Preparation of p-Phenylenediamine Modified Graphene Foam/Polyaniline@Epoxy Composite with Superior Thermal and EMI Shielding Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
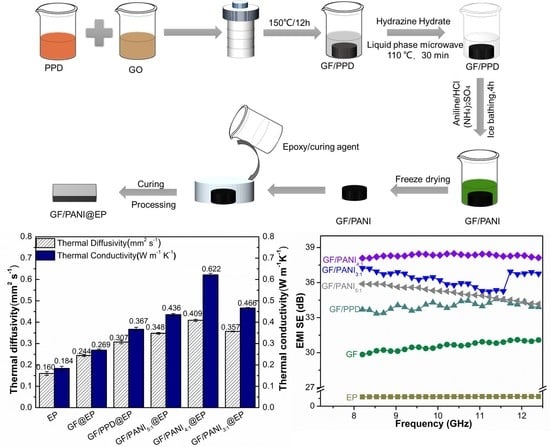
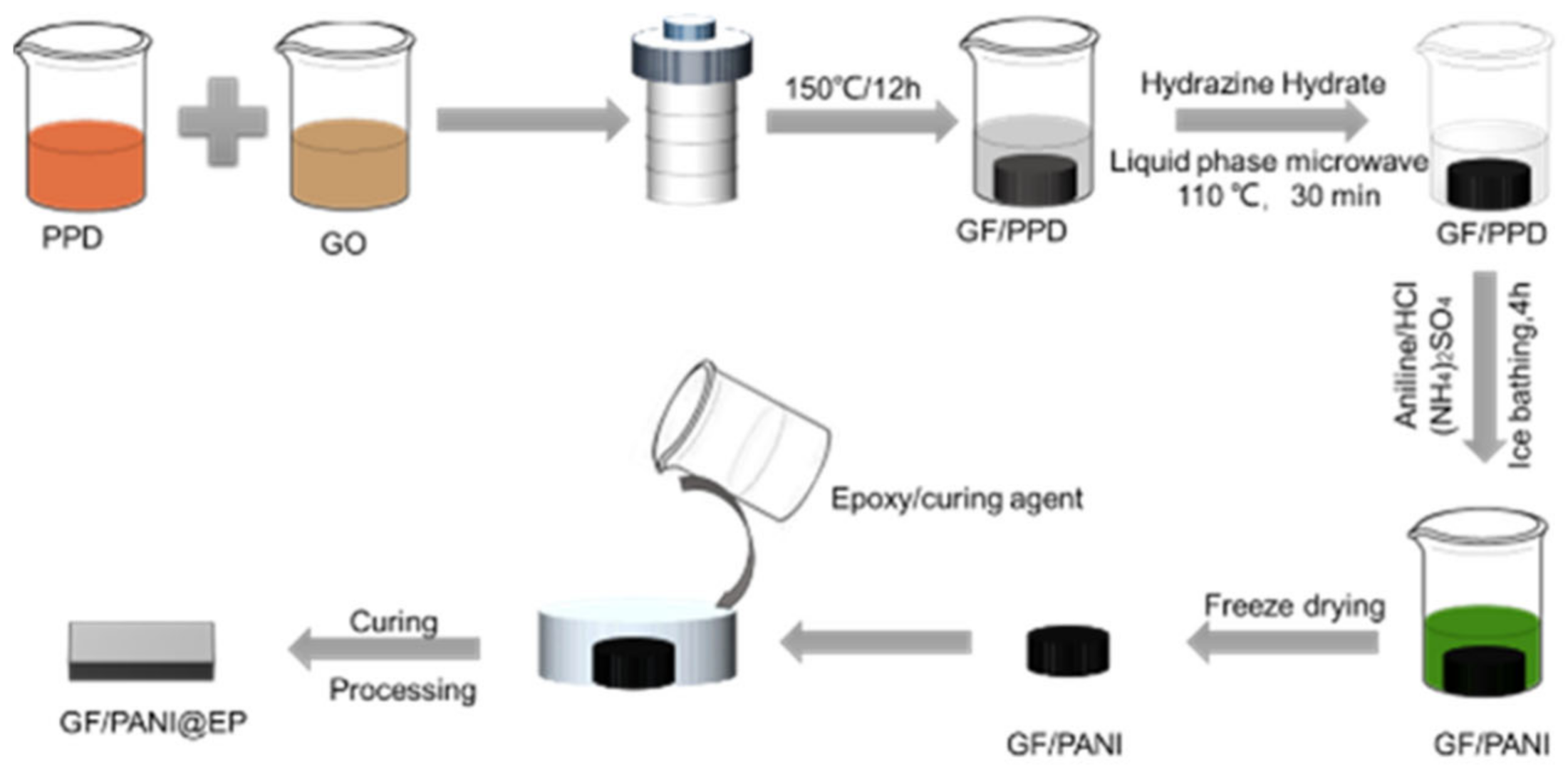
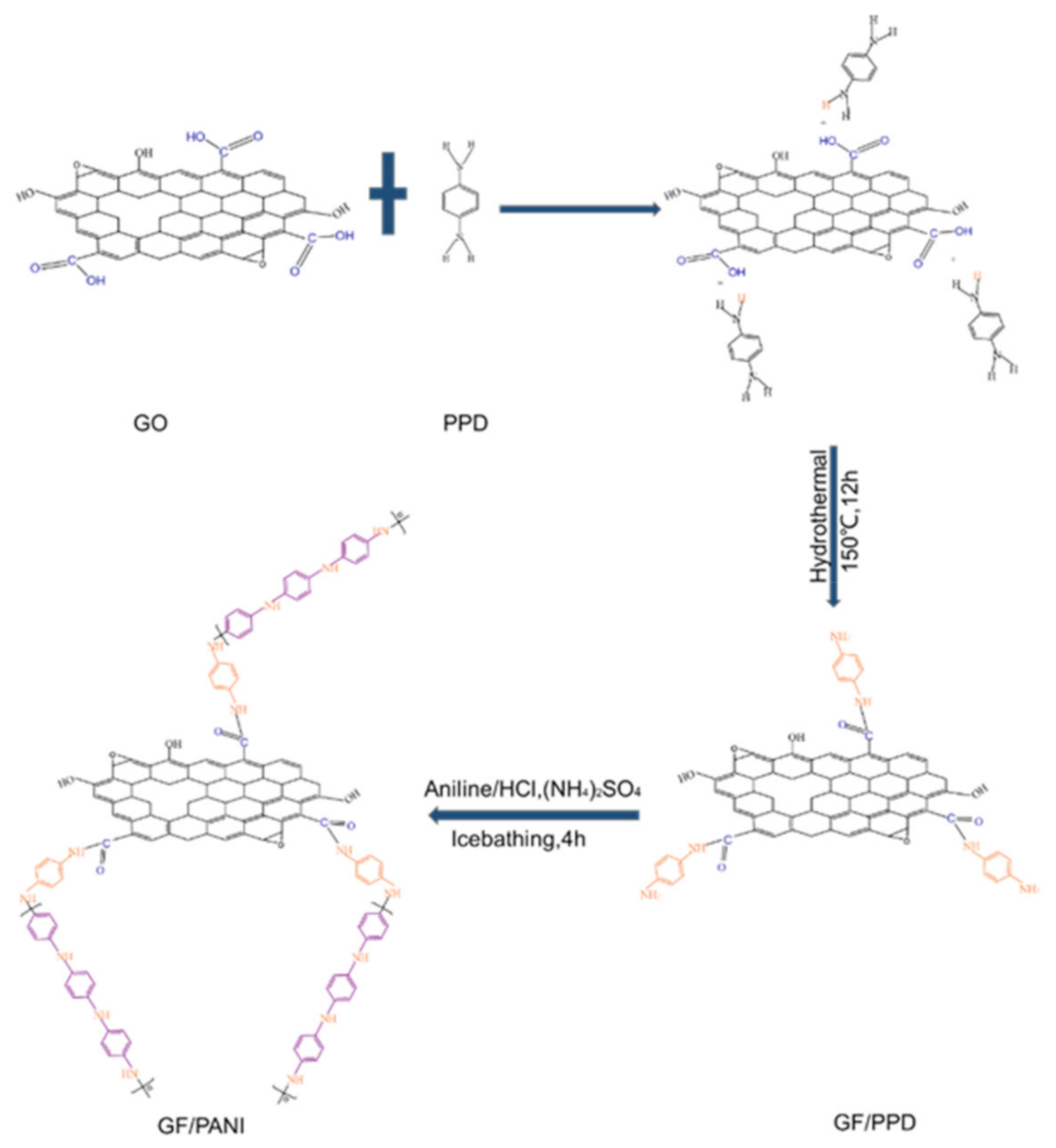
2.2. The Modification of PPD and the Preparation of GF/PANI Filler
2.3. Preparation of GF/PANI@EP Composite
2.4. Characterization
3. Results and Discussion
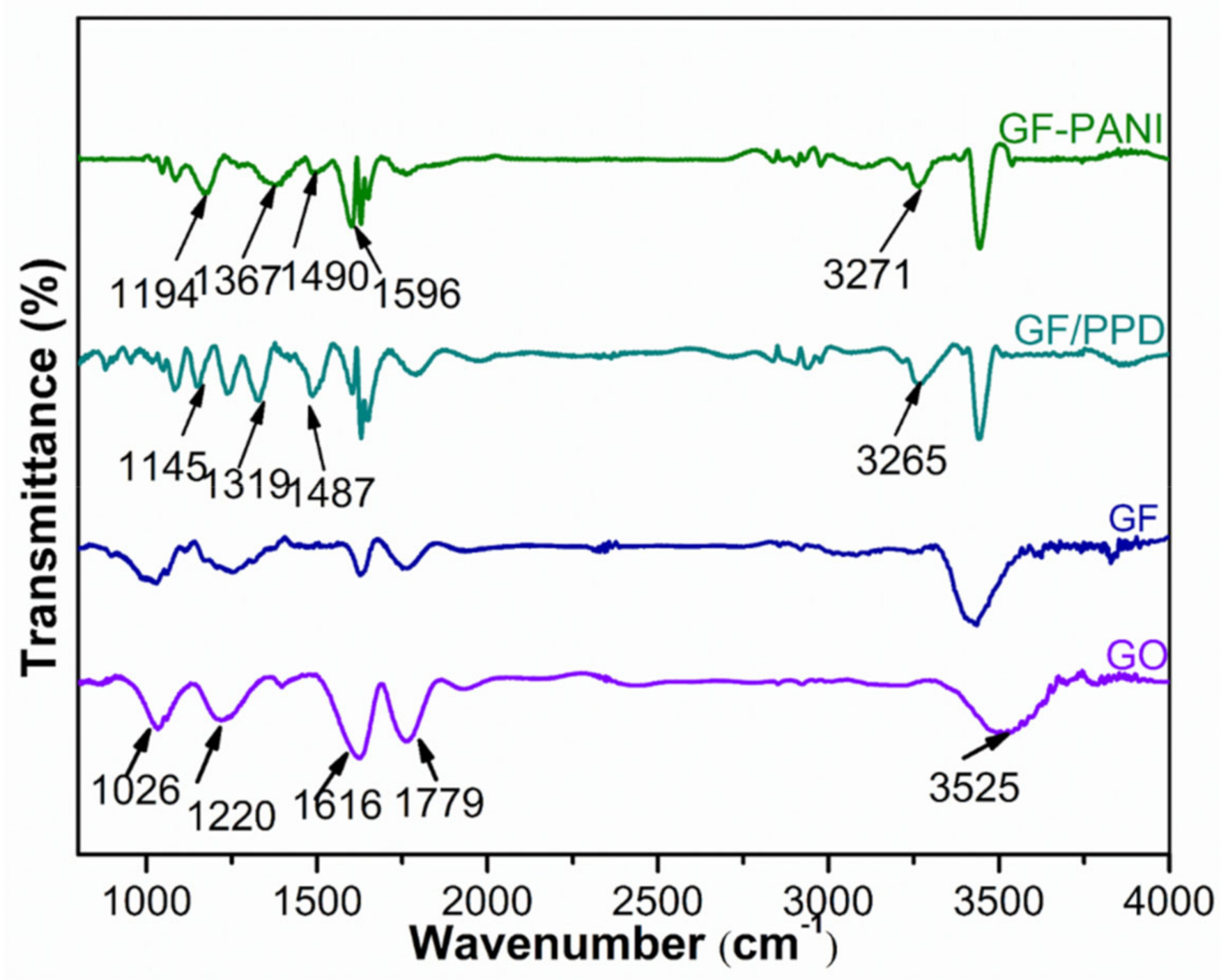
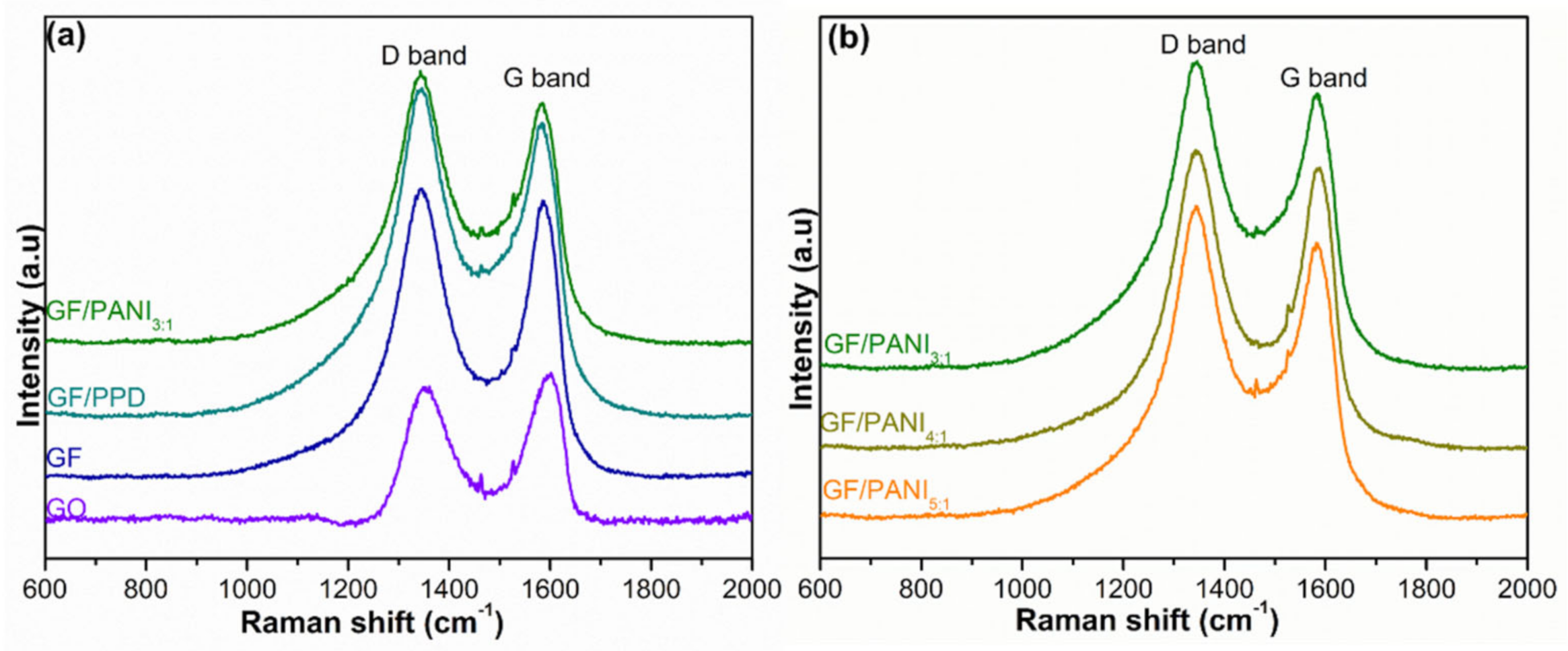
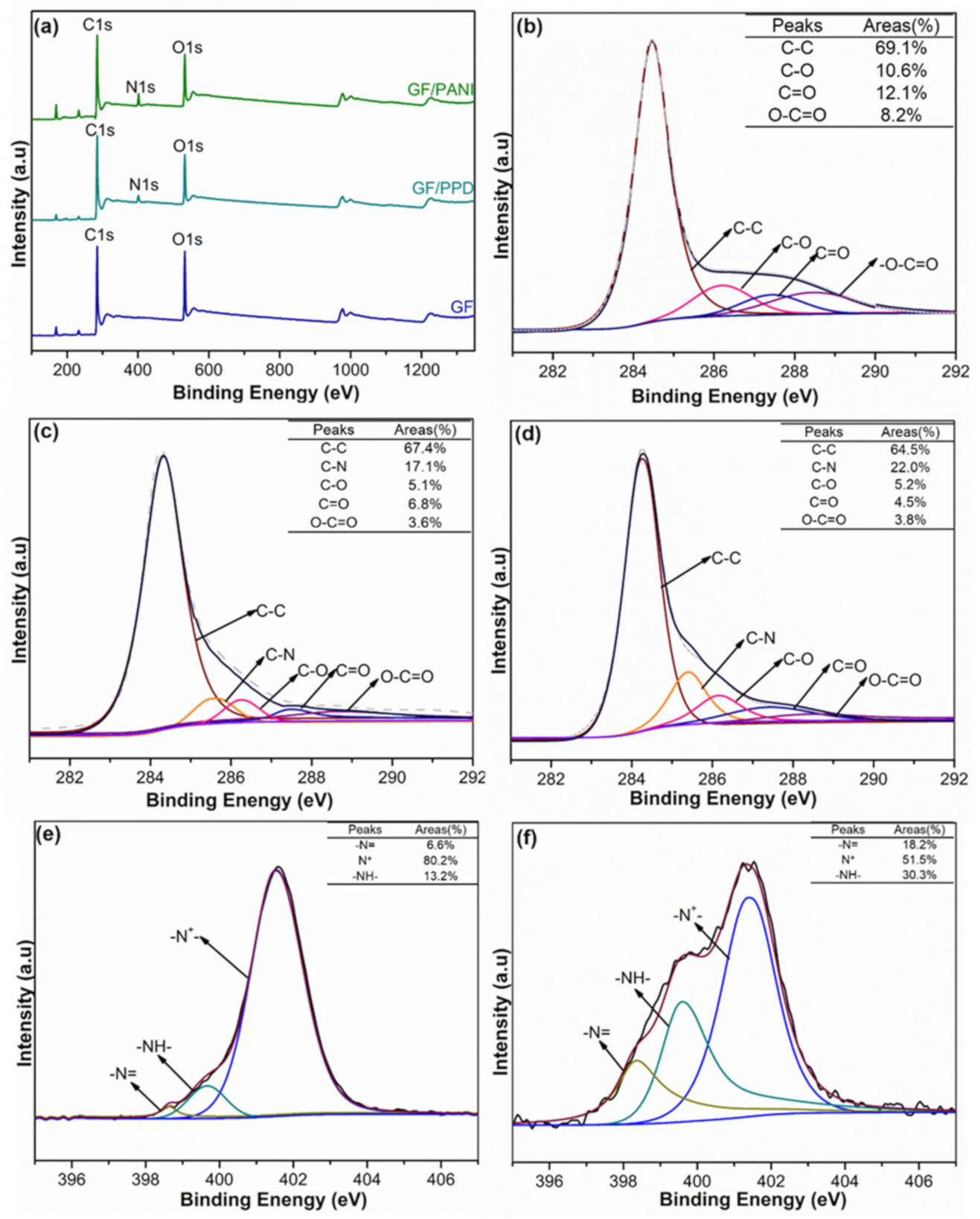
3.1. Structure and Characterization
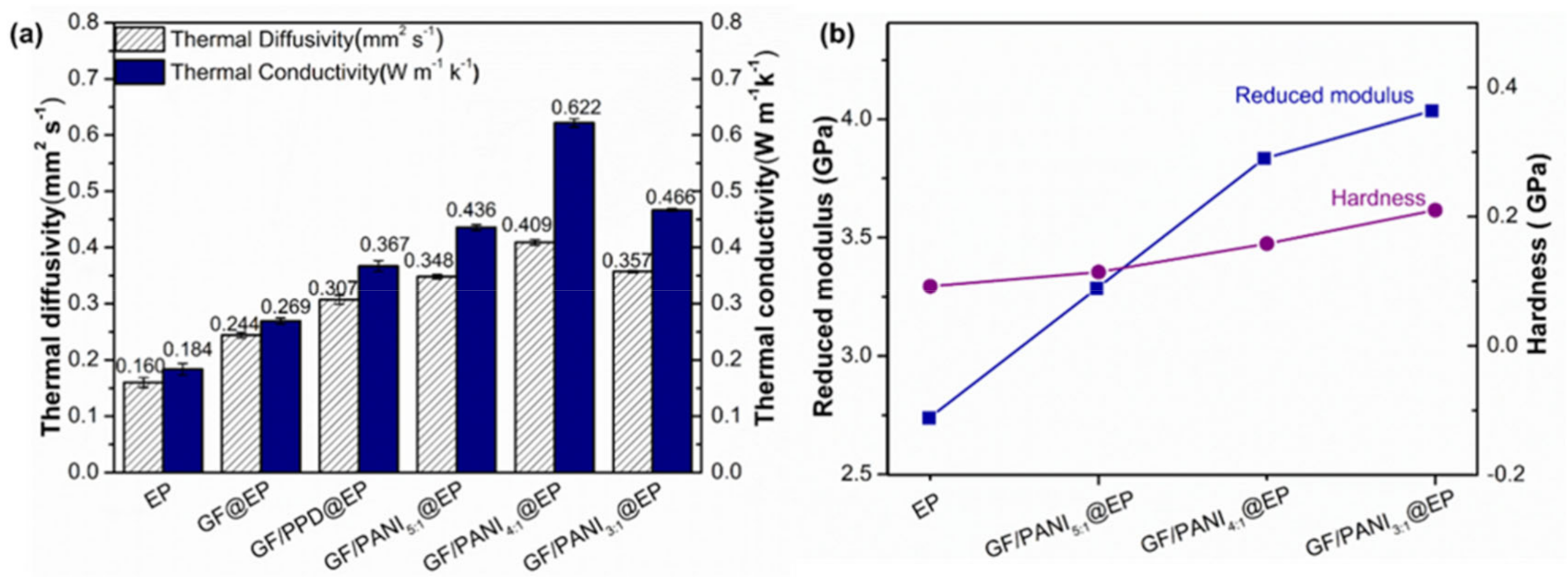
3.2. Thermal Conductivity and Mechanical Properties
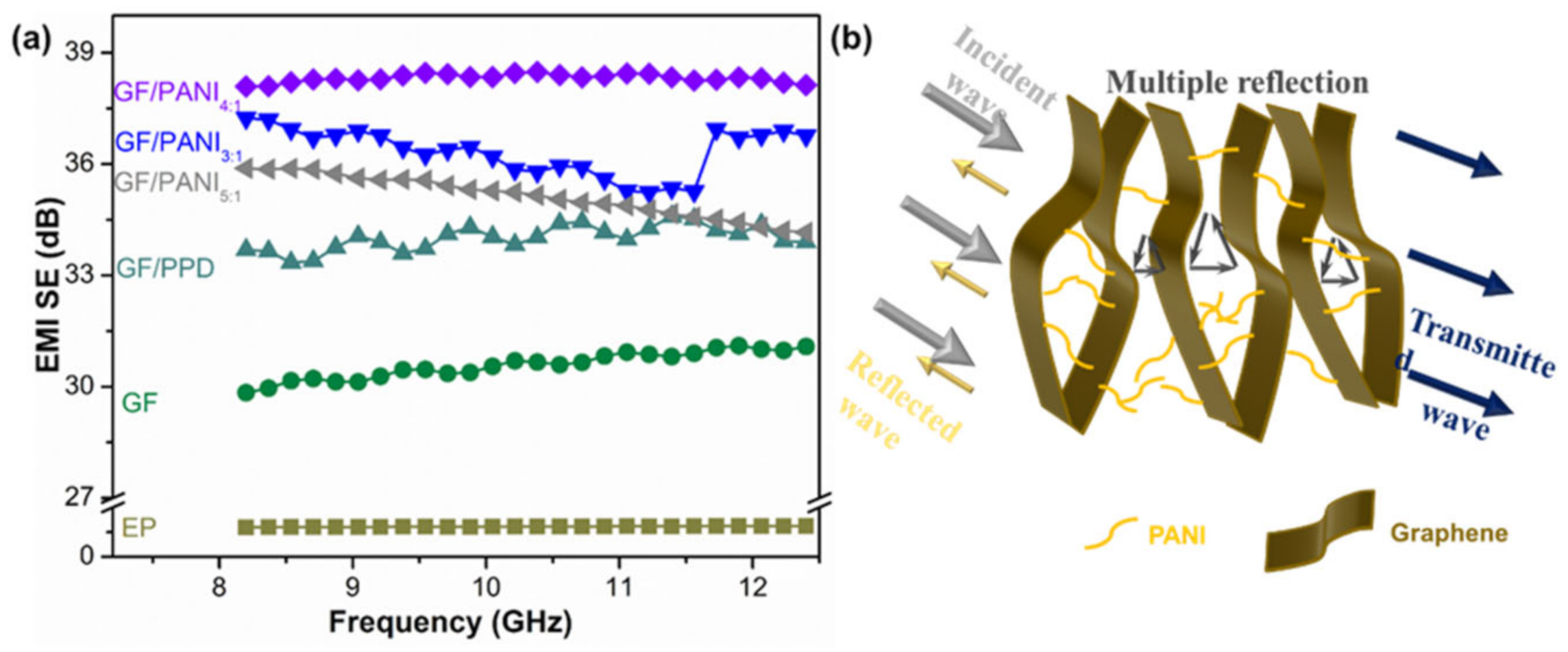
3.3. Electromagnetic Shielding Performance
3.4. Mechanism Diagram
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal conductivity of polymer-based composites: Fundamentals and applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar] [CrossRef]
- Sadasivuni, K.K.; Ponnamma, D.; Thomas, S.; Grohens, Y. Evolution from graphite to graphene elastomer composites. Prog. Polym. Sci. 2014, 39, 749–780. [Google Scholar] [CrossRef]
- Sarvar, F.; Whalley, D.C.; Conway, P.P. Thermal interface Materials-a review of the state of the art. IEEE 2006, 2, 1292–1302. [Google Scholar]
- Goyal, V.; Balandin, A.A. Thermal properties of the hybrid graphene-metal nano-micro-composites: Applications in thermal interface materials. Appl. Phys. Lett. 2012, 100, 073113. [Google Scholar] [CrossRef] [Green Version]
