Efficient Bulky Organo-Zinc Scorpionates for the Stereoselective Production of Poly(rac-lactide)s
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental
2.2.1. Nuclear Magnetic Resonance Spectroscopy (NMR)
2.2.2. Elemental Analysis
2.2.3. Gel Permeation Chromatography (GPC)
2.2.4. MALDI-TOF Mass Spectrometry
2.2.5. Crystallographic Refinement and Structure Solution
2.3. General Procedures
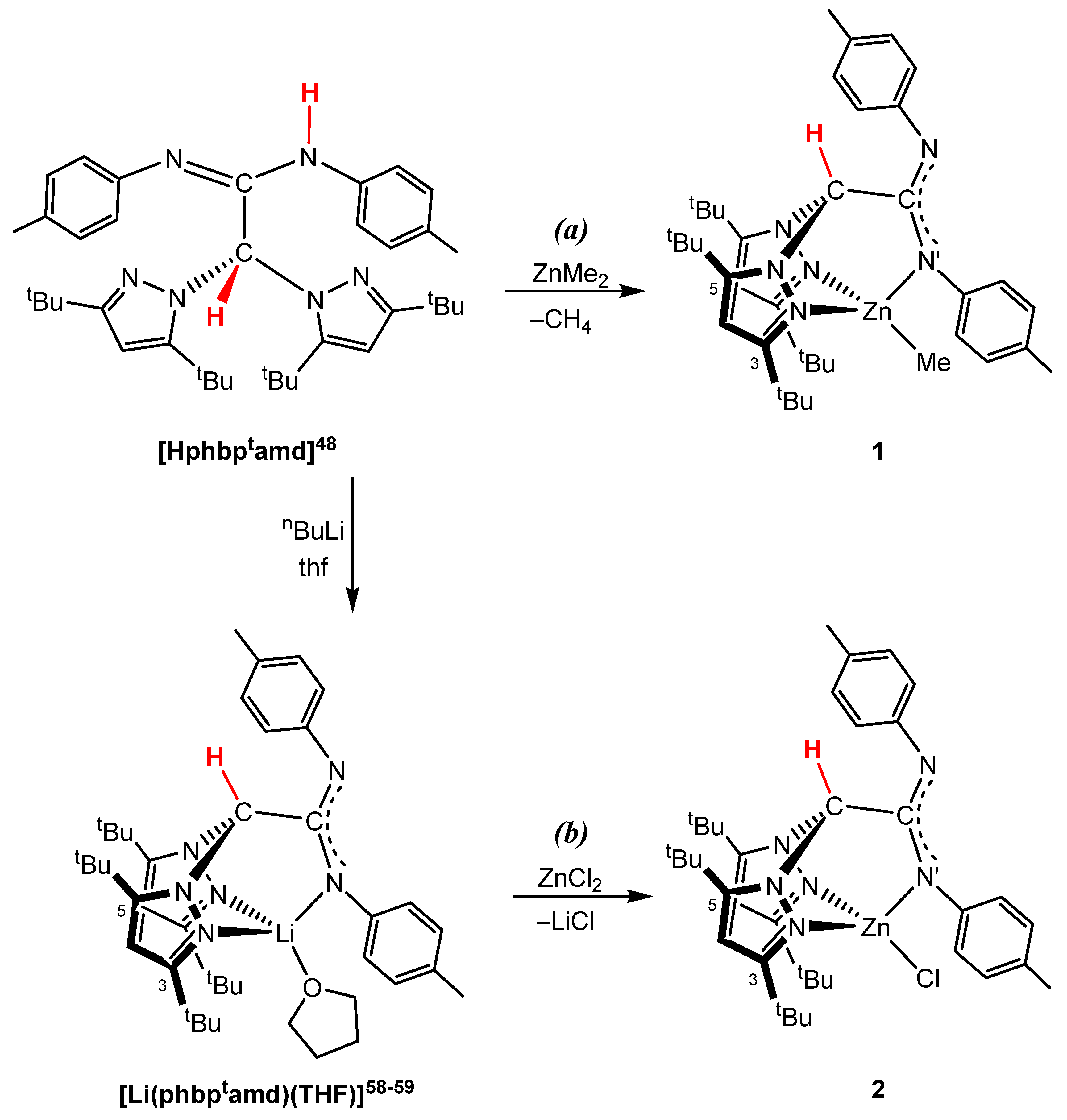
2.3.1. Preparation of Compounds 1–2
2.3.2. Typical Polymerisation Procedures
3. Results
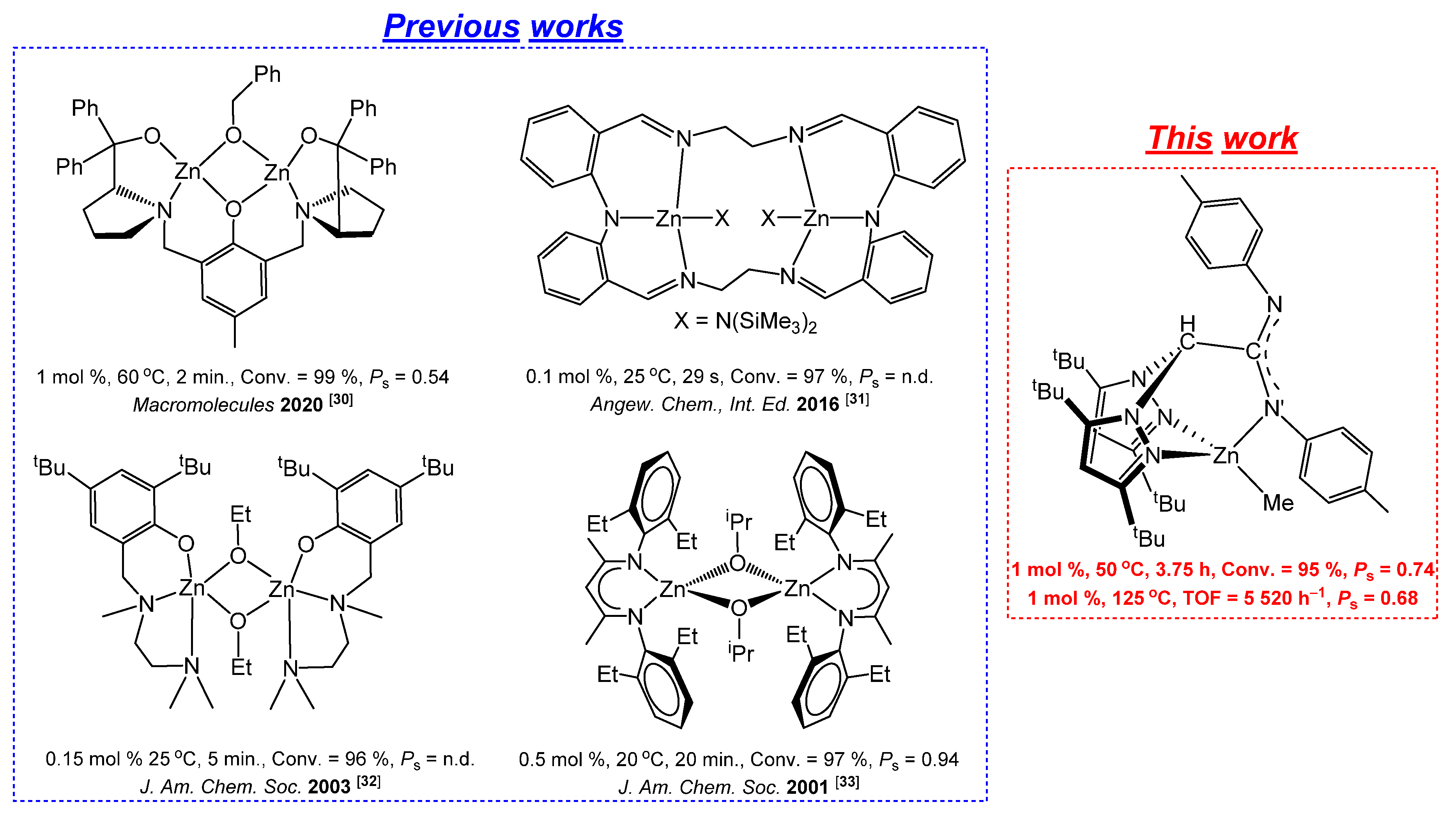
3.1. Synthesis and Characterisation of NNN’-Scorpionate Alkyl and Chloride Zinc Complexes 1 and 2
