto N, Kitai R, Suye S-i, Fujita S. Nanofiber-Mâché Hollow Ball Mimicking the Three-Dimensional Structure of a Cyst. Polymers. 2021; 13(14):2273. https://doi.org/10.3390/polym13142273
Chicago/Turabian StyleHuang, Wan-Ying, Norichika Hashimoto, Ryuhei Kitai, Shin-ichiro Suye, and Satoshi Fujita. 2021. "Nanofiber-Mâché Hollow Ball Mimicking the Three-Dimensional Structure of a Cyst" Polymers 13, no. 14: 2273. https://doi.org/10.3390/polym13142273
APA StyleHuang, W. -Y., Hashimoto, N., Kitai, R., Suye, S. -i., & Fujita, S. (2021). Nanofiber-Mâché Hollow Ball Mimicking the Three-Dimensional Structure of a Cyst. Polymers, 13(14), 2273. https://doi.org/10.3390/polym13142273