- Prasher, R. Thermal Interface Materials: Historical Perspective, Status, and Future Directions. Proc. IEEE 2006, 94, 1571–1586. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.K.; Calizo, I.; Teweldebrhan, D.; Pokatilov, E.P.; Nika, D.L.; A Balandin, A.; Bao, W.; Miao, F.; Lau, C.N. Extremely high thermal conductivity of graphene: Prospects for thermal management applications in nanoelectronic circuits. Appl. Phys. Lett. 2008, 92, 151911. [Google Scholar] [CrossRef]
- Liem, H.; Choy, H.S. Superior thermal conductivity of polymer nanocomposites by using graphene and boron nitride as fillers. Solid State Commun. 2013, 163, 41–45. [Google Scholar] [CrossRef]
- Malekpour, H.; Chang, K.-H.; Chen, J.-C.; Lu, C.-Y.; Nika, D.L.; Novoselov, K.S.; Balandin, A.A. Thermal Conductivity of Graphene Laminate. Nano Lett. 2014, 14, 5155–5161. [Google Scholar] [CrossRef]
- Shahil, K.M.F.; Balandin, A.A. Graphene-multilayer graphene nanocomposites as highly efficient thermal interface materials. Nano Lett. 2012, 12, 861–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Drzal, L.T.; Qin, Y.; Huang, Z. Multifunctional graphene nanoplatelets/cellulose nanocrystals composite paper. Compos. Part B Eng. 2015, 79, 521–529. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi-Moghadam, B.; Taheri, F. Effect of processing parameters on the structure and multi-functional performance of epoxy/GNP-nanocomposites. J. Mater. Sci. 2014, 49, 6180–6190. [Google Scholar] [CrossRef]