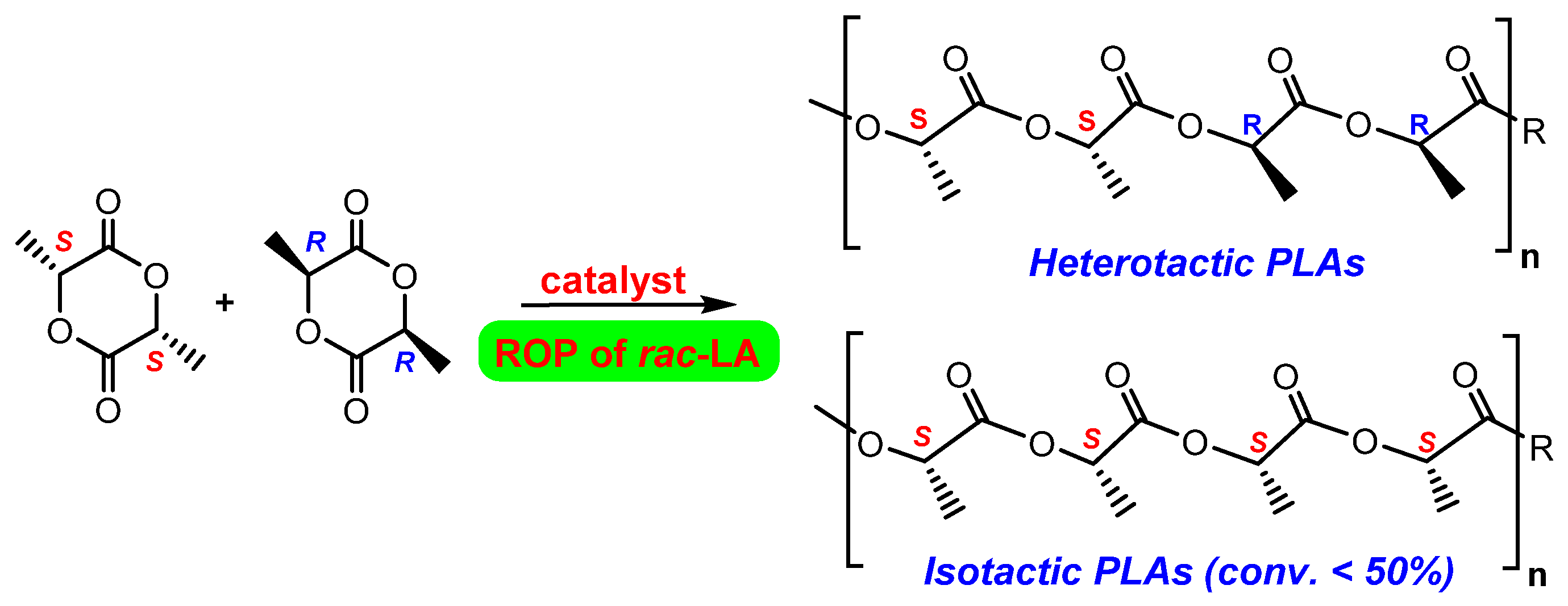
3.2. Catalytic Studies on the ROP of rac-LA for the Production of Poly(rac-lactide) 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amouroux, J.; Siffert, P.; Pierre Massué, J.; Cavadias, S.; Trujillo, B.; Hashimoto, K.; Rutberg, P.; Dresvin, S.; Wang, X. Carbon Dioxide: A new Material for Energy Storage. Prog. Nat. Sci. Mater. Int. 2014, 24, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Ellen MacArthur Foundation, Completing the Picture: How the Circular Economy Tackles Climate Change. 2019. Available online: https://www.ellenmacarthurfoundation.org/assets/downloads/Completing_The_Picture_How_The_Circular_Economy-_Tackles_Climate_Change_V3_26_September.pdf (accessed on 20 May 2021).
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current Applications of Poly(Lactic Acid) Composites in Tissue Engineering and Drug Delivery. Compos. Part B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Grand View Research, Polylactic Acid Market Size, Share&Trends Analysis Report By End-Use (Packaging, Textile, Agriculture, Automotive&Transport, Electronics), by Region (North America, APAC, Europe), and Segment Forecasts, 2021–2028. Available online: https://www.grandviewresearch.com/industry-analysis/polylactic-acid-pla-market (accessed on 12 April 2021).