- Zakaria, M.R.; Kudus, M.H.A.; Akil, H.M.; Thirmizir, M.Z.M. Comparative study of graphene nanoparticle and multiwall carbon nanotube filled epoxy nanocomposites based on mechanical, thermal and dielectric properties. Compos. Part B Eng. 2017, 119, 57–66. [Google Scholar] [CrossRef]
- Li, B.; Zhong, W.-H. Review on polymer/graphite nanoplatelet nanocomposites. J. Mater. Sci. 2011, 46, 5595–5614. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, Y.; Fang, H.; Ding, T.; Li, J.; Bai, S.-L. Simultaneous enhancement on thermal and mechanical properties of polypropylene composites filled with graphite platelets and graphene sheets. Compos. Part A Appl. Sci. Manuf. 2018, 112, 57–63. [Google Scholar] [CrossRef]
- Chen, Y.; Hou, X.; Liao, M.; Dai, W.; Wang, Z.; Yan, C.; Li, H.; Lin, C.-T.; Jiang, N.; Yu, J. Constructing a “pea-pod-like” alumina-graphene binary architecture for enhancing thermal conductivity of epoxy composite. Chem. Eng. J. 2020, 381, 122690. [Google Scholar] [CrossRef]
- Li, A.; Zhang, C.; Zhang, Y.F. Thermal conductivities of PU composites with graphene aerogels reduced by different methods. Compos. Part A Appl. Sci. Manuf. 2017, 103, 161–167. [Google Scholar] [CrossRef]
- Wei, S.; Yu, Q.; Fan, Z.; Liu, S.; Chi, Z.; Chen, X.; Zhang, Y.; Xu, J. Fabricating high thermal conductivity rGO/polyimide nanocomposite films via a freeze-drying approach. RSC Adv. 2018, 8, 22169–22176. [Google Scholar] [CrossRef] [Green Version]
- Hou, X.; Chen, Y.; Lv, L.; Dai, W.; Zhao, S.; Wang, Z.; Fu, L.; Lin, C.-T.; Jiang, N.; Yu, J. High-Thermal-Transport-Channel Construction within Flexible Composites via the Welding of Boron Nitride Nanosheets. ACS Appl. Nano Mater. 2019, 2, 360–368. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H.; Yin, L.; Zhao, J.; Xia, L.; Chen, L. Exceptionally high thermal conductivity of thermal grease: Synergistic effects of graphene and alumina. Int. J. Therm. Sci. 2015, 91, 76–82. [Google Scholar] [CrossRef]
- Renteria, J.; Legedza, S.; Salgado, R.; Balandin, M.P.; Ramirez, S.; Saadah, M.; Kargar, F.; Balandin, A.A. Magnetically-functionalized self-aligning graphene fillers for high-efficiency thermal management applications. Mater. Des. 2015, 88, 214–221. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Liu, Z.; Wang, S.; Zhang, Y. Preparation and properties of octadecylamine modified graphene oxide/styrene-butadiene rubber composites through an improved melt compounding method. J. Appl. Polym. Sci. 2016, 133, 42907. [Google Scholar] [CrossRef]
- Shah, R.; Kausar, A.; Muhammad, B.; Khan, M. Investigation on thermal conductivity and physical properties of polythiophene/p-phenylenediamine-graphene oxide and polythiophene-co-poly(methyl methacrylate)/p-phenylenediamine graphene oxide composites. Compos. Interface 2016, 23, 887–899. [Google Scholar] [CrossRef]