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The Path Forward for Biofuels and Biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Fevre, M.; Jones, G.O.; Waymouth, R.M. Catalysis as an Enabling Science for Sustainable Polymers. Chem. Rev. 2018, 118, 839–885. [Google Scholar] [CrossRef]
- Galbis, J.A.; García-Martín, M.D.G.; de Paz, M.V.; Galbis, E. Synthetic Polymers from Sugar-Based Monomers. Chem. Rev. 2016, 116, 1600–1636. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, S.M.; Kirillov, E.; Sarazin, Y.; Carpentier, J.-F. Beyond Stereoselectivity, Switchable Catalysis: Some of the Last Frontier Challenges in Ring-Opening Polymerization of Cyclic Esters. Chem. Eur. J. 2015, 21, 7988–8003. [Google Scholar] [CrossRef]
- Cameron, D.J.A.; Shaver, M.P. Aliphatic Polyester Polymer Stars: Synthesis, Properties and Applications in Biomedicine and Nanotechnology. Chem. Soc. Rev. 2011, 40, 1761–1776. [Google Scholar] [CrossRef] [Green Version]
- Di Lorenzo, M.L.; Androsch, R.; Abdel-Rahman, M.A.; Biernesser, A.B. Synthesis, Structure and Properties of Poly(Lactic Acid); Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Jiménez, A.; Peltzer, M.; Ruseckaite, R. Poly(Lactic Acid) Science and Technology: Processing, Properties, Additives and Applications; Royal Society of Chemistry: London, UK, 2015. [Google Scholar]
- Auras, R. Poly(Lactic Acid) Synthesis, Structures, Properties, Processing, and Applications; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Dubois, P.; Coulembier, O.; Raquez, J.-M. Handbook of Ring-Opening Polymerization; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Institute for Bioplastics and Biocomposites. Biopolymers-Facts and Statistics; Edition 2020; Institute for Bioplastics and Biocomposites: Hanover, Germany, 2020; Available online: https://www.ifbb-hannover.de/en/facts-and-statistics.html (accessed on 29 April 2021).
- Qi, J.; Zhang, Y.; Liu, X.; Zhang, Q.; Xiong, C. Preparation and Properties of A Biodegradable Poly(Lactide-Co-Glycolide)/Poly(Trimethylene Carbonate) Porous Composite Scaffold for Bone Tissue Engineering. New J. Chem. 2020, 44, 14632–14641. [Google Scholar] [CrossRef]
- Pêgo, A.P.; Siebum, B.; Van Luyn, M.J.A.; Gallego y Van Seijen, X.J.; Poot, A.A.; Grijpma, D.W.; Feijen, J. Preparation of Degradable Porous Structures Based on 1,3-Trimethylene Carbonate and D,L-Lactide (Co)Polymers for Heart Tissue Engineering. Tissue Eng. 2003, 9, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic Acid (PLA) Controlled Delivery Carriers for Biomedical Applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Bi, H.; Feng, T.; Li, B.; Han, Y. In Vitro and In Vivo Comparison Study of Electrospun PLA and PLA/PVA/SA Fiber Membranes for Wound Healing. Polymers 2020, 12, 839. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and New Opportunities on Barrier Performance of Biodegradable Polymers for Sustainable Packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Choi, W.; Richers, C.P. Ring-Opening Polymerization of Cyclic Monomers by Biocompatible Metal Complexes. Production of Poly(Lactide), Polycarbonates, and Their Copolymers. Macromolecules 2007, 40, 3521–3523. [Google Scholar] [CrossRef]
- Liu, N.; Liu, D.; Liu, B.; Zhang, H.; Cui, D. Stereoselective Polymerization of rac-Lactide Catalyzed by Zwitterionic Calcium Complexes. Polym. Chem. 2021, 12, 1518–1525. [Google Scholar] [CrossRef]