- Shao, D.; Hou, G.; Li, J.; Wen, T.; Ren, X.; Wang, X. PANI/GO as a super adsorbent for the selective adsorption of uranium (VI). Chem. Eng. J. 2014, 255, 604–612. [Google Scholar] [CrossRef]
- Wang, T.; Sun, H.; Peng, T.; Liu, B.; Hou, Y.; Lei, B. Preparation and characterization of polyaniline/p-phenylenediamine grafted graphene oxide composites for supercapacitors. J. Mol. Struct. 2020, 1221, 128835. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, W.L.; Choi, H.J. Facile fabrication of self-assembled PMMA/graphene oxide composite particles and their electroresponsive properties. Colloid Polym. Sci. 2012, 291, 955–962. [Google Scholar] [CrossRef]
- Shahnaz, G.S.; Emadi, R.; Mehdi, A.; Sorour, S. Fabrication and characterization of polyvinylpyrrolidone-eggshell membrane-reduced graphene oxide nanofibers for tissue engineering applications. Polymers 2021, 13, 913. [Google Scholar]
- Wang, Y.; Yu, Y.; Hu, X.; Feng, A.; Jiang, F.; Song, L. p-Phenylenediamine strengthened graphene oxide for the fabrication of superhydrophobic surface. Mater. Des. 2017, 127, 22–29. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, J.; Li, N.; Shi, M.; Ma, H.; Yan, B.; Wang, W.; Huang, W.; Ye, M. Amino-functionalization of graphene sheets and the fabrication of their nanocomposites. Polym. Compos. 2010, 31, 1987–1994. [Google Scholar] [CrossRef]
- Hung, W.-S.; Tsou, C.-H.; de Guzman, M.; An, Q.-F.; Liu, Y.-L.; Zhang, Y.-M.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Cross-Linking with Diamine Monomers to Prepare Composite Graphene Oxide-Framework Membranes with Varying d-Spacing. Chem. Mater. 2014, 26, 2983–2990. [Google Scholar] [CrossRef]