- Bhattacharjee, J.; Harinath, A.; Sarkar, A.; Panda, T.K. Alkaline Earth Metal-Mediated Highly Iso-selective Ring-Opening Polymerization of rac-Lactide. Chem. Asian J. 2020, 15, 860–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devaine-Pressing, K.; Oldenburg, F.J.; Menzel, J.P.; Springer, M.; Dawe, L.N.; Kozak, C.M. Lithium, Sodium, Potassium and Calcium Amine-Bis(Phenolate) Complexes in the Ring-Opening Polymerization of rac-Lactide. Dalton Trans. 2020, 49, 1531–1544. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, J.-F.; Sarazin, Y. Alkaline-Earth Metal Complexes in Homogeneous Polymerization Catalysis. In Alkaline-Earth Metal Compounds: Oddities and Applications; Harder, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 141–189. [Google Scholar]
- Devaine-Pressing, K.; Lehr, J.H.; Pratt, M.E.; Dawe, L.N.; Sarjeant, A.A.; Kozak, C.M. Magnesium Amino-Bis(Phenolato) Complexes for the Ring-Opening Polymerization of rac-Lactide. Dalton Trans. 2015, 44, 12365–12375. [Google Scholar] [CrossRef] [Green Version]
- Schofield, A.D.; Barros, M.L.; Cushion, M.G.; Schwarz, A.D.; Mountford, P. Sodium, Magnesium and Zinc Complexes of Mono(Phenolate) Heteroscorpionate Ligands. Dalton Trans. 2009, 1, 85–96. [Google Scholar] [CrossRef]
- Cowan, J.A. The Biological Chemistry of Magnesium; VCH: New York, NY, USA, 1995. [Google Scholar]
- Campbell, N.A. Biology, 3rd ed.; Benjamin Cummings Publishing Company: Redwood City, CA, USA, 1993. [Google Scholar]
- Gruszka, W.; Walker, L.C.; Shaver, M.P.; Garden, J.A. In Situ Versus Isolated Zinc Catalysts in the Selective Synthesis of Homo and Multi-block Polyesters. Macromolecules 2020, 53, 4294–4302. [Google Scholar] [CrossRef]
- Thevenon, A.; Romain, C.; Bennington Michael, S.; White Andrew, J.P.; Davidson Hannah, J.; Brooker, S.; Williams Charlotte, K. Dizinc Lactide Polymerization Catalysts: Hyperactivity by Control of Ligand Conformation and Metallic Cooperativity. Angew. Chem. Int. Ed. 2016, 55, 8680–8685. [Google Scholar] [CrossRef] [Green Version]
- Williams, C.K.; Breyfogle, L.E.; Choi, S.K.; Nam, W.; Young, V.G.; Hillmyer, M.A.; Tolman, W.B. A Highly Active Zinc Catalyst for the Controlled Polymerization of Lactide. J. Am. Chem. Soc. 2003, 125, 11350–11359. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, B.M.; Cheng, M.; Moore, D.R.; Ovitt, T.M.; Lobkovsky, E.B.; Coates, G.W. Polymerization of Lactide with Zinc and Magnesium β-Diiminate Complexes: Stereocontrol and Mechanism. J. Am. Chem. Soc. 2001, 123, 3229–3238. [Google Scholar] [CrossRef]
- Ding, K.; Miranda, M.O.; Moscato-Goodpaster, B.; Ajellal, N.; Breyfogle, L.E.; Hermes, E.D.; Schaller, C.P.; Roe, S.E.; Cramer, C.J.; Hillmyer, M.A.; et al. Roles of Monomer Binding and Alkoxide Nucleophilicity in Aluminum-Catalyzed Polymerization of ε-Caprolactone. Macromolecules 2012, 45, 5387–5396. [Google Scholar] [CrossRef]
- Normand, M.; Dorcet, V.; Kirillov, E.; Carpentier, J.-F. {Phenoxy-Imine}Aluminum Versus-Indium Complexes for the Immortal ROP of Lactide: Different Stereocontrol, Different Mechanisms. Organometallics 2013, 32, 1694–1709. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, S.; Zhou, S. Aluminum Alkyl Complexes: Synthesis, Structure, and Application in ROP of Cyclic Esters. Dalton Trans. 2016, 45, 4471–4485. [Google Scholar] [CrossRef] [PubMed]
- Aluthge, D.C.; Ahn, J.M.; Mehrkhodavandi, P. Overcoming Aggregation in Indium Salen Catalysts for Isoselective Lactide Polymerization. Chem. Sci. 2015, 6, 5284–5292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Roux, E. Recent Advances on Tailor-Made Titanium Catalysts for Biopolymer Synthesis. Coord. Chem. Rev. 2016, 306, 65–85. [Google Scholar] [CrossRef]