- Zhang, K.; Li, H.; Dong, Y.Z.; Zhang, H.; Zhao, W.; Zhao, S.; Choi, H.J. Jellyfish-shaped p-phenylenediamine functionalized graphene oxide-g-polyaniline fibers and their electrorheology. Polymer 2019, 168, 29–35. [Google Scholar] [CrossRef]
- Manna, B.; Retna Raj, C. Covalent functionalization and electrochemical tuning of reduced graphene oxide for the bioelectrocatalytic sensing of serum lactate. J. Mater. Chem. B. 2016, 4, 4585–4593. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Veerapandian, M.; Mohan, R.; Kim, S.J. Investigation of Raman and photoluminescence studies of reduced graphene oxide sheets. Appl. Phys. A. Mater. 2012, 106, 501–506. [Google Scholar] [CrossRef]
- Wu, H.; Lu, L.; Zhang, Y.; Sun, Z.; Qian, L. A facile method to prepare porous graphene with tunable structure as electrode materials for immobilization of glucose oxidase. Colloids Surf. A Physicochem. Eng. Asp. 2016, 502, 26–33. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, H.; Zhao, Z.; Zhang, L.; Ma, L.; Zhao, G.; Song, G.; Li, X. Investigation of carbon nanotube grafted graphene oxide hybrid aerogel for polystyrene composites with reinforced mechanical performance. Polymers 2021, 13, 735. [Google Scholar] [CrossRef]