- Bakewell, C.; White, A.J.P.; Long, N.J.; Williams, C.K. Metal-Size Influence in Iso-Selective Lactide Polymerization. Angew. Chem. Int. Ed. 2014, 53, 9226–9230. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Hong, C. An Overview of the Synthesis and Synthetic Mechanism of Poly (Lactic Acid). Mod. Chem. Appl. 2014, 2, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Honrado, M.; Sobrino, S.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Garcés, A.; Lara-Sánchez, A.; Rodríguez, A.M. Synthesis of an Enantiopure Scorpionate Ligand by a Nucleophilic Addition to a Ketenimine and a Zinc Initiator for the Isoselective ROP of rac-Lactide. Chem. Commun. 2019, 55, 8947–8950. [Google Scholar] [CrossRef]
- Otero, A.; Fernandez-Baeza, J.; Sanchez-Barba, L.F.; Sobrino, S.; Garces, A.; Lara-Sanchez, A.; Rodriguez, A.M. Mono- and Binuclear Chiral N,N,O-Scorpionate Zinc Alkyls as Efficient Initiators for the ROP of rac-Lactide. Dalton Trans. 2017, 46, 15107–15117. [Google Scholar] [CrossRef] [PubMed]
- Honrado, M.; Otero, A.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Garcés, A.; Lara-Sánchez, A.; Rodríguez, A.M. Synthesis and Dynamic Behavior of Chiral NNO-Scorpionate Zinc Initiators for the Ring-Opening Polymerization of Cyclic Esters. Eur. J. Inorg. Chem. 2016, 2016, 2562–2572. [Google Scholar] [CrossRef]
- Honrado, M.; Otero, A.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Garcés, A.; Lara-Sánchez, A.; Rodríguez, A.M. Copolymerization of Cyclic Esters Controlled by Chiral NNO-Scorpionate Zinc Initiators. Organometallics 2016, 35, 189–197. [Google Scholar] [CrossRef]
- Honrado, M.; Otero, A.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Garcés, A.; Lara-Sánchez, A.; Martínez-Ferrer, J.; Sobrino, S.; Rodríguez, A.M. New Racemic and Single Enantiopure Hybrid Scorpionate/Cyclopentadienyl Magnesium and Zinc Initiators for the Stereoselective ROP of Lactides. Organometallics 2015, 34, 3196–3208. [Google Scholar] [CrossRef]
- Otero, A.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Tejeda, J.; Honrado, M.; Garcés, A.; Lara-Sánchez, A.; Rodríguez, A.M. Chiral N,N,O-Scorpionate Zinc Alkyls as Effective and Stereoselective Initiators for the Living ROP of Lactides. Organometallics 2012, 31, 4191–4202. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, D.; Zhang, W.; Solan, G.A.; Ma, Y.; Sun, W.-H. Recent Progress in the Application of Group 1, 2&13 Metal Complexes as Catalysts for the Ring Opening Polymerization of Cyclic Esters. Inorg. Chem. Front. 2019, 6, 2619–2652. [Google Scholar] [CrossRef]
- Garcés, A.; Sánchez-Barba, L.F.; Fernández-Baeza, J.; Otero, A.; Fernández, I.; Lara-Sánchez, A.; Rodríguez, A.M. Organo-Aluminum and Zinc Acetamidinates: Preparation, Coordination Ability, and Ring-Opening Polymerization Processes of Cyclic Esters. Inorg. Chem. 2018, 57, 12132–12142. [Google Scholar] [CrossRef]
- Otero, A.; Fernández-Baeza, J.; Lara-Sánchez, A.; Sánchez-Barba, L.F. Metal Complexes with Heteroscorpionate Ligands Based on the Bis(Pyrazol-1-yl)Methane Moiety: Catalytic Chemistry. Coord. Chem. Rev. 2013, 257, 1806–1868. [Google Scholar] [CrossRef]
- Sanchez-Barba, L.F.; Alonso-Moreno, C.; Garcés, A.; Fajardo, M.; Fernandez-Baeza, J.; Otero, A.; Lara-Sanchez, A.; Rodriguez, A.M.; Lopez-Solera, I. Synthesis, Structures and Ring-Opening Polymerization Studies of New Zinc Chloride and Amide Complexes Supported by Amidinate Heteroscorpionate Ligands. Dalton Trans. 2009, 38, 8054–8062. [Google Scholar] [CrossRef] [PubMed]
- SAINT v8.37, Bruker-AXS; APEX3 v2016.1.0.; Bruker: Madison, WI, USA, 2016.