- Yuan, B.; Bao, C.; Song, L.; Hong, N.; Liew, K.M.; Hu, Y. Preparation of functionalized graphene oxide/polypropylene nanocomposite with significantly improved thermal stability and studies on the crystallization behavior and mechanical properties. Chem. Eng. J. 2014, 237, 411–420. [Google Scholar] [CrossRef]
- Prakash, K.; Masram, D.T. A reversible chromogenic covalent organic polymer for gas sensing applications. Dalton Trans. 2020, 49, 1007–1010. [Google Scholar]
- Yadav, D.; Awasthi, S.K. An unsymmetrical covalent organic polymer for catalytic amide synthesis. Dalton Trans. 2020, 49, 179–186. [Google Scholar] [CrossRef]
- Embrey, L.; Nautiyal, P.; Loganathan, A.; Idowu, A.; Boesl, B.; Agarwal, A. Three-dimensional graphene foam induces mul-tifunctionality in epoxy nanocomposites by simultaneous improvement in mechanical, thermal, and electrical properties. ACS Appl. Mater. Interfaces 2017, 9, 39717–39727. [Google Scholar] [CrossRef]
- Tarawneh, M.A.; Saraireh, S.A.; Chen, R.S.; Ahmad, S.H.; Al-Tarawni, M.A.M.; Al-Tweissi, M.; Yu, L.J. Mechanical, thermal, and conductivity performances of novel thermoplastic natural rubber/graphene nanoplates/polyaniline composites. J. Appl. Polym. Sci. 2020, 137, 48873. [Google Scholar] [CrossRef]
- Li, Y.; Sun, L.; Xu, F.; Wang, S.; Peng, Q.; Yang, Z.; He, X.; Li, Y. Electromagnetic and acoustic double-shielding graphene-based metastructures. Nanoscale 2019, 11, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, Y.; Wen, B. Enhanced electromagnetic interference shielding capability in bamboo fiber@polyaniline composites through microwave reflection cavity design. Compos. Sci. Technol. 