- SADABS; Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of Silver and Molybdenum Microfocus X-ray Sources for Single-Crystal Structure Determination. J. Appl. Crystallogr. 2015, 48, 3. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SHELX-2014, Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Rosen, T.; Goldberg, I.; Navarra, W.; Venditto, V.; Kol, M. Divergent [{ONNN}Mg–Cl] Complexes in Highly Active and Living Lactide Polymerization. Chem. Sci. 2017, 8, 5476–5481. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, P.M.; Fuchs, M.; Ohligschläger, A.; Rittinghaus, R.; McKeown, P.; Akin, E.; Schmidt, M.; Hoffmann, A.; Liauw, M.A.; Jones, M.D.; et al. Highly Active N,O Zinc Guanidine Catalysts for the Ring-Opening Polymerization of Lactide. ChemSusChem 2017, 10, 3547–3556. [Google Scholar] [CrossRef] [PubMed]
- Kremer, A.B.; Osten, K.M.; Yu, I.; Ebrahimi, T.; Aluthge, D.C.; Mehrkhodavandi, P. Dinucleating Ligand Platforms Supporting Indium and Zinc Catalysts for Cyclic Ester Polymerization. Inorg. Chem. 2016, 55, 5365–5374. [Google Scholar] [CrossRef] [PubMed]
- Tschan, M.J.L.; Guo, J.; Raman, S.K.; Brule, E.; Roisnel, T.; Rager, M.-N.; Legay, R.; Durieux, G.; Rigaud, B.; Thomas, C.M. Zinc and Cobalt Complexes Based on Tripodal Ligands: Synthesis, Structure and Reactivity toward Lactide. Dalton Trans. 2014, 43, 4550–4564. [Google Scholar] [CrossRef] [Green Version]
- Navarro, M.; Sánchez-Barba, L.F.; Garcés, A.; Fernández-Baeza, J.; Fernández, I.; Lara-Sánchez, A.; Rodríguez, A.M. Bimetallic Scorpionate-Based Helical Organoaluminum Complexes For Efficient Carbon Dioxide Fixation into a Variety of Cyclic Carbonates. Cat. Sci. Tech. 2020, 10, 3265–3278. [Google Scholar] [CrossRef]
- Garcés, A.; Sánchez-Barba, L.F.; Fernández-Baeza, J.; Otero, A.; Lara-Sánchez, A.; Rodríguez, A.M. Studies on Multinuclear Magnesium tert-Butyl Heteroscorpionates: Synthesis, Coordination Ability, and Heteroselective Ring-Opening Polymerization of rac-Lactide. Organometallics 2017, 36, 884–897. [Google Scholar] [CrossRef]
- Gong, Y.; Ma, H. High Performance Benzoimidazolyl-Based Aminophenolate Zinc Complexes for Isoselective Polymerization of rac-Lactide. Chem. Commun. 2019, 55, 10112–10115. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Ma, H. Ring-Opening Polymerization of rac-Lactide, Copolymerization of rac-Lactide and ε-Caprolactone by Zinc Complexes Bearing Pyridyl-Based Tridentate Amino-Phenolate Ligands. Eur. Polym. J. 2019, 119, 289–297. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Ma, H. Stereoselective Polymerization of rac-Lactide Catalyzed by Zinc Complexes with Tetradentate Aminophenolate Ligands in Different Coordination Patterns: Kinetics and Mechanism. Inorg. Chem. 2015, 54, 5839–5854. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-F.; Zhang, C.; Wang, Z.-X. Rapid and Controlled Polymerization of rac-Lactide Using N,N,O-Chelate Zinc Enolate Catalysts. Organometallics 2013, 32, 3262–3268. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, W.; Liu, D.; Li, S.; Liu, X.; Cui, D.; Chen, X. Magnesium and Zinc Complexes Supported by N,O-Bidentate Pyridyl Functionalized Alkoxy Ligands: Synthesis and Immortal ROP of ε-CL and l-LA. Organometallics 2012, 31, 4182–4190. [Google Scholar] [CrossRef]
- Inoue, S. Immortal Polymerization: The Outset, Development, and Application. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 2861–2871. [Google Scholar] [CrossRef]
- Baran, J.; Duda, A.; Kowalski, A.; Szymanski, R.; Penczek, S. Intermolecular Chain Transfer to Polymer with Chain Scission: General Treatment and Determination of kp/ktr in L,L-Lactide Polymerization. Macromol. Rapid Commun. 1997, 18, 325–333. [Google Scholar] [CrossRef]
- Alonso-Moreno, C.; Garcés, A.; Sánchez-Barba, L.F.; Fajardo, M.; Fernández-Baeza, J.; Otero, A.; Lara-Sánchez, A.; Antiñolo, A.; Broomfield, L.; López-Solera, M.I.; et al. Discrete Heteroscorpionate Lithium and Zinc Alkyl Complexes. Synthesis, Structural Studies, and ROP of Cyclic Esters. Organometallics 2008, 27, 1310–1321. [Google Scholar] [CrossRef]
- Drouin, F.; Oguadinma, P.O.; Whitehorne, T.J.J.; Prud’homme, R.E.; Schaper, F. Lactide Polymerization with Chiral β-Diketiminate Zinc Complexes. Organometallics 2010, 29, 2139–2147. [Google Scholar] [CrossRef]
- Sánchez-Barba, L.F.; Hughes, D.L.; Humphrey, S.M.; Bochmann, M. Ligand Transfer Reactions of Mixed-Metal Lanthanide/Magnesium Allyl Complexes with β-Diketimines: Synthesis, Structures, and Ring-Opening Polymerization Catalysis. Organometallics 2006, 25, 1012–1020. [Google Scholar] [CrossRef]


| Distances (Å) | Angles (°) | ||
|---|---|---|---|
| N(1)-Zn(1) | 2.167 (5) | C(39)-Zn(1)-N(5) | 128.2 (2) |
| N(3)-Zn(1) | 2.135 (5) | C(39)-Zn(1)-N(3) | 125.8 (2) |
| N(5)-Zn(1) | 2.034 (5) | N(5)-Zn(1)-N(3) | 90.8 (2) |
| C(39)-Zn(1) | 1.970 (7) | C(39)-Zn(1)-N(1) | 124.2 (2) |
| C(24)-N(6) | 1.295 (8) | N(5)-Zn(1)-N(1) | 91.76 (18) |
| C(24)-N(5) | 1.351 (8) | N(3)-Zn(1)-N(1) | 83.00 (18) |
| C(23)-C(24) | 1.540 (8) | N(6)-C(24)-N(5) | 135.5 (6) |
| N(6)-C(24)-C(23) | 110.3 (5) | ||
| N(5)-C(24)-C(23) | 114.1 (5) | ||
| N(2)-C(23)-N(4) | 110.6 (4) | ||
| N(2)-C(23)-C(24) | 114.9 (5) | ||
| N(4)-C(23)-C(24) | 106.7 (4) | ||
| Entry | Initiator | Temp (°C) | Time (h) | Yield (g) | Conv (%) b | Mn(theor.) (Da) c | Mn (Da) d | Mw/Mnd | Pse |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 50 | 1 | 0.17 | 13 | 1900 | 2500 | 1.05 | 0.74 |
| 2 | 1 | 50 | 2 | 0.35 | 27 | 3900 | 4800 | 1.09 | 0.73 |
| 3 | 1 | 50 | 3 | 0.90 | 70 | 10,100 | 10,400 | 1.12 | 0.74 |
| 4 | 1 | 50 | 3.75 | 1.23 | 95 | 13,700 | 13,000 | 1.13 | 0.74 |
| 5 | 2 | 60 | 6 | 0.67 | 52 | 7500 | 7200 | 1.12 | 0.72 |
| 6 | 1 + HOBn (1:5) | 50 | 1 | 0.78 | 60 | 1700 | 1900 | 1.08 | ND k |
| 7 | 1f | 50 | 7.5 | 2.41 | 93 | 26,800 | 25,200 | 1.12 | ND |
| 8 | 1 | 40 | 24 | — | traces | — | — | — | — |
| 9 | 1g | 50 | 8 | 0.25 | 19 | 2700 | 3100 | 1.06 | ND |
| 10 | 1h | 125 | 1 min | 1.19 | 92 | 13,200 | 12,600 | 1.28 | 0.68 |
| 11 | 2h | 125 | 2 min | 0.76 | 59 | 8500 | 9100 | 1.29 | 0.67 |
| 12 | 1i | 125 | 5 min | 0.54 | 42 | 6000 | 6400 | 1.35 | — |
| 13 | [Zn(Et)(tbptamd)] j, [67] | 110 | 48 | 0.81 | 63 | 9100 | 8700 | 1.11 | 0.68 |
| 14 | [Zn(Me)(bpzampe)] j, [42] | 20 | 4 | 1.10 | 85 | 12,200 | 12,300 | 1.15 | 0.62 |
| 15 | [Zn(CH2SiMe3)(R,R)-bpzmm)]2 j, [46] | 50 | 3.5 | 0.94 | 73 | 10,500 | 10,600 | 1.08 | 0.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, M.; Garcés, A.; Sánchez-Barba, L.F.; de la Cruz-Martínez, F.; Fernández-Baeza, J.; Lara-Sánchez, A. Efficient Bulky Organo-Zinc Scorpionates for the Stereoselective Production of Poly(rac-lactide)s. Polymers 2021, 13, 2356. https://doi.org/10.3390/polym13142356
Navarro M, Garcés A, Sánchez-Barba LF, de la Cruz-Martínez F, Fernández-Baeza J, Lara-Sánchez A. Efficient Bulky Organo-Zinc Scorpionates for the Stereoselective Production of Poly(rac-lactide)s. Polymers. 2021; 13(14):2356. https://doi.org/10.3390/polym13142356
Chicago/Turabian StyleNavarro, Marta, Andrés Garcés, Luis F. Sánchez-Barba, Felipe de la Cruz-Martínez, Juan Fernández-Baeza, and Agustín Lara-Sánchez. 2021. "Efficient Bulky Organo-Zinc Scorpionates for the Stereoselective Production of Poly(rac-lactide)s" Polymers 13, no. 14: 2356. https://doi.org/10.3390/polym13142356
APA StyleNavarro, M., Garcés, A., Sánchez-Barba, L. F., de la Cruz-Martínez, F., Fernández-Baeza, J., & Lara-Sánchez, A. (2021). Efficient Bulky Organo-Zinc Scorpionates for the Stereoselective Production of Poly(rac-lactide)s. Polymers, 13(14), 2356. https://doi.org/10.3390/polym13142356