2019, 178, 41–49. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, S.; Wang, X.; Chu, J.; Zhang, R.; Gong, M.; Wu, B.; Qu, M.; Li, Z.; Chen, Z. Covalently bonded polyaniline-reduced graphene oxide/single-walled carbon nanotubes nanocomposites: Influence of various dimensional carbon nanostructures on the electrochromic behavior of PANI. Polym. J. 2020, 52, 783–792. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Li, H.; Xiao, S.; Zhu, M.; Yang, J. Preparation of p-Phenylenediamine Modified Graphene Foam/Polyaniline@Epoxy Composite with Superior Thermal and EMI Shielding Performance. Polymers 2021, 13, 2324. https://doi.org/10.3390/polym13142324
Wang L, Li H, Xiao S, Zhu M, Yang J. Preparation of p-Phenylenediamine Modified Graphene Foam/Polyaniline@Epoxy Composite with Superior Thermal and EMI Shielding Performance. Polymers. 2021; 13(14):2324. https://doi.org/10.3390/polym13142324
Chicago/Turabian StyleWang, Liusi, Haoliang Li, Shuxing Xiao, Mohan Zhu, and Junhe Yang. 2021. "Preparation of p-Phenylenediamine Modified Graphene Foam/Polyaniline@Epoxy Composite with Superior Thermal and EMI Shielding Performance" Polymers 13, no. 14: 2324. https://doi.org/10.3390/polym13142324
APA StyleWang, L., Li, H., Xiao, S., Zhu, M., & Yang, J. (2021). Preparation of p-Phenylenediamine Modified Graphene Foam/Polyaniline@Epoxy Composite with Superior Thermal and EMI Shielding Performance. Polymers, 13(14), 2324. https://doi.org/10.3390/polym13142324